Sustainable Strategies for the Exploitation of End-of-Life Permanent Magnets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Process Description
2.1.1. Hydrometallurgical Process
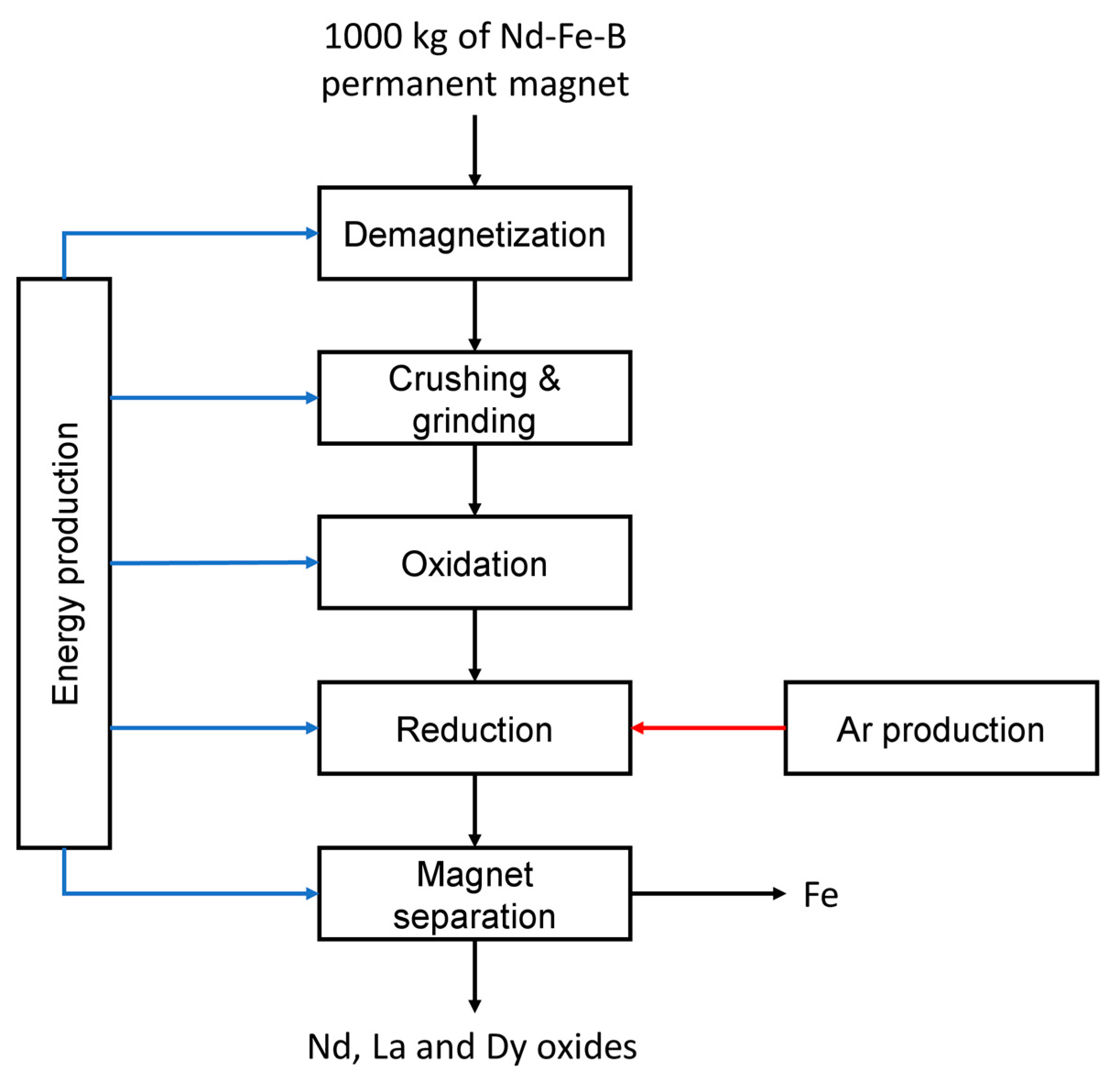
2.1.2. Pyrometallurgical Process
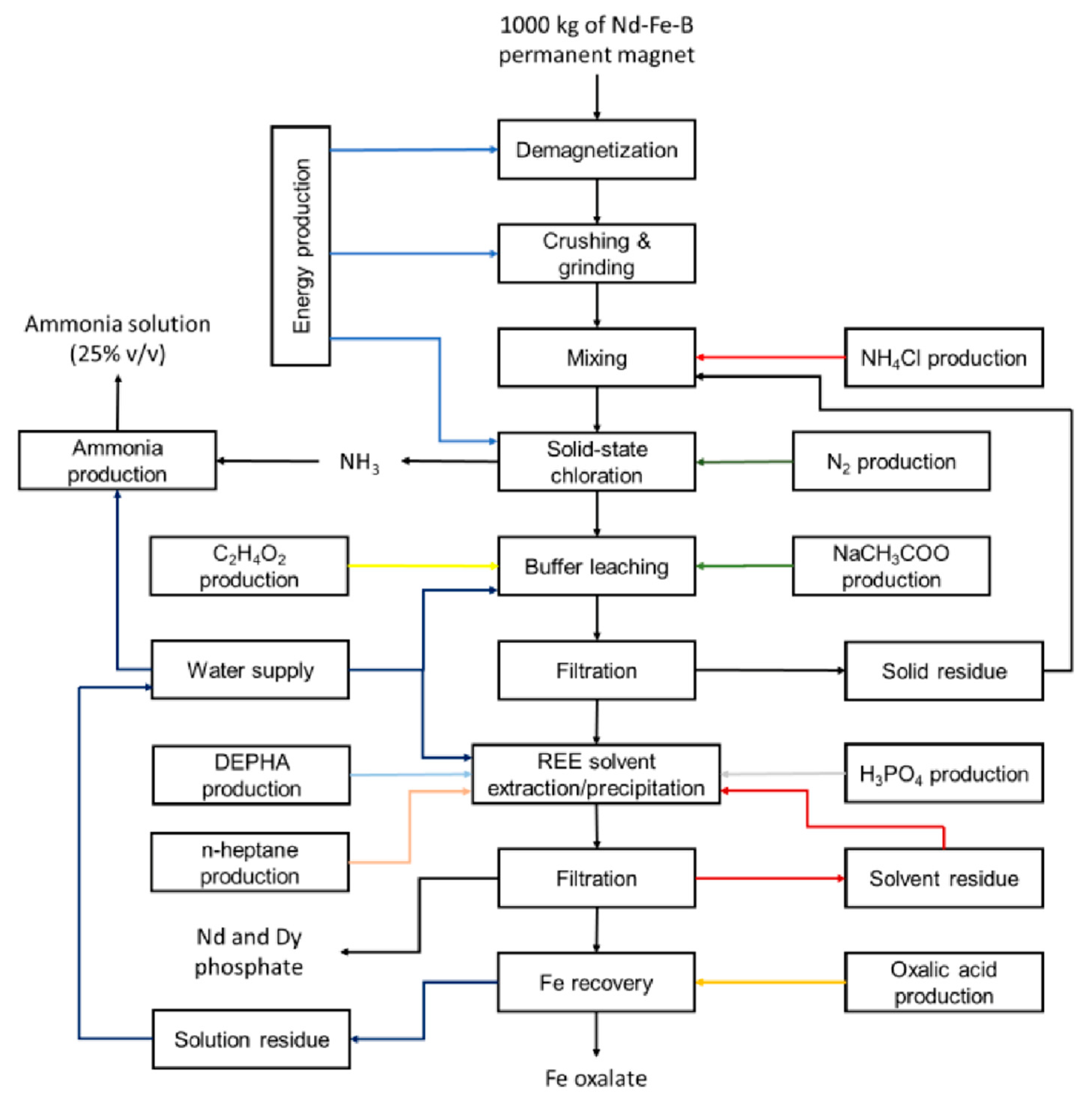
2.1.3. Solid-State Chlorination Process
2.2. Life Cycle Assessment
- Industrial machineries are considered to calculate the energy demand for the mechanical/physical treatments (e.g., demagnetisation, grinding, heating) [42];
- The 95% recirculation of the organic solvent for REE extraction is considered [11];
- The NH3 gas, generated during the solid-state chlorination, is recovered and used to produce an ammonia solution with a final concentration of 25% (v/v) and a purity higher than 99% [10];
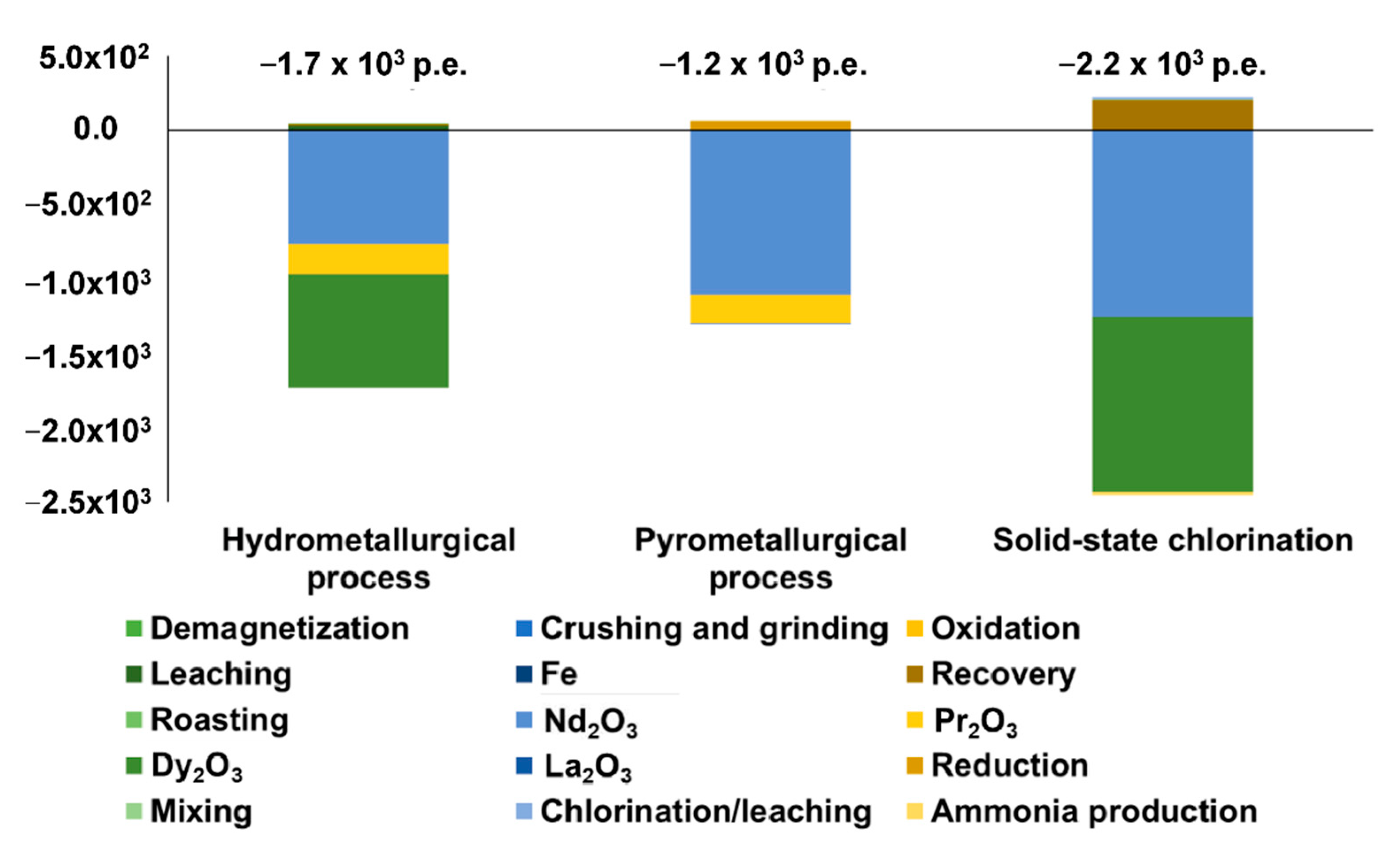
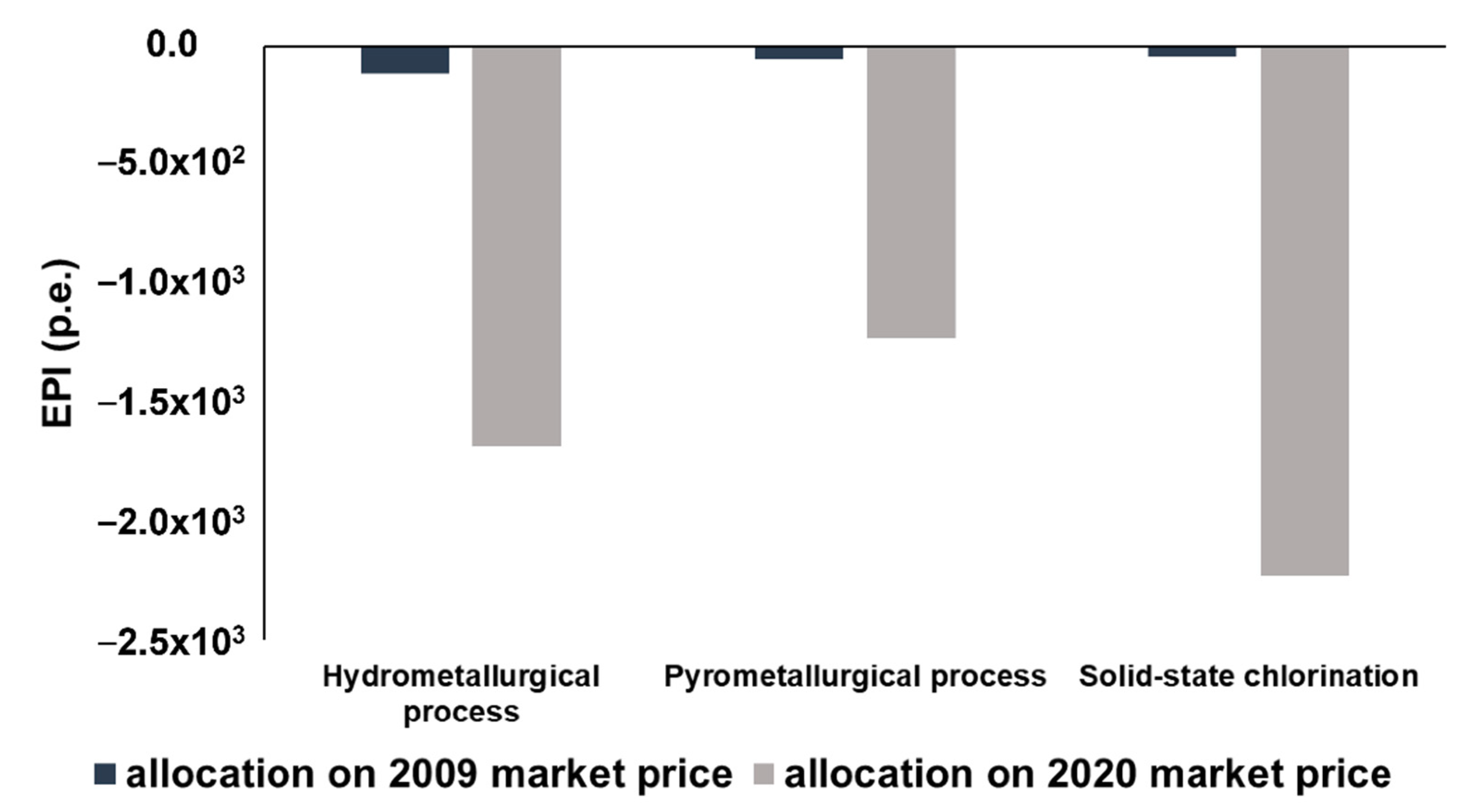
- Considering the absence of Nd, Pr and Dy oxides in the reference database, their environmental burdens have been estimated by the allocation of La2O3 impact, on the market price basis [49];
- The oxalic acid production process, considered for the analysis is that described by Santoro et al. (1999), using milk whey as carbon source [50].
3. Results
4. Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. The European Green Deal. Eur. Commun. 2019, 53, 24. [Google Scholar] [CrossRef]
- Oberle, B.; Bringezu, S.; Hatfileld-Dodds, S.; Hellweg, S.; Schandl, H.; Clement, J. Global Resources Outlook 2019; International Resource Panel, United Nations Environment Programme: Paris, France, 2020. [Google Scholar] [CrossRef]
- Nakamura, H. The current and future status of rare earth permanent magnets. Scr. Mater. 2018, 154, 273–276. [Google Scholar] [CrossRef]
- Yin, X.; Yue, M.; Lu, Q.; Liu, M.; Wang, F.; Qiu, Y.; Liu, W.; Zuo, T.; Zha, S.; Li, X.; et al. An efficient process for recycling Nd–Fe–B sludge as high-performance sintered magnets. Engineering 2020, 6, 165–172. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Van Gerven, T.; Jones, P.T.; et al. REE recovery from end-of-life NdFeB permanent magnet scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- München, D.D.; Veit, H.M. Neodymium as the main feature of permanent magnets from hard disk drives (HDDs). Waste Manag. 2017, 61, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, J.H.; Kleijn, R.; Yang, Y. Recycling as a strategy against rare earth element criticality: A systemic evaluation of the potential yield of NdFeB magnet recycling. Environ. Sci. Technol. 2013, 47, 10129–10136. [Google Scholar] [CrossRef] [PubMed]
- Pan, S. Rare Earth Permanent Magnet Alloys’ High Temperature Phase Transformation: In Situ and Dynamic Observation and Its Application in Material Design; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kumari, A.; Sinha, M.K.; Pramanik, S.; Sahu, S.K. Recovery of rare earths from spent NdFeB magnets of wind turbine: Leaching and kinetic aspects. Waste Manag. 2018, 75, 486–498. [Google Scholar] [CrossRef]
- Lorenz, T.; Bertau, M. Recycling of rare earth elements from FeNdB-Magnets via solid-state chlorination. J. Clean. Prod. 2019, 215, 131–143. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Birloaga, I.; De Michelis, I.; Ferella, F.; Innocenzi, V.; Ippolito, N.M.; Jimenez, C.P.; Vegliò, F.; Beolchini, F. Sustainability analysis of innovative technologies for the rare earth elements recovery. Renew. Sustain. Energy Rev. 2019, 106, 41–53. [Google Scholar] [CrossRef]
- Elwert, T.; Goldmann, D.; Roemer, F.; Schwarz, S. Recycling of NdFeB magnets from electric drive motors of (Hybrid) electric vehicles. J. Sustain. Metall. 2017, 3, 108–121. [Google Scholar] [CrossRef]
- Hurst, C. China’s Rare Earth Elements Industry: What Can the West Learn? Institute for the Analysis of Global Security: Washington, DC, USA, 2010. [Google Scholar]
- Sullivan, K.; Thomas, S.; Rosano, M. Using industrial ecology and strategic management concepts to pursue the sustainable development goals. J. Clean. Prod. 2018, 174, 237–246. [Google Scholar] [CrossRef]
- Okabe, T.H.; Shirayama, S. Method and Apparatus for Recovery of Rare Earth Element. U.S. Patent 8323592 B2, 4 December 2012. [Google Scholar]
- Gnanam, S.; Rajendran, V. Facile sol-gel preparation of Cd-doped cerium oxide (CeO2) nanoparticles and their photocatalytic activities. J. Alloys Compd. 2018, 735, 1854–1862. [Google Scholar] [CrossRef]
- Murase, K.; Machida, K.; Adachi, G. Recovery of rare metals from scrap of rare earth intermetallic material by chemical vapour transport. J. Alloys Compd. 1995, 217, 218–225. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Van Gerven, T. Recycling of NdFeB magnets using sulfation, selective roasting, and water leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Ellis, T.W.; Schmidt, F.A. Recycling of Rare Earth Metals from Rare Eart-Transition Metal Alloy Scrap by Liquid Metal Extraction. U.S. Patent 5,437,709, 1 August 1995. [Google Scholar]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Phase equilibrium of the system Ag-Fe-Nd, and Nd extraction from magnet scraps using molten silver. J. Alloys Compd. 2004, 379, 305–313. [Google Scholar] [CrossRef]
- Moore, M.; Gebert, A.; Stoica, M.; Uhlemann, M.; Löser, W. A route for recycling Nd from Nd-Fe-B magnets using Cu melts. J. Alloys Compd. 2015, 647, 997–1006. [Google Scholar] [CrossRef]
- Hua, Z.; Wang, J.; Wang, L.; Zhao, Z.; Li, X.; Xiao, Y.; Yang, Y. Selective extraction of rare earth elements from NdFeB scrap by molten chlorides. ACS Sustain. Chem. Eng. 2014, 2, 2536–2543. [Google Scholar] [CrossRef]
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Recycling of rare earths from scrap. In Handbook on the Physics and Chemistry of Rare Earths, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 43. [Google Scholar] [CrossRef]
- Saito, T.; Sato, H.; Ozawa, S.; Yu, J.; Motegi, T. The extraction of Nd from waste Nd-Fe-B alloys by the glass slag method. J. Alloys Compd. 2003, 353, 189–193. [Google Scholar] [CrossRef]
- Yang, Y.; Abrahami, S.; Xiao, Y. Recovery of rare earth elements from EOL permanent magnets with slag extraction. In Proceedings of the 3rd International Slag Valorisation Symposium, Leuven, Belgium, 19–20 March 2013; pp. 249–252. [Google Scholar]
- Belova, V.V. Development of solvent extraction methods for recovering rare earth metals. Theor. Found. Chem. Eng. 2017, 51, 599–609. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Rajesh Kumar, J.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Abrahami, S.T.; Xiao, Y.; Yang, Y. Rare-earth elements recovery from post-consumer hard-disc drives. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2015, 124, 106–115. [Google Scholar] [CrossRef]
- Lyman, J.W.; Palmer, G.R. Recycling of rare earths and iron from NdFeB magnet scrap. High Temp. Mater. Process. 1993, 11, 175–188. [Google Scholar] [CrossRef]
- Ellis, T.W.; Schmidt, F.A.; Jones, L.L. Methods and opportunities in the recycling of rare earth based materials. In Proceedings of the Metallurgical Society (TMS) conference on high performance composites, Rosemont, IL, USA, 10–15 October 1994. No. IS-M-796, CONF-9410184-5. [Google Scholar]
- Lee, J.; Jha, A.K.; Kumari, A.; Kumar, J.R.; Jha, M.K.; Kumar, V. Neodymium recovery by precipitation from synthetic leach liquor of concentrated rare earth mineral. J. Metall. Mater. Sci. 2011, 53, 349–354. [Google Scholar]
- Lee, C.H.; Chen, Y.J.; Liao, C.H.; Popuri, S.R.; Tsai, S.L.; Hung, C.E. Selective leaching process for neodymium recovery from scrap Nd-Fe-B magnet. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 5825–5833. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Field, K.D.; Emmert, M.H. Rare earth recovery from end-of-life motors employing green chemistry design principles. Green Chem. 2016, 18, 753–759. [Google Scholar] [CrossRef]
- Itakura, T.; Sasai, R.; Itoh, H. Resource recovery from Nd-Fe-B sintered magnet by hydrothermal treatment. J. Alloys Compd. 2006, 408–412, 1382–1385. [Google Scholar] [CrossRef]
- Tanaka, Y.; Zhang, Q.; Saito, F. Sono chemical recovery of metals from recording media. J. Chem. Eng. Japan. 2002, 35, 173–177. [Google Scholar] [CrossRef]
- Bian, Y.Y.; Guo, S.Q.; Xu, Y.L.; Tang, K.; Lu, X.G.; Ding, W.Z. Recovery of rare earth elements from permanent magnet scraps by pyrometallurgical process. Rare Met. 2015, 1-6. [Google Scholar] [CrossRef]
- Birloaga, I.P.; Vegliò, F. Hydrometallurgical Process for the Treatment of Permanent Magnets. IT102018 2018:000005178. WO2019215583A1, 2019. [Google Scholar]
- UNI EN ISO 14040:2006. Environmental Management—Life Cycle Assessment—Principles and Framework. 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 28 April 2021).
- ISO 14044:2006. Environmental Management—Life Cycle Assessment—Requirements and Guidelines. 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 28 April 2021).
- European Commission. Characterisation Factors of the ILCD Recommended Life Cycle Impact Assessment Methods; EUR 25167 EN; European Commission: Brussels, Belgium, 2012. [Google Scholar] [CrossRef]
- Zampori, L.; Pant, R. Suggestions for Updating the Product Environmental Footprint (PEF) Method; Publication Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Van der Hulst, M.K.; Huijbregts, M.A.J.; van Loon, N.; Theelen, M.; Kootstra, L.; Bergesen, J.D.; Hauck, M. A systematic approach to assess the environmental impact of emerging technologies: A case study for the GHG footprint of CIGS solar photovoltaic laminate. J. Ind. Ecol. 2020, 24, 1234–1249. [Google Scholar] [CrossRef]
- Caduff, M.; Huijbregts, M.A.J.; Koehler, A.; Althaus, H.J.; Hellweg, S. Scaling relationships in Life Cycle Assessment: The case of heat production from biomass and heat pumps. J. Ind. Ecol. 2014, 18, 393–406. [Google Scholar] [CrossRef]
- Gavankar, S.; Suh, S.; Keller, A.A. The role of scale and technology maturity in Life Cycle Assessment of emerging technologies: A case study on carbon nanotubes. J. Ind. Ecol. 2015, 19, 51–60. [Google Scholar] [CrossRef]
- Safaat, J.; Haque, N.; Rosano, M.; Biswas, W. Application of a life cycle assessment to compare environmental performance in coal mine tailings management. J. Environ. Manag. 2017, 199, 181–191. [Google Scholar] [CrossRef]
- Amato, A.; Mastrovito, M.; Becci, A.; Beolchini, F. Environmental sustainability analysis of case studies of agriculture residue exploitation. Sustainability 2021, 13, 3990. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.M.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and green rust: Synthesis, properties, and environmental applications of mixed-valent iron minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Y.; Wang, Y.; Zhao, J.; Chen, X.; Dai, Y.; Yao, Y. Enhanced electrochemical properties of iron oxalate with more stable Li+ ions diffusion channels by controlling polymorphic structure. Chem. Eng. J. 2020, 384, 123281. [Google Scholar] [CrossRef]
- ISE. Rare Earth Prices in January 2020. Available online: https://en.institut-seltene-erden.de/preise-fuer-seltene-erden-im-januar-2020/ (accessed on 28 April 2021).
- Santoro, R.; Cameselle, C.; Rodrigues-Couto, S.; Sanroman, A. Influence of milk whey, nitrogen and phosphorus concentration on oxalic acid production by Aspergillus Niger. Bioprocess Eng. 1991, 20, 1–5. [Google Scholar] [CrossRef]
- Belboom, S.; Szöcs, C.; Léonard, A. Environmental impacts of phosphoric acid production using di-hemihydrate process: A Belgian case study. J. Clean. Prod. 2015, 108, 978–986. [Google Scholar] [CrossRef]
- Schmidt, A.; Frydendal, J. Methods for calculating the environmental benefits of ‘green’ products. In Buying into the Environment, 1st ed.; Erdmenger, C., Ed.; Taylor & Francis Group: Sheffield, UK, 2003; p. 30. [Google Scholar]
- Frischknecht, R.; Braunschweig, A.; Hofstetter, P.; Suter, P. Human health damages due to ionising radiation in life cycle impact assessment. Environ. Impact Assess. Rev. 2000, 20, 159–189. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare Earth Elements: Industrial applications and economic dependency of Europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef] [Green Version]



| Process | Step | Input | Output |
|---|---|---|---|
| Hydrometallurgical process | Demagnetisation | 58 kWh | 1000 kg permanent magnet |
| Crushing and grinding | 10 kWh | 1000 kg magnet powder (<150 µm) | |
| Oxidation | 369 kWh | 1365 kg oxidized magnet powder | |
| Leaching | 13,600 kg water 250 kg HCl 164 kWh | 1080 kg solid residue (Fe2O3 93%) 13,100 kg leaching solution | |
| Precipitation | 257 kg oxalic acid 41 kWh | 662 kg REE oxalates 12,700 kg solution residue | |
| Roasting | 94 kWh | 304 kg REE oxides | |
| Pyrometallurgical process | Demagnetisation | 58 kWh | 1000 kg permanent magnet |
| Crushing and grinding | 10 kWh | 1000 kg magnet powder (<150 µm) | |
| Oxidation | 434 kWh | 1338 kg oxidized magnet powder | |
| Reduction | 6688 kWh 0.15 kg Ar | 1070 kg oxidized magnet powder + Fe | |
| Magnet separation | 1 kWh | 615 kg Fe 455 kg REE oxides | |
| Solid-state chlorination | Demagnetisation | 66 kWh | 1000 kg permanent magnet |
| Crushing and grinding | 100 kWh | 1000 kg magnet powder (<100 µm) | |
| Mixing | 1500 kg NH4Cl | 2500 kg magnet powder + NH4Cl | |
| Solid-state chlorination | 1740 kWh 116 kg N2 | 480 kg NH3 1990 kg magnet powder + NH4Cl | |
| Ammonia production | 1910 kg water | 1910 kg ammonia solution | |
| Buffer leaching | 21,000 kg water 630 kg C2H4O2 30 kg NaCH3COO · 2H2O | 316 kg magnet residue need a second solid-state chlorination 23,040 kg leaching solution | |
| REE solvent extraction/precipitation | 2259 kg DEPHA 1581 kg n-heptane 2032 kg H3PO4 5646 kg water | 463 kg REE phosphate 23,150 kg solution residue | |
| Fe recovery | 4630 kg oxalic acid | 1535 kg Fe oxalate 20,000 kg solution residue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becci, A.; Beolchini, F.; Amato, A. Sustainable Strategies for the Exploitation of End-of-Life Permanent Magnets. Processes 2021, 9, 857. https://doi.org/10.3390/pr9050857
Becci A, Beolchini F, Amato A. Sustainable Strategies for the Exploitation of End-of-Life Permanent Magnets. Processes. 2021; 9(5):857. https://doi.org/10.3390/pr9050857
Chicago/Turabian StyleBecci, Alessandro, Francesca Beolchini, and Alessia Amato. 2021. "Sustainable Strategies for the Exploitation of End-of-Life Permanent Magnets" Processes 9, no. 5: 857. https://doi.org/10.3390/pr9050857
APA StyleBecci, A., Beolchini, F., & Amato, A. (2021). Sustainable Strategies for the Exploitation of End-of-Life Permanent Magnets. Processes, 9(5), 857. https://doi.org/10.3390/pr9050857