Abstract
Mineral fertilization is considered to be useful for improving soil fertility and yields. However, its use is linked to global warming and soil and water pollution by its rapid mobilization. On the other hand, organic fertilization is recommended to maintain or improve soil organic carbon and total nitrogen stocks while contributing to climate change mitigation. The main goal of this study was to assess the effect of two different fertilizer types, mineral and organic, during three cowpea crop cycles on the soil’s physicochemical properties, enzyme activities, crop yield, crop quality and nutritional composition when considering two cowpea cultivars (Feijão frade de fio preto (FP) and Feijão frade de fio claro (FC)). The use of mineral fertilizers was seen to contribute to improved soil fertility due to the increase in soil properties, such as recalcitrant carbon, total nitrogen, ammonium content, available K and available Mg. On the other hand, organic fertilizers only increased the nitrate content in the soil. There were no differences in terms of cowpea crop yield, quality and nutritional composition by fertilizer type. Thus, both fertilizer types contributed to the same crop yield and quality, and thus the use of organic fertilizers can result in a sustainable alternative for maintaining cowpea crop yield and quality.
1. Introduction
Cowpea (Vigna unguiculata (L.) Walp.) was first cultivated in Africa, and its cultivation has extended to tropical and semi-arid regions of the world [1]. Its seeds, leaves and green pods are valued for their nutritional properties, since its grains are rich in protein (about 25%), vitamins, fibers, minerals and other nutrients [2,3]. This legume is a drought-tolerant and warm weather crop which can be used not only in human food, but also for animal feed and the recovery of soil fertility through its use as green manure [4]. Cowpea as a legume crop needs a symbiotic association with effective nitrogen-fixing bacteria to attain maximal benefits from symbiotic N2 fixation and thus save costly N fertilizers. Cowpea establishes symbiotic relationships with several species of the phyla Alpha- and Betaproteobacteria [5].
The use of mineral or organic fertilizers is directly related to soil fertility, soil carbon sequestration, greenhouse gas (GHG) emissions and crop yield. Mineral fertilizers play an important role in terms of the rapid increase of soil fertility and crop yield due to their high nutrient content and ease of availability [6]. However, this type of fertilization could not be the most effective or adequate, considering the maintenance of soil fertility over time, since an excessive application of mineral fertilizers can lead to low nutrient efficiency and deterioration of the soil and environment [7,8]. In terms of carbon sequestration, the use of mineral fertilizers has shown to have a rapid influence on the soil, since it can promote mineralization of a stable fraction of organic matter due mainly to their high N rates, or it can increase carbon stocks by plant biomass production [9,10,11]. On the other hand, the application of N-rich mineral fertilizers used to sustain crop yield are responsible for 60% of global anthropogenic N2O emissions. This greenhouse gas is primarily produced with the application of mineral fertilizers, providing a N source in a form directly available to the plant as NO3−, which can be reduced to gas by denitrifying microorganisms [12,13,14].
Organic fertilizers have higher organic matter and richer nutrient elements, although they are not readily available to the plant and they have to be microbially mineralized before becoming available to the plant [15]. In turn, the use of organic fertilizers has usually been associated with lower crop yields [16]. However, there are studies in which the application of manure acted as a better fertilizer than mineral fertilizers for increasing crop yields [17,18]. In terms of soil, organic fertilizers lead to the increase of carbon stocks, which enhances the benefits for plant growing and reduces soil erosion [19,20]. They can enhance the soil’s physical properties such as aggregate stability, as well as the soil’s biological and biochemical properties, and optimize the soil’s microbial community structure [21,22,23,24]. In contrast to mineral fertilizers, the use of organic fertilizers results in positive long-term effects [10]. In addition, they can reduce the environmental impact in terms of GHG emissions, since organic farming uses N more efficiently by increasing the soil organic carbon (SOC) and N storage, and thus it is responsible for the lower GHG emissions [25,26].
According to the latter approaches, where cowpea was studied mostly in terms of symbiotic efficiency, stress response, genetic and morphological diversity or its nutritional characteristics, a cowpea crop was cultivated using mineral and organic fertilizers for three years. The objectives of this study were to (1) assess the effect of mineral and organic fertilizers on soil organic carbon content and pools, soil nutrients, soil aggregation, soil enzyme activities and the crop yield and quality parameters, and (2) infer if there is a relationship between soil fertility and crop yield and quality. We hypothesized that the use of mineral fertilizers would increase cowpea crop yield, since this is a source of nutrients directly available to the plant. However, the use of organic fertilizers would result in higher organic carbon and nutrient contents and thus higher microbial activity.
2. Materials and Methods
2.1. Study Site and Experimental Design
This study was carried out in the same field in Cartagena in southeastern Spain (37°41′ N 0°57′ E) for three years. The field experiment was designed as a complete randomized block with four replications, and the plot had a size of 100 m2. Two local Portuguese cultivars (Feijão frade de fio preto (FP) and Feijão frade de fio claro (FC)) of cowpea (Vigna unguiculata (L.) Walp.) were grown during the spring and summer of 2014 (from 29 May 2014 to 13 August 2014), 2015 (from 3 June 2015 to 14 September 2015) and 2016 (from 1 June 2016 to 22 August 2016). This crop was drip irrigated, and two fertilizer types (mineral and organic) were used.
The soil was a Haplic Calcisol [27] with a clay loam texture, the main characteristics of which are shown in Table 1. The mean annual temperature of the study area was 18 °C, and the mean annual precipitation was 275 mm. Annual potential evapotranspiration surpassed 900 mm. Cowpea seeds were sown with a spacing of 100 cm between rows and 20 cm between plants (5 plants m−2). No herbicide treatment was provided, and the crops were kept free of weeds through hand-hoeing when necessary.

Table 1.
Main soil characteristics. Values are mean ± standard deviation (n = 4).
Every year, a surface application of 16,000 kg ha−1 of goat and sheep manure was carried out in all plots before sowing. This manure had the following characteristics: pH = 8.32 ± 0.07; electrical conductivity = 21.2 ± 1.01 mS cm−1; total organic carbon content = 307 ± 11 g kg−1; total nitrogen content = 13.4 ± 0.7 g kg−1; P2O5 content = 1.8 ± 0.08%; and K2O content = 4.6 ± 0.2%. Fertilizer application in the cowpea plots started between two or three weeks after sowing and continued until harvesting. In the cowpea crops, 30 kg ha−1 of N and 2.4 kg ha−1 of P2O5 were applied by mineral fertigation of ammonium nitrate (33.5% N) and monoammonium phosphate (61% P2O5, 12% N) and a commercial liquid organic fertilizer (Bombardier, Agroquímicos los Triviños, Spain; 10.7% w/v N, 0.7% w/v P2O5) in the organic fertigation. Cowpea residues were removed from the field and not applied in the soil as green manure. The irrigation was established on the basis of the evapotranspiration rate, crop coefficient and climatic conditions of rainfall.
2.2. Soil and Plant Sampling
The soil was sampled after harvesting the cowpea crop for three successive years. All plots were sampled at 0–20 cm (Ap horizon). Three random soil samples per plot were collected and homogenized to obtain a composite sample, which was air-dried for 7 days, sieved (<2 mm) and stored at room temperature until analyzed. Enzyme activities were also measured in the air-dried samples, since this property is medium-term stable in stored air-dried samples of Mediterranean semiarid soils [28].
The cowpea crop yield was determined by weighing all the pods in each plot harvested when the seeds were dried at the end of the crop cycle. The weight of 100 seeds and protein content in the seeds were recorded as crop quality parameters.
2.3. Soil Analyses
The following parameters were measured: bulk density by the cylinder method [29]; soil pH and electrical conductivity (EC) in deionized water (1:2.5 and 1:5 w/v, respectively); soil texture by the Bouyoucos method [30]; equivalent calcium carbonate using the volumetric method (Bernard calcimeter) [31]; SOC by the wet oxidation method using K2Cr2O7 [32]; recalcitrant carbon (RC) and labile carbon (LC) by the method of double acid hydrolysis [33]; aggregate stability (AS) by the method proposed by [34], based on the application of simulated rainfall with a known intensity; total nitrogen (Nt) by the Kjeldahl method [35]; cation exchange capacity using BaCl2 as an exchangeable salt [36]; NO3−, extracted with deionized water in a 1:10 soil–extractant ratio [37] and measured by ion chromatography (Metrohm 861); NH4+, extracted with 2 M KCl in a 1:10 soil–extractant ratio [37] and colorimetrically measured [38]; and available phosphorus (P), extracted according to the Burriel-Hernando method [39] using a Burriel-Hernando solution (0.2 g CaCO3, 0.17 g MgCO3, 5 mL glacial acetic acid and 0.2 mL H2SO4 in 2 L deionized water) in a 1:25 soil–extractant ratio. The available boron (B) was determined in the deionized water extract [40]. Exchangeable Ca, Mg, Na and K were determined in the BaCl2 extract for CEC, and the P, B, Ca, Mg, Na and K concentrations were measured using ICP-MS (Agilent 7500CE). The β-glucosidase activity was based on the determination of p-nitrophenol released after incubation at 37 °C with β-D-glucopyranoside [41], while β-glucosaminidase activity was based on the determination of p-nitrophenol released after incubation with p-nitrofenil-β-D-glucopyranoside at 37 °C [42]. The dehydrogenase activity was determined using p-iodonitrotetrazolium chloride as a substrate and measuring the absorbance of the iodonitrotetrazolium formazam (INTF) produced [43]. The arylesterase activity was based on the determination of p-nitrophenol released after incubation with p-nitrophenyl acetate at 37 °C [44]. The cellulase activity was assessed by the determination of gearbox sugars using amorphous cellulose as a substrate [45,46], and the urease activity was based on the determination of ammonium released after incubation of the soil with urea at 37 °C [47].
2.4. Plant Analyses
The plant samples were oven dried and ground (A11 Basic, IKA, Staufen, Germany) before incinerating at 500 °C. The ashes were dissolved in 0.6 N HNO3 and analyzed for P, Ca, Mg, Na and K by ICP-MS (7500 CE, Agilent, Madrid, Spain). The nitrogen (N) content was determined by the Kjeldahl method [35]. NO3− was extracted with deionized water in a 1:50 plant–extractant ratio [37] and measured by ion chromatography (Metrohm 861, Metrohm Hispania, Madrid, Spain). The total organic carbon (TOC) was quantified by the total combustion.
2.5. Statistical Analyses
The data were checked to ensure normal distribution using the Kolmogorov–Smirnov test and log-transformed when necessary to ensure normal distribution. The data were submitted to two-way repeated ANOVA measures, with the year (2014, 2015 and 2016) as a within-subject factor and the cowpea cultivar (FP and FC) and fertilizer type (mineral and organic) as between-subject factors. The relationships among the properties were studied using Pearson’s correlations. A principal components analysis (PCA) was performed with all data to study the structure of dependence and correlation established among the variables studied with both fertilizer types. Statistical analyses were performed with IBM SPSS version 22 software for Windows (IBM, New Yok, United States).
3. Results
3.1. Soil Physicochemical Properties
As a general pattern, the crop year significantly affected all soil properties, with general variations along the three crop cycles in most properties (Table 2 and Table 3). The SOC, Nt, AS, RC, NO3− and P were higher in 2016 than in the first two years. The NH4+ showed significantly higher values during the first year, while LC, Ca, Mg, K and Na showed significantly higher values during the second year.

Table 2.
Soil organic carbon, total nitrogen, aggregates stability, recalcitrant carbon, labile carbon, ammonium and nitrate contents in the soil of cowpea crops using mineral and organic fertilizers over three years. Values are mean ± standard deviation (n = 4).

Table 3.
Exchangeable calcium, magnesium, potassium and available phosphorus in the soil of cowpea crops using mineral and organic fertilizers over three years. Values are mean ± standard deviation (n = 4).
The cowpea cultivar only significantly influenced the RC and NH4+ (Table 2). Both soil properties were higher in the soils cultivated with FC than FP in 2015 and 2016. With regard to fertilizer type, the use of mineral fertilizers significantly contributed to higher values of Nt, RC, NH4+, Mg and K, mainly in 2015 and 2016 compared with the organic fertilizers. However, the NO3− content was higher using organic rather than mineral fertilizers. There was no significant effect of fertilizer type on the SOC, AS, LC, Ca, P and Na. The interaction of the year and fertilizer type was significant with respect to AS, RC and NO3−. AS and NO3− showed the highest values using organic fertilizers during 2016, while RC showed the highest values when using mineral fertilizers during 2016. The interaction of the year and cowpea cultivar was significant for the RC, which was higher in the soil cultivated with FC during 2016 than during the last two years. The interaction of the cowpea cultivar and fertilizer type did not affect any of the soil properties. Finally, the interaction of the three factors (year, cowpea cultivar and fertilizer type) was significant for NH4+, with the highest values in the FP cultivar using mineral fertilizers during 2016.
Nt was positively correlated with the RC (r = 0.82, p < 0.01). The Ca content was positively correlated with the Mg (r = 0.96, p < 0.01) and K contents (r = 0.85, p < 0.01). The Na content was negatively correlated with the available P (r = −0.83, p < 0.01).
3.2. Soil Enzyme Activities
Enzyme activities were significantly influenced by the crop year (Table 4). The β-glucosidase and cellulase activities showed the highest values during 2014. The dehydrogenase and arylesterase activities showed the highest values during 2015 and 2016, respectively. The activity of β-glucosaminidase was significantly higher during 2015 and 2016. The cowpea cultivar only significantly influenced β-glucosaminidase activity, with higher values in the FP than in the FC cultivar. The fertilizer type only significantly influenced the β-glucosidase activity, which was higher with the use of mineral fertilizer. The only interaction between factors that was significant was that of the year and fertilizer type for arylesterase activity. Arylesterase showed significantly higher activity in the cowpea cultivated with organic fertilizers during the first year. Dehydrogenase was positively correlated with Ca (r = 0.87, p < 0.01), Mg (r = 0.90, p < 0.01) and K (r = 0.88, p < 0.01). The other enzyme activities were not correlated with any other property.

Table 4.
Dehydrogenase, β-glucosaminidase, β-glucosidase, arylesterase and cellulase activities in the soil of cowpea crops using mineral and organic fertilizers for three successive years. Values are mean ± standard deviation (n = 4).
3.3. Cowpea Yield, Crop Quality and Nutritional Characteristics
As a general trend, the year had a significant effect on the cowpea yield and quality and on the nutritional characteristics (Table 5 and Table 6). The cowpea crop showed the lowest yield in 2015, which was related to a serious virosis episode in this crop during this crop cycle. The cowpea yield and weight of 100 seeds were highest during 2016, while the Ca, Mg, K and P contents showed higher values during 2015. The cowpea cultivar significantly affected the cowpea yield and weight of 100 seeds. The cowpea yield was significantly higher in the FC cultivar, while the weight of 100 seeds was higher in the FP cultivar. The fertilizer type did not significantly affect the cowpea yield, crop quality or nutritional characteristics, indicating that both organic and conventional fertilizers contributed to the same crop yield and quality every year. The interaction of the year and fertilizer was significant for Ca, Mg, K and P, which showed higher values using organic fertilizers during 2015. The other interactions among factors were not significant. The cowpea yield was negatively correlated with Ca (r = −0.83, p < 0.01), Mg (r = −0.81, p < 0.01), K (r = −0.88, p < 0.01) and P (r = −0.86, p < 0.01).

Table 5.
Crop yield and quality of cowpea crops using mineral and organic fertilizers for three successive years. Values are mean ± standard deviation (n = 4).

Table 6.
Nutrient content of cowpea seeds using mineral and organic fertilizers for three successive years. Values are mean ± standard deviation (n = 4).
3.4. Interrelationships between Soil and Crop Properties
The PCA performed with the studied soil physicochemical properties, enzyme activities, cowpea crop yield, crop quality and nutritional characteristics using both fertilizer types showed that 83.3% of the total variation could be explained by the first five PCs. PC1 (which explained 33.2% of the variation) and PC2 (which explained 19.1% of the variation) did not separate the mineral and organic fertilizers (Figure 1A). PC1 was related to soil-exchangeable K, Mg and Ca, soil NH4+, the nutrient content in the seeds and soil enzyme activities (Table 7). PC2 was related to soil B, soil AS, exchangeable Na, bioavailable P and enzyme activities. PC3, which explained 15.5% of the variation, slightly separated the mineral (positive scores) and organic fertilizers (negative scores) (Figure 1B). PC3 was related with higher values of EC, Nt and SOC and lower values of pH (Table 7). PC4 (which explained 9.5% of the variation) and PC5 (which explained 5.9% of the variation) did not separate the mineral and organic fertilizers (Figure 1B,C) and were related to the protein content in the seeds, the weight of the seeds (PC4) and the soil LC (PC5). Therefore, PCA confirmed the lack of differences between both types of fertilizers in terms of crop properties and most soil properties during the three crop cycles.
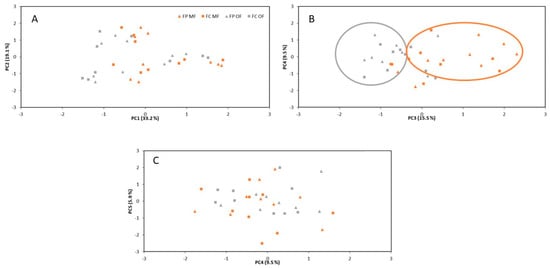
Figure 1.
PCA factor scores of variations in the soil’s physicochemical properties, enzyme activities, cowpea crop yield, crop quality and nutritional characteristics, considering different cowpea cultivar and fertilizer types. The figure type represents the cowpea cultivar (triangle: FP cowpea cultivar; and square: FC cowpea cultivar), and the color represents the fertilizer type (orange: mineral fertilizer; and gray: organic fertilizer). FP: Feijão frade de fio preto; FC: Feijão frade de fio claro; MF: mineral fertilizer; and OF: organic fertilizer. (A) **; (B) **; (C) **. ** p < 0.01.

Table 7.
Matrix of the PCA of the soil’s physicochemical properties, enzyme activities, crop yield, crop quality and nutritional characteristics of the cowpea crop using different fertilizer types for three years.
4. Discussion
The inclusion of a cowpea crop for three years increased the soil’s properties, accompanied by an enhance in the crop yield and quality over time. Cultivation of the cowpea acts as a source of different types of C through rhizodeposition, which stimulates the growth of rhizosphere microorganisms [48] and facilitates nutrient availability for the plant [49]. In addition to this, we observed the effects of the specific cowpea cultivar on the soil properties, soil enzyme activities and crop yield and quality. This is not surprising, as previous reports have observed that the release of root exudates is controlled by the plant genotype [50,51]; thus, the plant genotype could have different effects on the soil properties and soil enzyme activities. On the other hand, this effect would be even larger if cowpea residues were used as green manure, increasing the nutrients’ availability through mineralization of its residues [52,53,54]. In this regard, in this study, we observed a negative relationship between the exchangeable cations and crop yield. In fact, the lowest cowpea crop yield was observed during 2015, which coincided with the highest nutrient availability in the soil (Table 3 and Table 5). This could be explained by the fact that soil sampling was carried out after two weeks of harvesting, and thus the higher the cowpea production and the higher the quantity of nutrients absorbed from the soil by the plants to sustain that production, reducing their concentrations in the soil at the end of the crop cycle. These results indicate that there was no nutrient limitation under either of the fertilized systems, conventional or organic. Since soil nutrients are the main factors limiting the crop yield, a high crop yield mainly depends on nutrient availability through fertilizer application [55], also being highly efficient with the use of organic fertilizers.
As far as the fertilizer type is concerned, both had a similar effect on the microbial activity, with similar soil enzyme activities. In turn, this study showed through PCA that the application of mineral and organic fertilizers had the same effect on the soil’s properties (Figure 1). However, mineral fertilizers contributed to higher RC and Nt contents, which was not associated with a higher crop yield. This may be explained by the fact that the application of mineral fertilizers provides a source of N directly available for crop growth, and this N input acts as a driver for the C cycle in soils since it increases biomass production. Thus, it maintains and builds up the SOC stocks [56,57,58]. On the other hand, organic fertilizers contributed to an increase in NO3− content in the soil. This result may be explained by the fact that the application of fertilizers in the cowpea crop concluded three weeks before soil sampling, and the use of organic fertilizers produced a greater residual effect than mineral fertilizers by continuous mineralization and nitrification in the soil [59]. In fact, some authors have reported that the residual effect from mineral N fertilizers after a long-term fertilization experiment was low, or even that no effect was observed [60,61,62].
The lack of the expected results with improved soil physicochemical and biological properties with the use of organic fertilizers could be explained by the fact that these fertilizers release nutrients slowly, and only a fraction of the N and other nutrients become available to plants in the first year after application [63,64]. However, the nutrient availability was enough to provide high crop yields, with similar values to those with mineral fertilizers. In terms of crop yield, the literature reviewed showed that mineral fertilization of the soil is useful for improving yields through the increase of SOC and N stocks [65,66]. However, this type of fertilization could not be the most effective in light of a high environmental cost [67]. In addition to this, a higher soil N concentration following mineral fertilization may induce microbe-mediated N transformation processes, leading to high N2O emissions [12] or the lack of synchrony between the N release and crop demand, which can stimulate N2O emissions [68]. As a consequence, the use of organic fertilizers has been strongly recommended as a replacement for some or all mineral fertilizers, since this kind of fertilization not only includes mitigation of global warming [69], but their use also results in the reduction of NO3− and can improve crop yields. Despite this, the results of our study indicated, on one hand, that the use of organic fertilizers led to a higher NO3− content in the soil due to a greater residual effect, and on the other hand, there were no significant differences in the crop yield, crop quality or nutritional characteristics due to the fertilizer type (Figure 1). In this context, the use of organic fertilizers may be beneficial to crop yields in the long term [70]. However, there are a number of scientific papers which conclude that the use of organic fertilizers alone as substitutes for mineral fertilizers is not enough to maintain the productivity of high-yielding crops [71,72], while an effective solution could be the partial substitution of mineral fertilizers with organic fertilizers [73]. Irrigation and fertilization are two important factors for obtaining higher productivity [74]. Nevertheless, some other factors such as the cultivar, climate and soil type are also responsible for changing the yield. In this study, we confirmed that the crop yield was indeed influenced by the cowpea cultivar.
5. Conclusions
In conclusion, our results show that the cowpea cultivar influences the soil’s physicochemical properties and crop yield and quality. Mineral fertilizers increased the RC, nitrogen forms (Nt and NH4+) and nutrient availability (Mg and K), but the concentration of soil nutrients with organic fertilizers was also suitable for providing high crop yield and quality. As a consequence, there were no significant differences in the cowpea yield, crop quality or nutritional characteristics by fertilizer type. Thus, although mineral fertilizers increased the soil organic matter and some nutrients in the short term, the nutrient availability and microbial activity were high with the use of both types of fertilizers, leading to the same crop yield.
Author Contributions
Conceptualization, J.A.F. and R.Z.; methodology, R.Z.; software, R.Z.; validation, J.A.F., R.Z. and Á.F.; formal analysis, V.S.-N.; investigation, V.S.-N.; resources, J.A.F.; data curation, V.S.-N.; writing—original draft preparation, V.S.-N.; writing—review and editing, J.A.F., R.Z. and Á.F.; visualization, J.A.F. and R.Z.; supervision, J.A.F. and R.Z.; project administration, J.A.F.; funding acquisition, J.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Commission through the Seventh Framework Programme project n° 613781 “Enhancing of legumes growing in Europe through sustainable cropping for protein supply for food and feed” (EUROLEGUME).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boukar, O.; Coulibaly, O.; Fatokun, C.; Lopez, K.; Tamò, M. Innovative Research along the Cowpea Value Chain, Proceedings of the Fifth World Cowpea Conference on Improving Livelihoods in the Cowpea Value Chain through Advancement in Science, Saly, Senegal, 26 September–1 October 2010; Boukar, O., Coulibaly, O., Fatokun, C.A., Lopez, K., Tamò, M., Eds.; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2012. [Google Scholar]
- Alayande, L.B.; Mustapha, K.B.; Dabak, J.D.; Ubom, G.A. Comparison of nutritional values of brown and white beans in Jos North Local Government markets. Afr. J. Biotechnol. 2012, 11, 10135–10140. [Google Scholar] [CrossRef]
- Avanza, M.; Acevedo, B.; Chaves, M.; Añón, M. Nutritional and anti-nutritional components of four cowpea varieties under thermal treatments: Principal component analysis. Food Sci. Technol. 2013, 51, 148–157. [Google Scholar] [CrossRef]
- Rivas, R.; Falcão, H.M.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C.; Santos, M.G. Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S. Afr. J. Bot. 2016, 103, 101–107. [Google Scholar] [CrossRef]
- Sawada, H.; Kuykendall, L.D.; Young, J.M. Changing concepts in the systematics of bacterial nitrogen-fixing legume symbionts. J. Gen. Appl. Microbiol. 2003, 49, 155–179. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xang, X.J.; Xu, M.G.; Huang, S.M.; Liu, H.; Peng, C. Soil organic carbon dynamics under long-term fertilizations in arable land of northerm China. Biogeosci. Discuss. 2009, 6, 6539–6577. [Google Scholar]
- El-Metwally, I.M.; Abdelhamid, M.T. Weed control under integrated nutrient management systems in faba bean (Vicia faba) production in Egypt. Planta. Daninha. 2008, 26, 585–594. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Goulding, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Leita, L.; De Nobili, M.; Mondini, C.; Muhlbachova, G.; Marchiol, L.; Bragato, G.; Contin, M. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biol. Fertil Soils 1999, 28, 371–376. [Google Scholar] [CrossRef]
- Parham, J.A.; Deng, S.P.; Raun, W.R.; Johnson, G.V. Long-term cattle manure application in soil. I. Effect on soil phosphorus levels, microbial biomass C, and dehydrogenase and phosphatase activity. Biol. Fertil Soils 2002, 35, 328–337. [Google Scholar]
- Tong, X.; Xu, M.; Wang, X.; Bhattacharyya, R.; Zhang, W.; Cong, R. Long-term fertilization effects on organic carbon fractions in a red soil of China. Catena 2014, 113, 251–259. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycle 2002, 16, 1058. [Google Scholar] [CrossRef]
- Meng, L.; Ding, W.; Cai, Z. Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol. Biochem. 2005, 37, 2037–2045. [Google Scholar] [CrossRef]
- Yan, G.; Yao, Z.; Zheng, X.; Liu, C. Characteristics of annual nitrous and nitric oxide emissions from major cereal crops in the North China Plain under alternative fertilizer management. Agric. Ecosyst. Environ. 2015, 207, 67–78. [Google Scholar] [CrossRef]
- Seufert, V.M.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Alaru, M.; Talgre, A.; Eremeev, V.; Tein, B.; Luik, A.; Nemvalts, A.; Loit, E. Crop yield and supply of nitrogen compared in conventional and organic farming systems. Agric. Food Sci. 2014, 23, 317–326. [Google Scholar] [CrossRef]
- Jannoura, R.; Joergensen, R.G.; Bruns, C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur. J. Agron. 2014, 52, 259–270. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Brunori, E.; Farina, R.; Biasi, R. Sustainable viticulture: The carbon-sink function of the vineyard agro-ecosystem. Agric. Ecosyst. Environ. 2016, 223, 10–21. [Google Scholar] [CrossRef]
- Puig-Montserrat, X.; Stefanescu, C.; Torre, I.; Palet, J.; Fàbregas, E.; Dantart, J.; Arrizabalaga, A.; Flaquer, C. Effects of organic and conventional crop management on vineyard biodiversity. Agric. Ecosyst. Environ. 2017, 243, 19–26. [Google Scholar] [CrossRef]
- Mäder, P.; Fliebbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Poulton, P.R. Long-term management impacts on soil C, N and physical fertility. Soil Tillage Res. 2006, 91, 30–38. [Google Scholar] [CrossRef]
- Wang, W.; Niu, J.; Zhou, X.; Wang, Y. Long-term change in land management from subtropical wetland to paddy field shifts soil microbial community structure as determined by PLFA and T-RFLP. Pol. J. Ecol. 2011, 59, 37–44. [Google Scholar]
- Bottinelli, N.; Angers, D.A.; Hallaire, V.; Michot, D.; Le Guillou, C.; Cluzeau, D.; Heddadj, D.; Menasseri-Aubry, S. Tillage and fertilization practices affect soil aggregate stability in a Humic Cambisol of Northwest France. Soil Tillage Res. 2017, 170, 14–17. [Google Scholar] [CrossRef]
- Abbona, E.A.; Sarandón, S.J.; Marasas, M.E.; Astier, M. Ecological sustainability evaluation of traditional management in different vineyard systems in Berisso, Argentina. Agric. Ecosyst. Environ. 2007, 119, 335–345. [Google Scholar] [CrossRef]
- Chiriacò, M.V.; Belli, C.; Chiti, T.; Trotta, C.; Sabbatini, S. The potential carbon neutrality of sustainable viticulture showed through a comprehensive assessment of the greenhouse gas (GHG) budget of wine production. J. Clean. Prod. 2019, 225, 435–450. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources, International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Reports; FAO: Rome, Italy, 2014; Volume 106. [Google Scholar]
- Zornoza, R.; Mataix-Solera, J.; Guerrero, C.; Arcenegui, V.; Mataix-Beneyto, J. Storage Effects on Biochemical Properties of Air-Dried Soil Samples from Southeastern Spain. Arid Land Res. Manag. 2009, 23, 213–222. [Google Scholar] [CrossRef]
- Campbell, D.J.; Hensall, J.K. Bulk density. In Soil Analysis; Smith, K.A., Mullis, C.E., Eds.; Marcel Dekker: New York, NY, USA, 1991; pp. 329–366. [Google Scholar]
- Dewis, J.; Freitas, F. Physical and chemical methods of soil and water analyses. FAO Soils Bulletin 1970, 10, 275. [Google Scholar]
- Cobertera, E. Edafología Aplicada; Ediciones Cátedra: Madrid, Spain, 1993. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Labile, recalcitrant, and inert organic matter in Mediterranean forest soils. Soil Biol. Biochem. 2007, 39, 202–215. [Google Scholar] [CrossRef]
- Roldán, A.; García-Orenes, F.; Lax, A. An incubation experiment to determinate factor involving aggregation changes in an arid soil receiving urban refuse. Soil Biol. Biochem. 1994, 26, 1699–1707. [Google Scholar] [CrossRef]
- Hoeger, R. Büchi Training Papers: Nitrogen Determination According to Kjeldahl; BÜCHI Labortechnik AG: Flawil, Switzerland, 1998. [Google Scholar]
- Roig, A.; Romero, M.; Lax, A.; Fernández, F.G. Estudio comparativo de métodos de determinación de capacidad de cambio catiónica en suelos calizos. Anal. Edaf. Agrobiol. 1980, 39, 2021–2032. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen inorganic forms, Methods of soil analysis part 2 Chemical and microbiological properties, second edition. Agronomy 1982, 9, 643–698. [Google Scholar]
- Kandeler, E.; Gerber, E. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Díez, J.A. Consideraciones sobre la utilización de la técnica extractiva de Burriel-Hernando para la evaluación de fósforo asimilable en suelos. Anal. Edaf. Agrobiol. 1982, 41, 1345–1353. [Google Scholar]
- Porta, J.; López-Acevedo, M.; Rodríguez, R. Técnicas y Experimentos en Edafología; Collegi Official d’Enginyers Agrònoms de Catalunya: Barcelona, Spain, 1986; p. 282. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes in Methods of Soil Analyses part 2: Chemical and Microbiological Properties. Soil Sci. Soc. Am. J. 1982, 903–947. [Google Scholar] [CrossRef]
- Parham, J.A.; Deng, S.P. Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol. Biochem. 2000, 32, 1183–1190. [Google Scholar] [CrossRef]
- Von Merci, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fert. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Zornoza, R.; Landi, L.; Nannipieri, P.; Renella, G. A protocol for the assay of arylesterase activity in soil. Soil Biol. Biochem. 2009, 41, 659–662. [Google Scholar] [CrossRef]
- García-Álvarez, A.; Ibáñez, J.J. Seasonal fluctuations and crop influence on microbiota and enzyme activity in fully developed soils of central Spain. Arid. Soil Res. Rehab. 1994, 8, 161–178. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for determination of glucose. J. Biol. Chem. 1994, 153, 375–380. [Google Scholar] [CrossRef]
- Nannipieri, P.; Johnson, R.L.; Paul, E.A. Criteria for measurement of microbial growth and activity in soil. Soil Biol. Biochem. 1978, 10, 223–229. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Mwafulirwa, L.; Baggs, E.M.; Russell, J.; George, T.; Morley, N.; Sim, A.; Cant, C.D.L.F.; Paterson, E. Barley genotype influences stabilization of rhizodeposition-derived C and soil organic matter mineralization. Soil Biol. Biochem. 2016, 95, 60–69. [Google Scholar] [CrossRef]
- Merbach, W.; Schulze, J.; Richert, M.; Rrocco, E.; Mengel, K. A comparison of different 15N application techniques to study the N net rhizodeposition in the plant–soil system. J. Plant Nutr. Soil Sci. 2000, 163, 375–379. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 2003, 23, 375–393. [Google Scholar] [CrossRef]
- Bukert, A.; Bationo, A.; Possa, K. Mechanism of residue Mulch-induced cereal growth increases in West-African. Soil Sci. Soc. Am. J. 2000, 64, 1–42. [Google Scholar]
- Shah, Z.; Shah, S.H.; Peoples, M.B.; Schwenke, G.D.; Herriedge, D.F. Crop residue and fertilizer N effects on nitrogen fixation and yields of legume-cereal rotations and soil organic fertility. Field Crops Res. 2003, 83, 1–11. [Google Scholar] [CrossRef]
- Shafi, M.; Bakht, J.; Jan, M.T.; Shah, Z. Soil C and N dynamics and maize (Zea may L.) yields as affected by cropping systems and residue management in North-western Pakistan. Soil Tillage Res. 2007, 94, 520–529. [Google Scholar] [CrossRef]
- Galloway, N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 80, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wang, X.; Han, B.; Ouyang, Z.; Duan, X.; Zheng, H.; Miao, H. Soil carbon sequestrations by nitrogen fertilizer application, straw return and no-tillage in China’s cropland. Glob. Chang. Biol. 2009, 15, 281–305. [Google Scholar] [CrossRef]
- Ladha, J.K.; Reddy, C.K.; Padre, A.T.; van Kessel, C. Role of nitrogen fertilization in sustaining organic matter in cultivated soils. J. Environ. Qual. 2011, 40, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Mazzoncini, M.; Sapkota, T.B.; Bàrberi, P.; Antichi, D.; Risaliti, R. Long-term effect of tillage, nitrogen fertilization and cover crops on soil organic carbon and total nitrogen content. Soil Tillage Res. 2011, 114, 165–174. [Google Scholar] [CrossRef]
- Quemada, M.; Menacho, E. Soil respiration 1 year after sewage sludge application. Biol. Fertil Soils 2001, 33, 344–346. [Google Scholar] [CrossRef]
- Cela, S.; Santiveri, F.; Lloveras, J. Residual effects of pig slurry and mineral nitrogen fertilizer on irrigated wheat. Eur. J. Agron. 2011, 34, 257–262. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, B.; Yang, X.; Zhou, J. Fate of residual 15N-labeled fertilizer in dryland farming systems on soils of contrasting fertility. Soil Sci. Plant Nutr. 2015, 61, 846–855. [Google Scholar] [CrossRef]
- Riley, H. Residual value of inorganic fertilizer and farmyard manure for crop yields and soil fertility after long-term use on a loam soil in Norway. Nutr. Cycl. Agroecosystems 2016, 104, 25–37. [Google Scholar] [CrossRef]
- Khaliq, A.; Abbasi, M.K.; Hussain, T. Effects of integrated use of organic and inorganic nutrient sources with effective microorganisms (EM) on seed cotton yield in Pakistan. Bioresour. Technol. 2006, 97, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Maltas, A.; Charles, R.; Jeangros, B.; Sinaj, S. Effect of organic fertilizers and reduced-tillage on soil properties, crop nitrogen response and crop yield: Results of a 12-year experiment in Changins, Switzerland. Soil Tillage Res. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, M.; He, X.; Zhang, W.; Huang, S.; Yang, X.; Liu, H.; Peng, C.; Shirato, Y.; Iizumi, T.; et al. Soil organic carbon sequestration in upland soils of northern China under variable fertilizer management and climate change scenarios. Glob. Biogeochem. Cycles 2014, 28, 319–333. [Google Scholar] [CrossRef]
- Cucci, G.; Lacolla, G.; Summo, C.; Pasqualone, A. Effect of organic and mineral fertilization on faba bean (Vicia faba L.). Sci. Hortic. 2019, 243, 338–343. [Google Scholar] [CrossRef]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Develop. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Berry, P.M.; Sylvester-Bradley, R.; Philipps, L.; Hatch, D.J.; Cuttle, S.P.; Rayans, F.W.; Gosling, P. Is the productivity of organic farms restricted by the supply of available nitrogen. Soil Use Manag. 2002, 18, 248–255. [Google Scholar] [CrossRef]
- Yang, B.; Xiong, Z.; Wang, J.; Xu, X.; Huang, Q.; Shen, Q. Mitigating net global warming potential and greenhouse gas intensities by substituting chemical nitrogen fertilizers with organic fertilization strategies in rice-wheat annual rotation systems in China: A 3-year field experiment. Ecol. Eng. 2015, 81, 289–297. [Google Scholar] [CrossRef]
- Tirol-Padre, A.; Ladha, J.K.; Regmi, A.P.; Bhandari, A.L.; Inubushi, K. Organic amendments affect soil parameters in two long-term rice-wheat experiments. Soil Sci. Soc. Am. J. 2007, 71, 442–452. [Google Scholar] [CrossRef]
- Amoach, A.A.; Senge, M.; Miyagawa, S.; Itou, K. Effects of soil fertility management on growth, yield, and water-use efficiency of maize (Zea mays L.) and selected soil properties. Commun. Soil Sci. Plant Anal. 2012, 43, 924–935. [Google Scholar] [CrossRef]
- Bravo, K.; Toselli, M.; Baldi, E.; Marcolini, G.; Sorrenti, G.; Quartieri, M.; Marangoni, B. Effect of organic fertilization on carbon assimilation and partitioning in bearing nectarine trees. Sci. Hortic. 2012, 137, 100–106. [Google Scholar] [CrossRef]
- Lu, H.J.; Ye, Z.Q.; Zhang, X.L.; Lin, X.Y.; Ni, W.Z. Growth and yield responses of crops and macronutrient balance influenced by commercial organic manure used as a partial substitute for chemical fertilizers in an intensive vegetable cropping system. Phys. Chem. Earth Parts A B C 2011, 36, 387–394. [Google Scholar] [CrossRef]
- Liang, H.; Hu, K.; Batchelor, W.D.; Hu, K.; Qi, Z.; Li, B. An integrated soil-crop system model for water and nitrogen management in North China. Sci. Rep. 2016, 6, 25755. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).