Enhanced Resistance to Amylolysis in Rice Kernels through Interaction with Chlorogenic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Rice-CGA Complex Samples
2.2. Determination of CGA Content in the Complex Samples
2.3. Determination of Antioxidant Activity by TEAC Method
2.4. Determination of Swelling Ability and Water Solubility Index
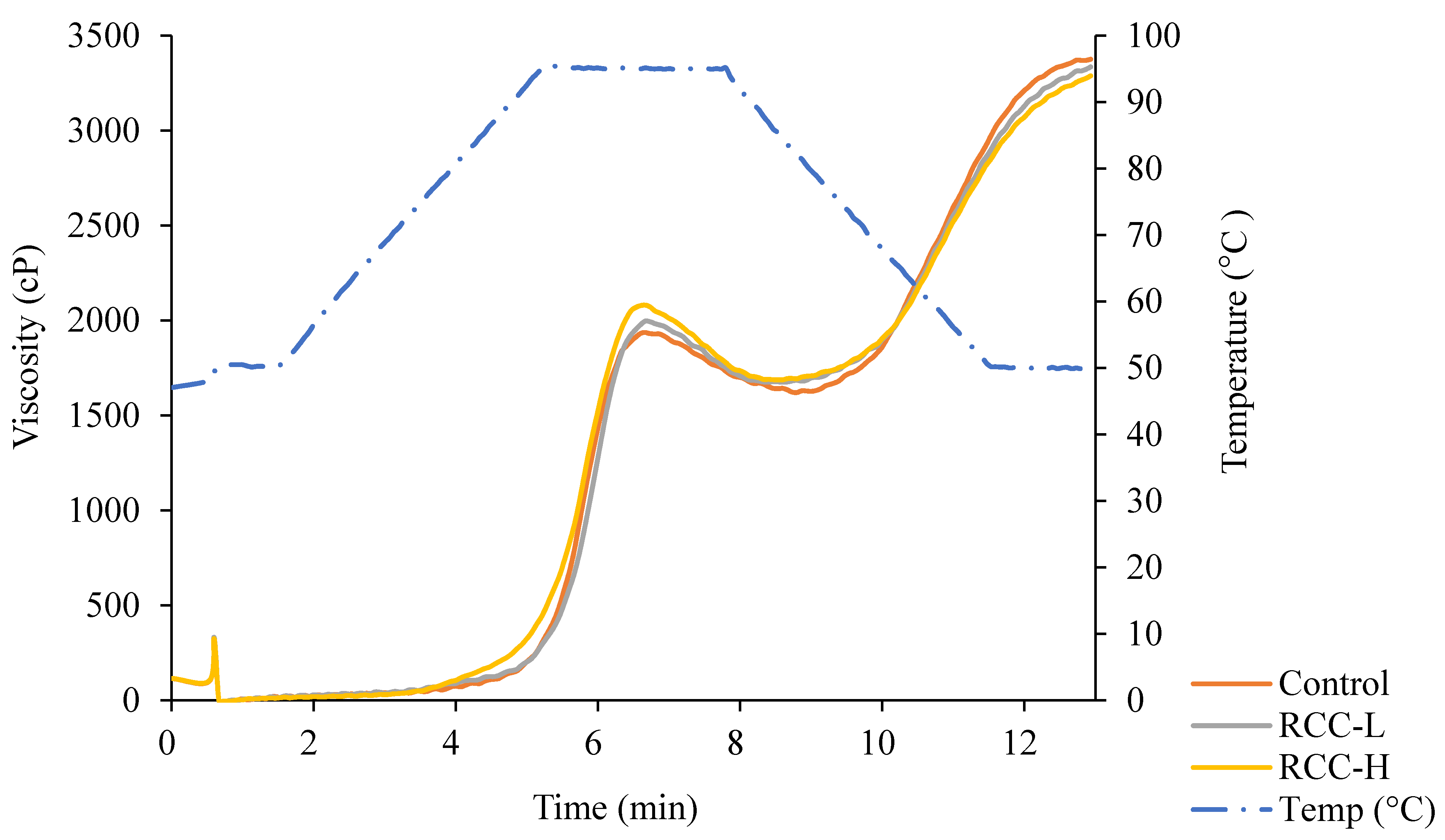
2.5. Determination of Pasting Properties
2.6. Determination of Resistance to Amylolysis
2.7. Determination of In Vivo Starch Digestibility
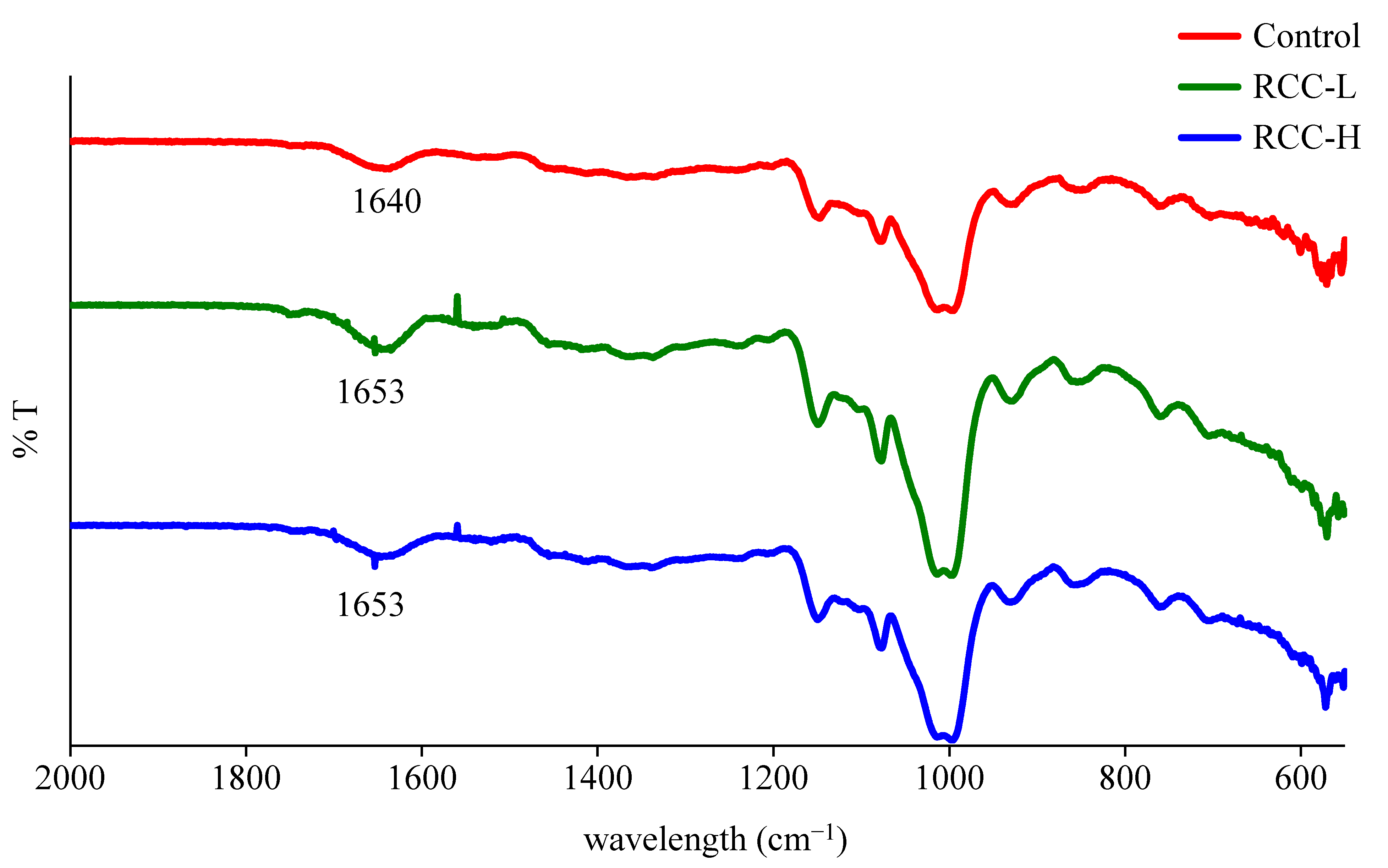
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
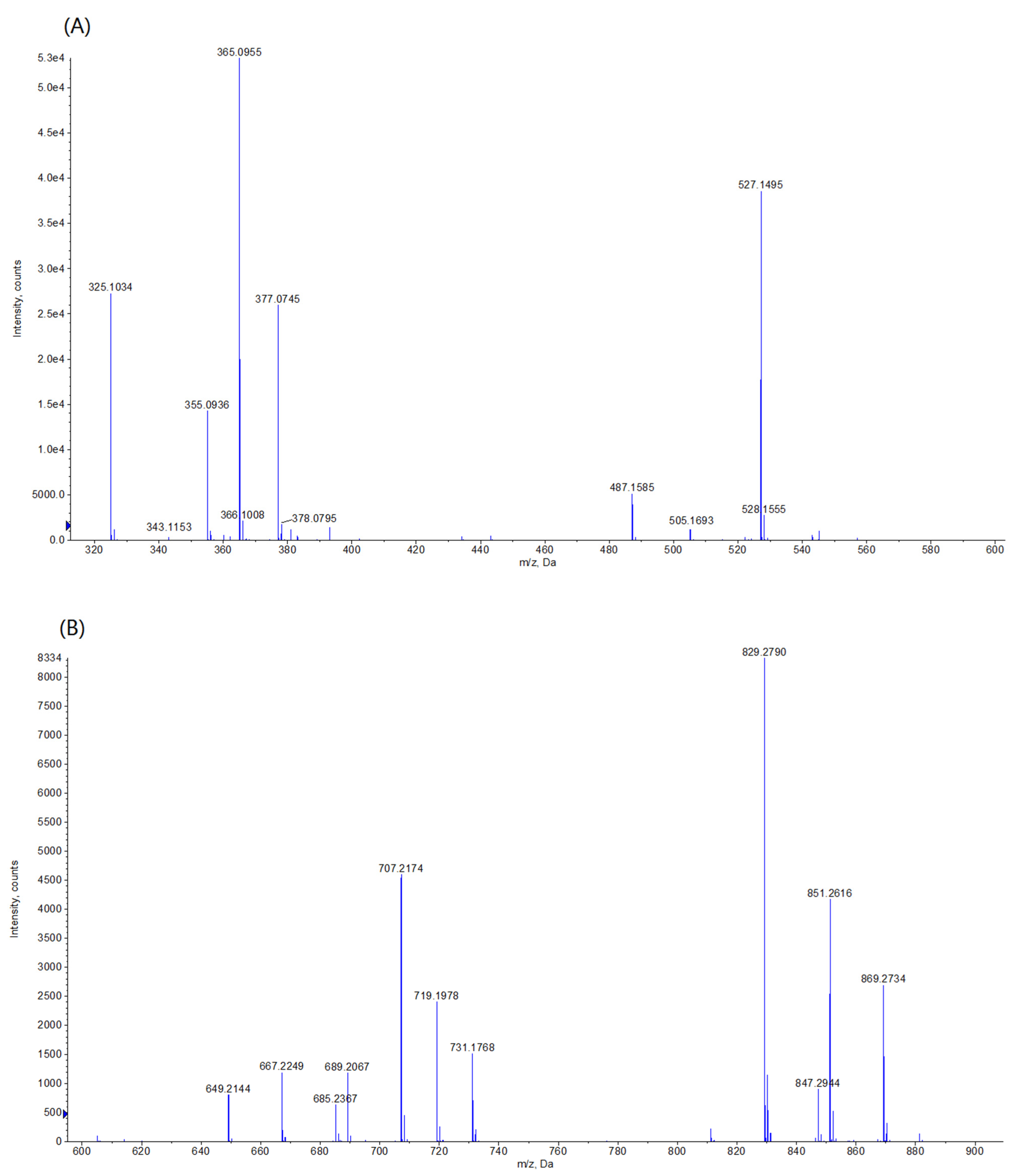
2.9. Electrospray Ionization Time-of-Flight Mass Spectrometry (ESI-TOF-MS) Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. CGA-Binding Capacity in the Complex
3.2. TEAC Value
3.3. Swelling Ability and Water Solubility Index
3.4. In Vitro and In Vivo Starch Digestibility
3.5. Characterization of Interactions
3.6. Pasting Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Kendall, C.W.; Emam, A.; Augustin, L.S.; Jenkins, D.J. Resistant starches and health. J. AOAC Int. 2004, 87, 769–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.Y.; Sun, N.N.; Chau, C.F. Application of corona electrical discharge plasma on modifying the physicochemical properties of banana starch indigenous to Taiwan. J. Food Drug Anal. 2018, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.W. The inhibition of digestive enzymes by polyphenolic compounds. In Nutritional and Toxicological Significance of Enzyme Inhibitors in Foods; Springer: Boston, MA, USA, 1986; pp. 509–516. [Google Scholar]
- Sun, L.; Gidley, M.J.; Warren, F.J. Tea polyphenols enhance binding of porcine pancreatic α-amylase with starch granules but reduce catalytic activity. Food Chem. 2018, 258, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, R.; Zhu, F. Physicochemical interactions of maize starch with ferulic acid. Food Chem. 2016, 199, 372–379. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. J. Enzyme Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, X.; Kong, X.; Chen, S.; Xu, E.; Liu, D.; Ogawa, Y.; Ye, X.; Tian, J. Introduction of chlorogenic acid during extrusion affects the physicochemical properties and enzymatic hydrolysis of rice flour. Food Hydrocoll. 2021, 116, 106652. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.A.; Davis, M.J.; Gopinathan, M.; Milner, M. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.Y.; Chang, C.R.; Chang, T.J.; Chang, Y.J.; Liew, Y.; Chau, C.F. Changes in physicochemical properties of corn starch upon modifications by atmospheric pressure plasma jet. Food Chem. 2019, 283, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.F.; Huang, Y.L.; Lee, M.H. In vitro hypoglycemic effects of different insoluble fiber-rich fractions prepared from the peel of Citrus sinensis L. cv. Liucheng. J. Agric. Food Chem. 2003, 51, 6623–6626. [Google Scholar] [CrossRef]
- Matthews, J.; Altman, D.; Campbell, M.; Royston, P. Analysis of Serial Measurements in Medical Research. Br. Med. J. 1990, 300, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, N.; Lu, X.; Hu, Y.; Kitts, D.D. Application of attenuated total reflectance—Fourier transformed infrared (ATR-FTIR) spectroscopy to determine the chlorogenic acid isomer profile and antioxidant capacity of coffee beans. J. Agric. Food Chem. 2016, 64, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Saura-Calixto, F. Macromolecular antioxidants or non-extractable polyphenols in fruit and vegetables: Intake in four European countries. Int. Food Res. J. 2015, 74, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and antioxidant activity of the complex of tea polyphenols and oat β-glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; D’Arcy, B.R.; Gidley, M.J. Polyphenol-cellulose interactions: Effects of pH, temperature and salt. Int. J. Food Sci. Technol. 2016, 51, 203–211. [Google Scholar] [CrossRef]
- Nguela, J.M.; Poncet-Legrand, C.; Sieczkowski, N.; Vernhet, A. Interactions of grape tannins and wine polyphenols with a yeast protein extract, mannoproteins and β-glucan. Food Chem. 2016, 210, 671–682. [Google Scholar] [CrossRef]
- Simanaviciute, D.; Klimaviciute, R.; Rutkaite, R. Equilibrium adsorption of caffeic, chlorogenic and rosmarinic acids on cationic cross-linked starch with quaternary ammonium groups. Int. J. Biol. Macromol. 2017, 95, 788–795. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Yang, R.; Liu, C.; Hu, X.; Luo, S.; Gong, E.; Ye, J. The relationship between reducing sugars and phenolic retention of brown rice after enzymatic extrusion. J. Cereal Sci. 2017, 74, 244–249. [Google Scholar] [CrossRef]
- Oladele, A.K.; Duodu, K.G.; Emmambux, N.M. Hydrolysis and antioxidant activity of starch modified with phenolic extracts from grape pomace and sorghum bran under alkaline conditions. Carbohydr. Polym. 2020, 240, 116291. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Wani, T.A.; Wani, I.A.; Masoodi, F.A. Comparative study of the physicochemical properties of rice and corn starches grown in Indian temperate climate. J. Saudi Soc. Agric. Sci. 2016, 15, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of Cereal starches II. Waxy rice Starches. Cereal Chem. 1990, 67, 551–557. [Google Scholar]
- Srichuwong, S.; Suharti, C.; Mishima, T.; Isono, M.; Hisamatsu, M. Starches from different botanical sources II: Contribution of starch structure to swelling and pasting properties. Carbohydr. Polym. 2005, 62, 25–34. [Google Scholar] [CrossRef]
- Gunaratne, A.; Corke, H. Influence of prior acid treatment on acetylation of wheat, potato and maize starches. Food Chem. 2007, 105, 917–925. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon tannin decreased the glycemic response through decreasing the digestibility of starch and inhibiting α-amylase, α-glucosidase, and intestinal glucose uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef] [Green Version]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam Repub. Iran 2017, 31, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalita, D.; Kaushik, N.; Mahanta, C.L. Physicochemical, morphological, thermal and IR spectral changes in the properties of waxy rice starch modified with vinyl acetate. J. Food Sci. Technol. 2014, 51, 2790–2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.Q.; Lu, J.P.; Li, X.H.; Tong, Z.F. Effect of mechanical activation on physico-chemical properties and structure of cassava starch. Carbohydr. Polym. 2007, 68, 128–135. [Google Scholar] [CrossRef]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.M.; Coimbra, M.A. Data on coffee composition and mass spectrometry analysis of mixtures of coffee related carbohydrates, phenolic compounds and peptides. Data Brief. 2017, 13, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef]
- Adebowale, A.A.; Sanni, L.O.; Awonorin, S.O. Effect of texture modifiers on the physicochemical and sensory properties of dried fufu. Food Sci. Technol. Int. 2005, 11, 373–382. [Google Scholar] [CrossRef]
- Seetharaman, K.; Tziotis, A.; Borras, F.; White, P.J.; Ferrer, M.; Robutti, J. Thermal and functional characterization of starch from Argentinean corn. Cereal Chem. 2001, 78, 379–386. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Norulaini, N.N.; Omar, A.M.; Yamauchi, H.; Noda, T. RVA analysis of mixtures of wheat flour and potato, sweet potato, yam, and cassava starches. Carbohydr. Polym. 2007, 69, 784–791. [Google Scholar] [CrossRef]
| Samples # | Heating Time (H) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| RCC-L | 2.27 ± 0.13 a,m | 2.60 ± 0.05 a,n | 2.61 ± 0.23 a,n | 2.78 ± 0.20 a,n |
| RCC-H | 3.44 ± 0.91 a,m | 3.95 ± 0.13 b,n | 3.86 ± 0.14 b,n | 3.85 ± 0.27 b,n |
| Samples | TEAC # (nmol Trolox Equivalent/g) | Swelling Ability (g/g) | Water Solubility Index (%) |
|---|---|---|---|
| Control | 236 ± 7 a | 11.13 ± 0.04 a | 8.53 ± 2.04 a |
| RCC-L | 570 ± 3 b | 11.93 ± 0.23 b | 10.20 ± 1.27 a,b |
| RCC-H | 572 ± 3 b | 12.14 ± 0.21 b | 12.44 ± 0.68 b |
| Samples | In Vitro Amylolytic Hydrolysis (Mg/g RCC) | AUC # |
|---|---|---|
| Control | 487.2 ± 77.7 a | 3920 ± 715 a |
| RCC-L | 308.4 ± 24.5 b | 3842 ± 351 a |
| RCC-H | 283.0 ± 35.3 b | 3018 ± 293 b |
| Samples | Peak Viscosity (cP) | Breakdown (cP) | Final Viscosity (cP) | Setback (cP) | Pasting Temperature (°C) |
|---|---|---|---|---|---|
| Control | 1937 a | 316 a | 3376 a | 1775 a | 93.3 a |
| RCC-L | 1997 a,b | 322 a | 3336 a,b | 1661 b | 93.4 a |
| RCC-H | 2081 b | 394 b | 3288 b | 1601 b | 90.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-L.; Huang, Y.-W.; Wu, M.-Z.; Wu, T.-Y.; Lai, P.-S.; Sun, N.-N.; Saw, C.-Y.; Li, C.-W.; Chau, C.-F. Enhanced Resistance to Amylolysis in Rice Kernels through Interaction with Chlorogenic Acid. Processes 2021, 9, 788. https://doi.org/10.3390/pr9050788
Li Y-L, Huang Y-W, Wu M-Z, Wu T-Y, Lai P-S, Sun N-N, Saw C-Y, Li C-W, Chau C-F. Enhanced Resistance to Amylolysis in Rice Kernels through Interaction with Chlorogenic Acid. Processes. 2021; 9(5):788. https://doi.org/10.3390/pr9050788
Chicago/Turabian StyleLi, Yi-Ling, Yu-Wen Huang, Min-Zi Wu, Tsung-Yen Wu, Ping-Shan Lai, Nan-Nong Sun, Chiou-Yeong Saw, Chia-Wei Li, and Chi-Fai Chau. 2021. "Enhanced Resistance to Amylolysis in Rice Kernels through Interaction with Chlorogenic Acid" Processes 9, no. 5: 788. https://doi.org/10.3390/pr9050788
APA StyleLi, Y.-L., Huang, Y.-W., Wu, M.-Z., Wu, T.-Y., Lai, P.-S., Sun, N.-N., Saw, C.-Y., Li, C.-W., & Chau, C.-F. (2021). Enhanced Resistance to Amylolysis in Rice Kernels through Interaction with Chlorogenic Acid. Processes, 9(5), 788. https://doi.org/10.3390/pr9050788







