Utilization of Barley Straw as Feedstock for the Production of Different Energy Vectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Characterization
2.2. Experimental Plan
2.3. Explosive Decompression Pretreatment
2.4. Enzymatic Hydrolysis and Fermentation
2.5. Biomethane Potential
2.6. Characterization of Combustion Behavior
2.7. Sample Analysis Methods
2.8. Biogas Calculations
2.9. Energy Balance Calculations
3. Results and Discussion
3.1. Biomass Characterization
3.2. Bioethanol Production Potential
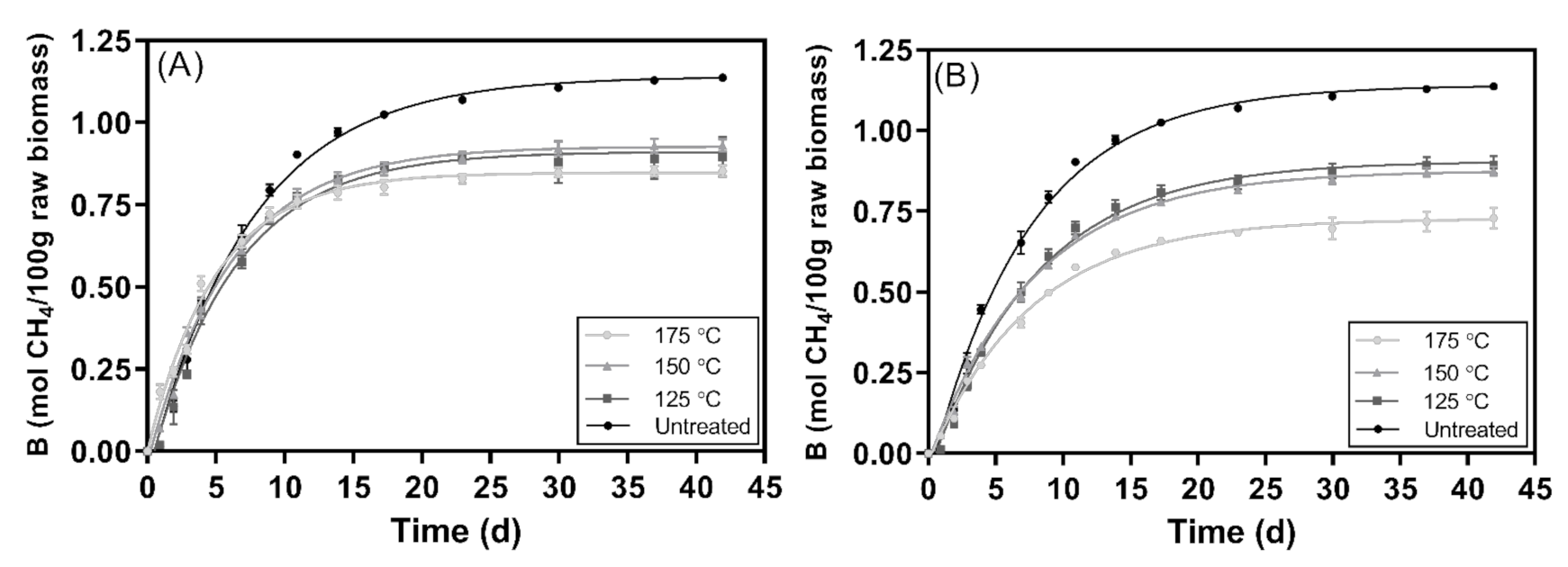
3.3. Methane Recovery
Digestion Time
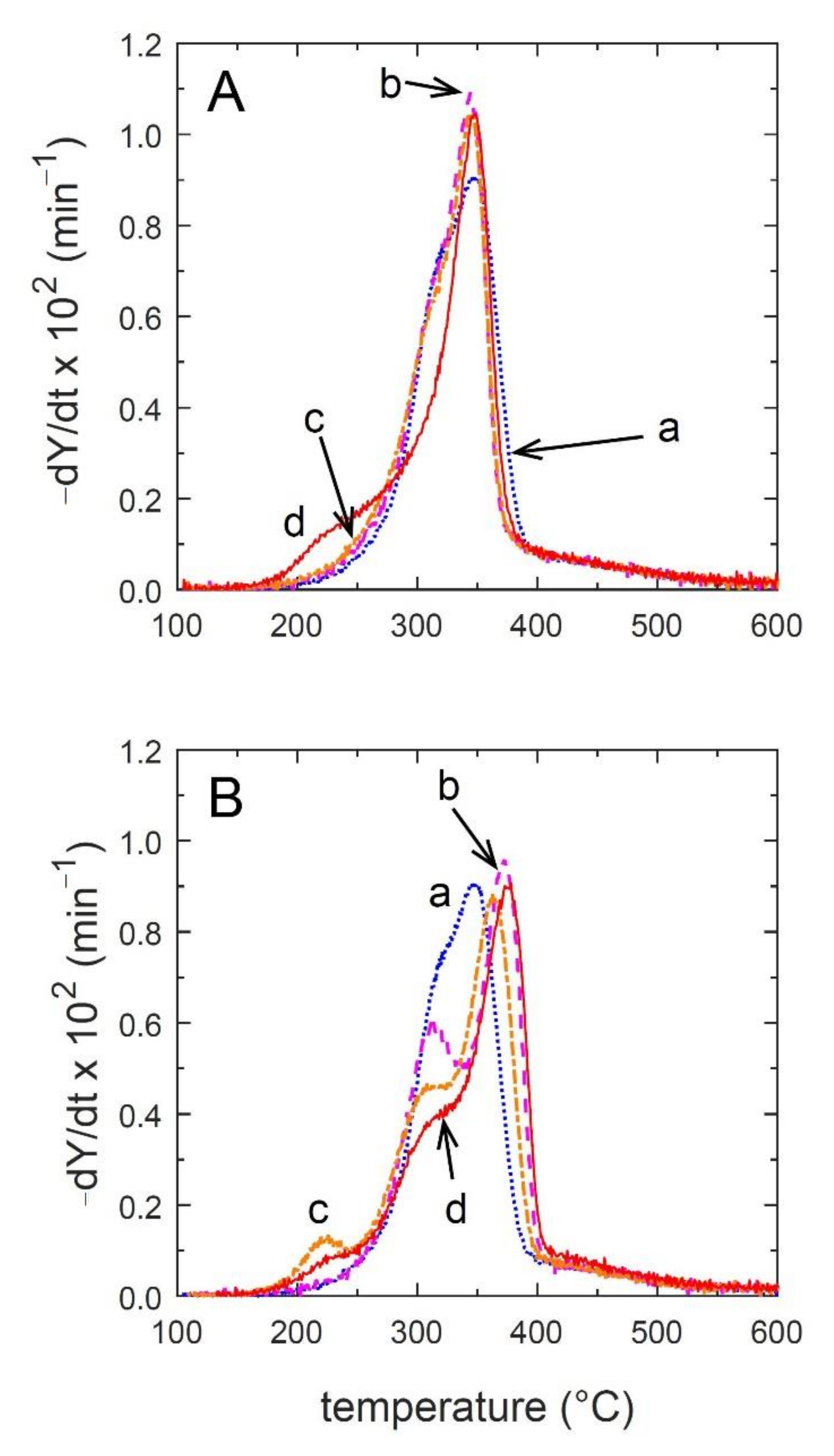
3.4. Combustion Characteristics
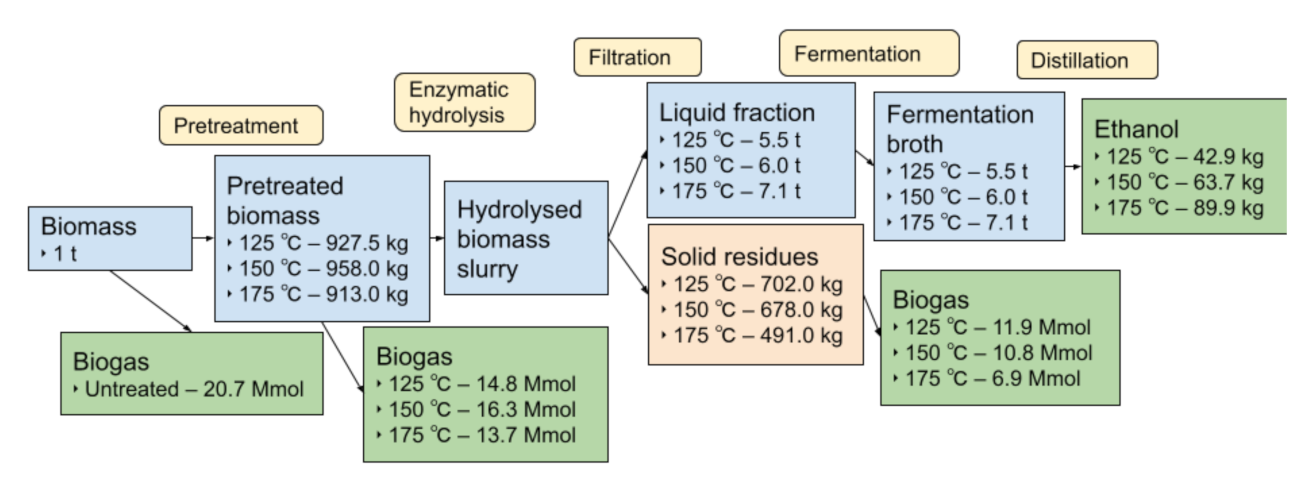
3.5. Energy Balance and Analysis of Different Routes
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Union 2018.
- Directive (EU) 2015/1513 of the European Rarliament and of the Council of 9 september 2015 amending directive 98/70/ec relating to the quality of petrol and diesel fuels and amending directive 2009/28/ec on the promotion of the use of energy from renewable sources. 2015.
- Ghosh, S.; Chowdhury, R.; Bhattacharya, P. Sustainability of cereal straws for the fermentative production of second generation biofuels: A review of the efficiency and economics of biochemical pretreatment processes. Appl. Energy 2017, 198, 284–298. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Bacovsky, D.; Ludwiczek, N.; Ognissanto, M.; Wörgetter, M. Status of Advanced Biofuels Demonstration Facilities in 2012. A Bioenergy Task 39; IEA: Paris, France, 2013. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renew. Sustain. Energy Rev. 2019, 101, 590–599. [Google Scholar] [CrossRef]
- Kreuger, E.; Sipos, B.; Zacchi, G.; Svensson, S.-E.; Björnsson, L. Bioconversion of industrial hemp to ethanol and methane: The benefits of steam pretreatment and co-production. Bioresour. Technol. 2011, 102, 3457–3465. [Google Scholar] [CrossRef]
- Hatzis, C.; Riley, C.; Philippidis, G. Detailed Material Balance and Ethanol Yield Calculations for the Biomass-to-Ethanol Conversion Process. In Seventeenth Symposium on Biotechnology for Fuels and Chemicals: Presented as Volumes 57 and 58 of Applied Biochemistry and Biotechnology; Wyman, C.E., Davison, B.H., Eds.; Humana Press: Totowa, NJ, USA, 1996; pp. 443–459. [Google Scholar]
- Kaur, M.; Kumar, M.; Singh, D.; Sachdeva, S.; Puri, S.K. A sustainable biorefinery approach for efficient conversion of aquatic weeds into bioethanol and biomethane. Energy Convers. Manag. 2019, 187, 133–147. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Potential of bioethanol production waste for methane recovery. Energy 2019, 173, 133–139. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Ivanova, A.; Atouguia, G.; Ávila, I.; Raud, M.; Orupõld, K.; Kikas, T. The effect of flue gas explosive decompression pretreatment on methane recovery from bioethanol production waste. Ind. Crop. Prod. 2019, 127, 66–72. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef]
- Elsayed, M.; Abomohra, A.E.-F.; Ai, P.; Wang, D.; El-Mashad, H.M.; Zhang, Y. Biorefining of rice straw by sequential fermentation and anaerobic digestion for bioethanol and/or biomethane production: Comparison of structural properties and energy output. Bioresour. Technol. 2018, 268, 183–189. [Google Scholar] [CrossRef]
- Raud, M.; Olt, J.; Kikas, T. N2 explosive decompression pretreatment of biomass for lignocellulosic ethanol production. Biomass Bioenergy 2016, 90, 1–6. [Google Scholar] [CrossRef]
- Raud, M.; Rooni, V.; Kikas, T. Explosive decompression pretreatment: Nitrogen vs. compressed air. Agron. Res. 2016, 14, 569–578. [Google Scholar]
- Tutt, M.; Raud, M.; Kahr, H.; Pointner, M.; Olt, J.; Kikas, T. Nitrogen explosion pretreatment of lignocellulosic material for bioethanol production. Energy Sources Recovery Util. Env. Eff. 2016, 38, 1785–1789. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Telliard, W.A. Method 1684: Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids; US Environmental Protection Agency: Washington, DC, USA, 2001.
- Lane, D.J.H.A.; Sippula, O.; Lähde, A.; Mesceriakovas, A.; Peräniemi, S.; Jokiniemi, J. Thermal separation of metal values from municipal solid waste incineration fly ash. J. Cleaner Prod. 2020, 253, 120014. [Google Scholar] [CrossRef]
- NREL. Determination of Ash in Biomass, Laboratory Analytical Procedure (LAP): Golden, CO, USA, 2005.
- Lu, J.; Li, X.; Zhao, J.; Qu, Y. Enzymatic Saccharification and Ethanol Fermentation of Reed Pretreated with Liquid Hot Water. J. Biomed. Biotechnol. 2012, 2012, 276278. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Ahlgren, E.O.; Shukla, P.R. Future biogas resource potential in India: A bottom-up analysis. Renew. Energy 2019, 141, 379–389. [Google Scholar] [CrossRef]
- Gabisa, E.W.; Gheewala, S.H. Potential, environmental, and socio-economic assessment of biogas production in Ethiopia: The case of Amhara regional state. Biomass Bioenergy 2019, 122, 446–456. [Google Scholar] [CrossRef]
- Han, M.; Kang, K.E.; Kim, Y.; Choi, G.-W. High efficiency bioethanol production from barley straw using a continuous pretreatment reactor. Process Biochem. 2013, 48, 488–495. [Google Scholar] [CrossRef]
- Kikas, T.; Tutt, M.; Raud, M.; Alaru, M.; Lauk, R.; Olt, J. Basis of Energy Crop Selection for Biofuel Production: Cellulose vs. Lignin. Int. J. Green Energy 2014. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Conde-Mejía, C.; Jiménez-Gutiérrez, A.; El-Halwagi, M. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Raud, M.; Krennhuber, K.; Jäger, A.; Kikas, T. Nitrogen explosive decompression PRE-TREATMENT: An alternative to steam explosion. Energy 2019, 177, 175–182. [Google Scholar] [CrossRef]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: Performance and microbial characteristics analysis. PLoS ONE 2014, 9, e102548. [Google Scholar] [CrossRef]
- Agudelo, R.A.; García-Aparicio, M.P.; Görgens, J.F. Steam explosion pretreatment of triticale (× Triticosecale Wittmack) straw for sugar production. New Biotechnol. 2016, 33, 153–163. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Ferrero, S.; Oriani, L.; Ottonello, P.; Torre, P.; Cherchi, F. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012, 46, 25–35. [Google Scholar] [CrossRef]
- Oliveira, F.M.V.; Pinheiro, I.O.; Souto-Maior, A.M.; Martin, C.; Gonçalves, A.R.; Rocha, G.J.M. Industrial-scale steam explosion pretreatment of sugarcane straw for enzymatic hydrolysis of cellulose for production of second generation ethanol and value-added products. Bioresour. Technol. 2013, 130, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef]
- Tutt, M.; Kikas, T.; Kahr, H.; Pointner, M.; Kuttner, P.; Olt, J. Using steam explosion pretreatment method for bioethanol production from floodplain meadow hay. Agron. Res. 2014, 12, 417–424. [Google Scholar]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Raud, M.; Rooni, V.; Kikas, T. The Efficiency of Nitrogen and Flue Gas as Operating Gases in Explosive Decompression Pretreatment. Energies 2018, 11, 2074. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar]
- Montgomery, L.F.R.; Bochmann, G. Pretreatment of Feedstock for Enhanced Biogas Production. In IEA Bioenergy Task 37—Energy from Biogas; Baxter, D., Ed.; IEA Bioenergy: Paris, France, 2014; ISBN 978-1-910154-05-2. [Google Scholar]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Tan, H.; Hui, S.e. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. The implications of chlorine-associated corrosion on the operation of biomass-fired boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]
- Hupa, M. Ash-Related Issues in Fluidized-Bed Combustion of Biomasses: Recent Research Highlights. Energy Fuels 2012, 26, 4–14. [Google Scholar] [CrossRef]
- Sippula, O.; Lamberg, H.; Leskinen, J.; Tissari, J.; Jokiniemi, J. Emissions and ash behavior in a 500kW pellet boiler operated with various blends of woody biomass and peat. Fuel 2017, 202, 144–153. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Tran, K.-Q.; Skreiberg, Ø. Comparative study on the thermal degradation of dry- and wet-torrefied woods. Appl. Energy 2017, 185, 1051–1058. [Google Scholar] [CrossRef]
- Gudka, B.; Jones, J.M.; Lea-Langton, A.R.; Williams, A.; Saddawi, A. A review of the mitigation of deposition and emission problems during biomass combustion through washing pre-treatment. J. Energy Inst. 2016, 89, 159–171. [Google Scholar] [CrossRef]


| Pretreatment Temperature | Moisture (%) | Ash (%) | Lignin (%) | Cellulose (%) | Hemicellulose (%) | TS (g kg−1) | VS (g kgTS−1) |
|---|---|---|---|---|---|---|---|
| Untreated biomass | 6.8 ± 0.2 | 3.4 | 7.8 ± 0.2 | 40.0 ± 0.2 | 27.2 ± 0.8 | 931 ± 0.8 | 963 ± 0.5 |
| Pretreated samples | |||||||
| 125 °C | 6.7 ± 0.2 | 3.2 | 8.7 ± 0.6 | 41.1 ± 0.4 | 26.6 ± 0.7 | 927 ± 1 | 960 ± 0.4 |
| 150 °C | 6.6 ± 0.2 | 3.8 | 8.9 ± 0.2 | 39.3 ± 0.6 | 21.1 ± 0.8 | 929 ± 2 | 963 ± 0.5 |
| 175 °C | 5.4 ± 0.2 | 2.6 | 11.3 ± 0.2 | 40.3 ± 0.2 | 6.5 ± 1.0 | 939 ± 0.4 | 962 ± 0.7 |
| Hydrolyzed samples | |||||||
| 125 °C | 6.7 ± 0.2 | 2.2 | 9.4 ± 0.7 | 39.8 ± 0.3 | 20.2 ± 0.7 | 931 ± 1 | 976 ± 0.2 |
| 150 °C | 5.9 ± 0.2 | 2.2 | 11.6 ± 0.6 | 40.8 ± 0.1 | 28.0 ± 0.5 | 928 ± 0.4 | 973 ± 0.4 |
| 175 °C | 5.4 ± 0.2 | 2.6 | 12.1 ± 0.5 | 38.9 ± 0.6 | 21.5 ± 0.3 | 938 ± 2 | 974 ± 0.5 |
| Sample | Pretreatment Temperature | Bmax | 85% Bmax | 95% Bmax | ||
|---|---|---|---|---|---|---|
| mol CH4 100 g−1 | mol CH4 100 g−1 | Days | mol CH4 100 g−1 | Days | ||
| Untreated | - | 1.14 ± 0.02 a,b | 0.97 | 14.3 | 1.1 | 22.1 |
| Pretreated | 125 °C | 0.91 ± 0.02 c | 0.77 | 12.2 | 0.86 | 18.8 |
| 150 °C | 0.93 ± 0.01 d | 0.79 | 11.7 | 0.88 | 18.3 | |
| 175 °C | 0.85 ± 0.01 e | 0.72 | 9.47 | 0.81 | 14.9 | |
| Solid fraction of the post-hydrolysis broth | 125 °C | 0.91 ± 0.01 f | 0.77 | 15.1 | 0.86 | 23.2 |
| 150 °C | 0.87 ± 0.01 | 0.74 | 14.8 | 0.83 | 22.9 | |
| 175 °C | 0.73 ± 0.01 | 0.62 | 14.4 | 0.69 | 22.3 | |
| Untreated | Pretreated | Pretreated and Hydrolyzed | |||||
|---|---|---|---|---|---|---|---|
| 125 °C | 150 °C | 175 °C | 125 °C | 150 °C | 175 °C | ||
| Proximate analysis | |||||||
| Volatile matter (%) | 80.5 | 79.6 | 79.7 | 79.9 | 85.4 | 82.3 | 82.8 |
| Fixed carbon (%) | 15.8 | 16.3 | 16.3 | 16.2 | 12.1 | 15.1 | 14.6 |
| Ash (%) | 3.7 | 4.1 | 4.0 | 3.9 | 2.5 | 2.6 | 2.6 |
| Ultimate analysis | |||||||
| C (% w/w) | 47.7 | 47.5 | 47.5 | 48.1 | 49.2 | 49.0 | 51.7 |
| H (% w/w) | 5.9 | 5.9 | 5.9 | 5.9 | 6.0 | 6.0 | 6.1 |
| N (% w/w) | 0.76 | 0.96 | 0.91 | 0.92 | 1.07 | 1.03 | 1.43 |
| S (% w/w) | 0.10 | 0.11 | 0.11 | 0.11 | 0.08 | 0.09 | 0.12 |
| Inorganic element analysis | |||||||
| Si (% w/w) | 0.66 | 0.61 | 0.67 | 0.64 | 0.85 | 0.64 | 0.81 |
| K (% w/w) | 0.63 | 0.75 | 0.74 | 0.76 | 0.05 | 0.22 | 0.06 |
| Ca (% w/w) | 0.37 | 0.43 | 0.37 | 0.54 | 0.34 | 0.23 | 0.37 |
| Cl (% w/w) | 0.10 | 0.17 | 0.14 | 0.19 | <0.1 | <0.1 | <0.1 |
| P (mg kg−1) | 897 | 1015 | 1142 | 1015 | 488 | 651 | 424 |
| Mg (mg kg−1) | 858 | 862 | 948 | 782 | 320 | 436 | 201 |
| Al (mg kg−1) | 99 | 125 | 138 | 156 | 202 | 241 | 235 |
| Fe (mg kg−1) | 70 | 122 | 145 | 128 | 181 | 160 | 225 |
| Na (mg kg−1) | 59 | 81 | 73 | 54 | 45 | 230 | 40 |
| Zn (mg kg−1) | <40 | <40 | <40 | 42 | <40 | <40 | 47 |
| Ti (mg kg−1) | <40 | <40 | <40 | <40 | <40 | <40 | <40 |
| Gross calorific value (MJ kg−1) | 18.9 | 19.0 | 19.0 | 19.3 | 19.7 | 19.7 | 21.0 |
| Net calorific value (MJ kg−1) | 17.6 | 17.6 | 17.7 | 18.0 | 18.3 | 18.3 | 19.6 |
| Untreated Biomass | 125 °C | 150 °C | 175 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | Biogas | Combustion | Bioethanol | Biogas | Combustion | Bioethanol | Biogas | Combustion | Bioethanol | Biogas | Combustion |
| 1 | - | 16 194.7 | - | - | - | - | - | - | - | - | - |
| 2 | 9273.6 | - | - | - | - | - | - | - | - | - | - |
| 3 | - | - | - | - | −1452.3 | - | - | −915.2 | - | - | −532.1 |
| 4 | - | - | - | 6648.3 | - | - | 7296.1 | - | - | 6135.4 | - |
| 5 | - | - | 1157.4 | - | 3724.9 | 1720.6 | - | 2882.2 | 2428.0 | - | 3578.0 |
| 6 | - | - | 1157.4 | 5346.4 | - | 1720.6 | 4859.9 | - | 2428.0 | 3079.6 | - |
| 7 | - | - | 1157.4 | - | - | 1720.6 | - | - | 2428.0 | - | - |
| Total Energy from All Outputs | |||||||||||
| Untreated Biomass | 125°C | 150°C | 175°C | ||||||||
| 1 | 16,194.7 | - | - | - | |||||||
| 2 | 9273.6 | - | - | - | |||||||
| 3 | - | −1452.3 | −915.2 | −532.1 | |||||||
| 4 | - | 6648.3 | 7296.1 | 6135.4 | |||||||
| 5 | - | 4882.4 | 4602.9 | 6006.0 | |||||||
| 6 | - | 6503.9 | 6580.7 | 5507.6 | |||||||
| 7 | - | 1157.4 | 1720.8 | 2428.0 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raud, M.; Rocha-Meneses, L.; Lane, D.J.; Sippula, O.; Shurpali, N.J.; Kikas, T. Utilization of Barley Straw as Feedstock for the Production of Different Energy Vectors. Processes 2021, 9, 726. https://doi.org/10.3390/pr9040726
Raud M, Rocha-Meneses L, Lane DJ, Sippula O, Shurpali NJ, Kikas T. Utilization of Barley Straw as Feedstock for the Production of Different Energy Vectors. Processes. 2021; 9(4):726. https://doi.org/10.3390/pr9040726
Chicago/Turabian StyleRaud, Merlin, Lisandra Rocha-Meneses, Daniel J. Lane, Olli Sippula, Narasinha J. Shurpali, and Timo Kikas. 2021. "Utilization of Barley Straw as Feedstock for the Production of Different Energy Vectors" Processes 9, no. 4: 726. https://doi.org/10.3390/pr9040726
APA StyleRaud, M., Rocha-Meneses, L., Lane, D. J., Sippula, O., Shurpali, N. J., & Kikas, T. (2021). Utilization of Barley Straw as Feedstock for the Production of Different Energy Vectors. Processes, 9(4), 726. https://doi.org/10.3390/pr9040726









