Purification and Characterization of Fractions Containing Polysaccharides from Talinum triangulare and Their Immunomodulatory Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Extraction of Polysaccharides of T. triangulare (TTP) by the Heat Reflux Extraction (HRE) Method
2.3. Extraction of TTP by the Ultrasonic Wave-Assistant Extraction (UAE) Method
2.4. Cell Assays
2.4.1. TTP-Treated RAW264.7 Media and Nitric Oxide (NO) Assay
2.4.2. HeLa Cell Survival Rates Cultured with Various RAW264.7 CMs
2.4.3. Cell Survival, Nitrite and Cytokine Productions in Different Fractions of LTTP-Treated RAW264.7 CMs
2.4.4. SW620 Cell Survivals Following Cultured with RAW264.7 Condition Media Pre-Treated with LTTP Fractions
2.5. Isolation and Purification of LTTP by Anion-Exchange Chromatography
2.6. Analysis of LTTPs
2.6.1. Chemical Composition
2.6.2. Determinations of Mw of Polysaccharides
2.7. Infrared (IR) Spectroscopy Analysis
2.8. Statistical Analyses
3. Results and Discussion
3.1. Optimal HRE Condition for TTP Extraction
3.2. Optimal UAE Condition for TTP Extraction
3.3. Cell Culture and Nitrite Productions of TTPs
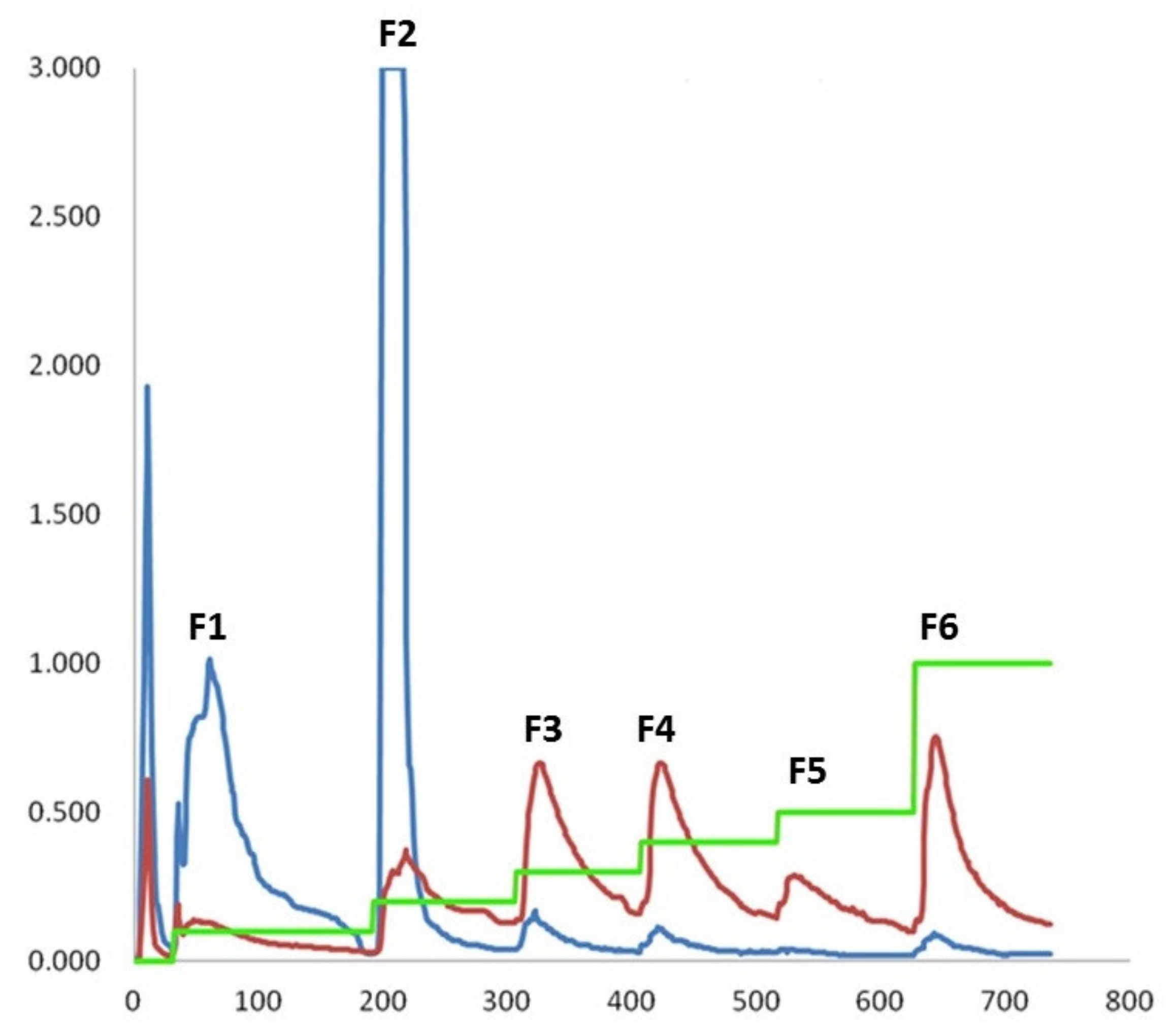
3.4. Separation and Fractionation of LTTP
3.5. Chemical Components of Different Fractions of LTTP
3.6. Molecular Weights of Polysaccharides
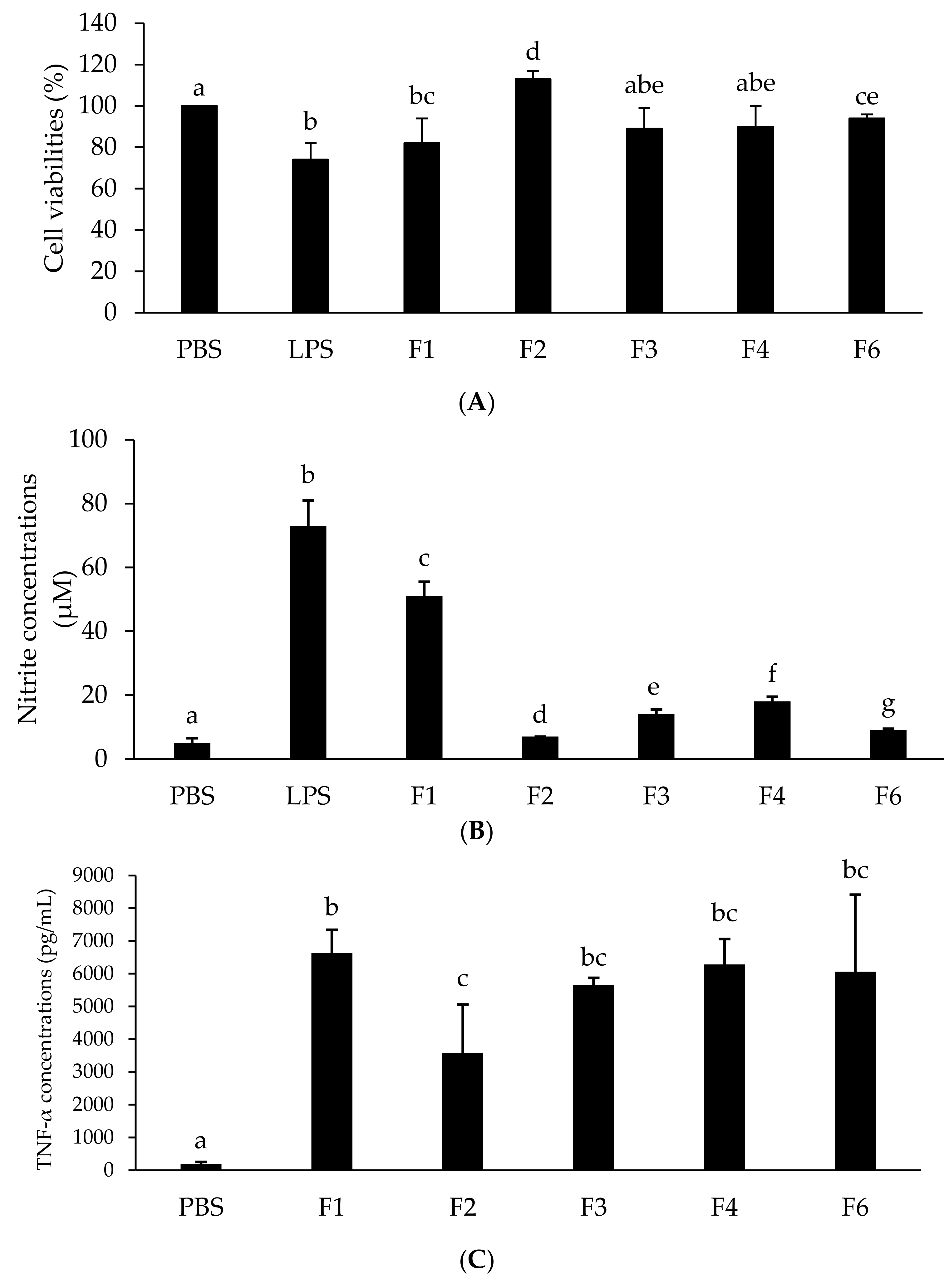
3.7. Effect of Different LTTP Fractions on Growth of Macrophages
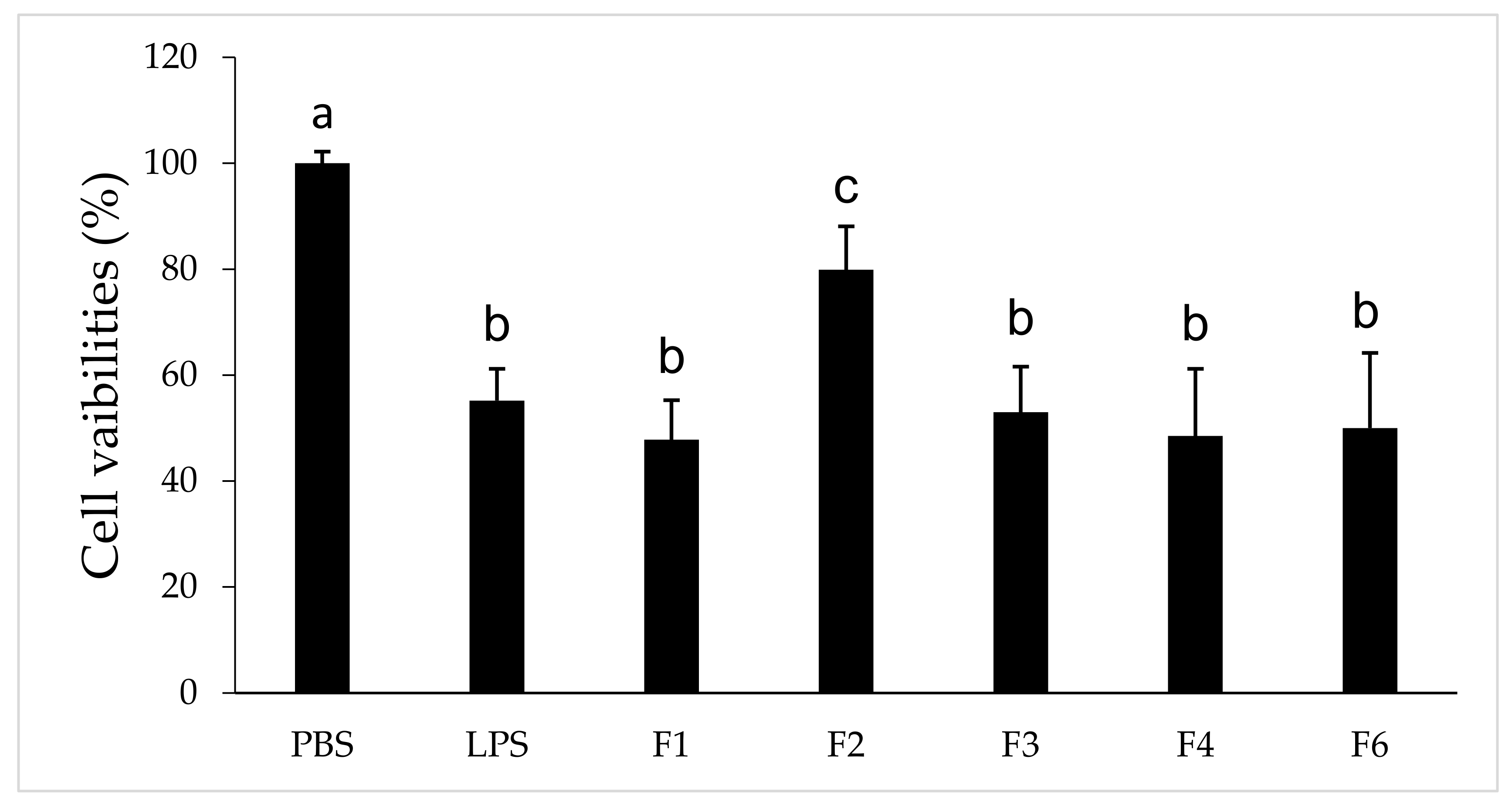
3.8. SW620 Cell Survivals Following Cultured with Condition Media of RAW264.7 Macrophages Pre-Treated with Different LTTP Fractions
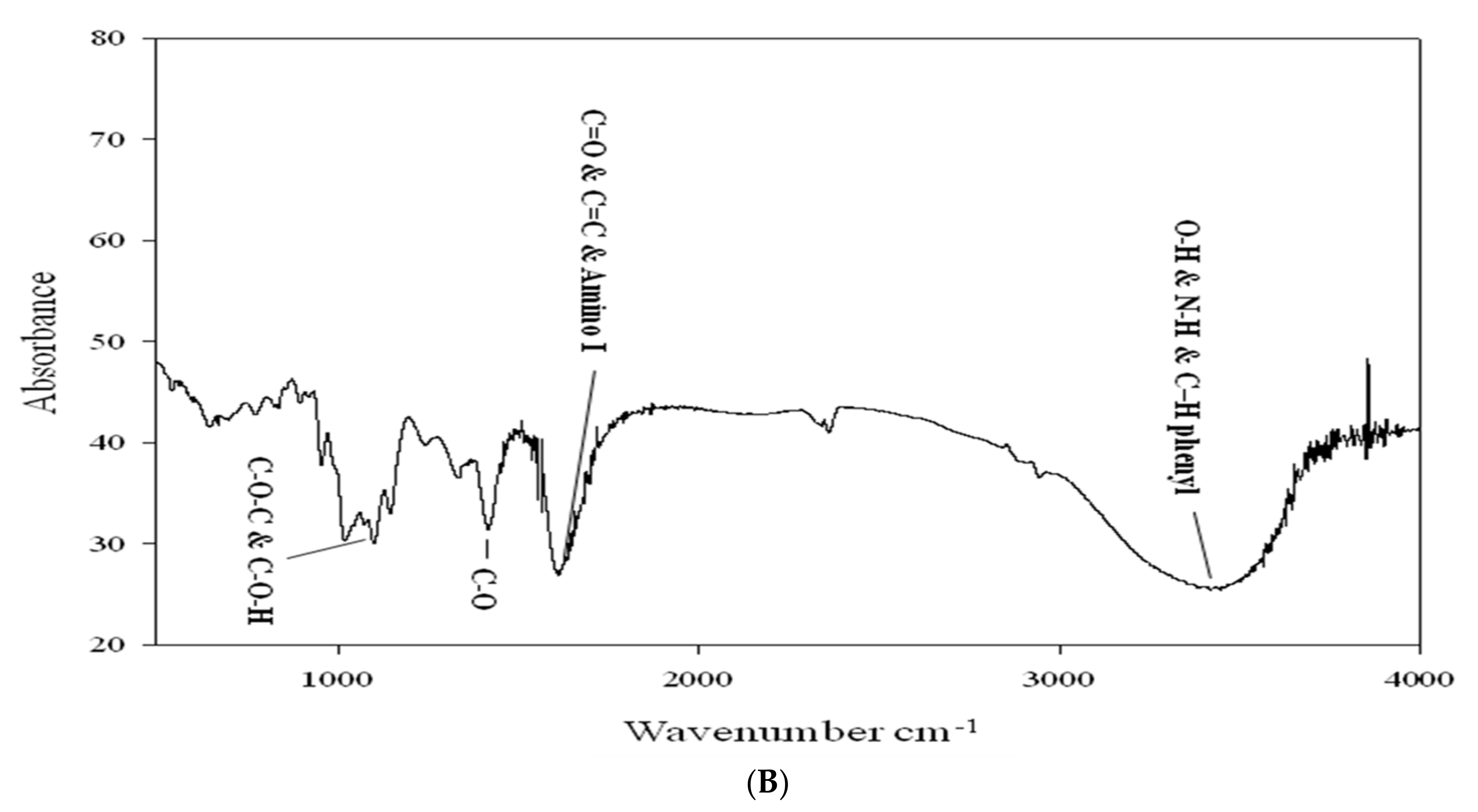
3.9. Fourier-Transformed Infrared (FTIR) Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kala, C.; Ali, S.S.; Khan, N.A. Immunostimulatory potential of n-butanolic fraction of hydroalcoholic extract of Costus speciosus koen Rhizome. Int. J. Pharm. Sci. Res. 2015, 6, 2886–2892. [Google Scholar]
- Wang, S.Y.; Hsu, M.L.; Hsu, H.C.; Tzeng, C.H.; Lee, S.S.; Shiao, M.S. The antitumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2003, 60, 258–274. [Google Scholar]
- Zhang, C.; Huang, K. Characteristic immunostimulation by MAP, a polysaccharide isolated from the mucus of the loach, Misgurnus anguillicaudatus. Carbohydr. Polym. 2005, 59, 75–82. [Google Scholar] [CrossRef]
- Agbonon, A.; Eklu-Gadegbeku, K.; Aklikokou, K.; Gbeassor, M.; Akpagana, K.; Tam, T.W.; Arnason, J.T.; Foster, B.C. In vitro inhibitory effect of West African medicinal and food plants on human cytochrome P450 3A subfamily. J. Ethnopharmacol. 2010, 128, 390–394. [Google Scholar] [CrossRef]
- Fenny, K.L.; Andreanus, A.S.; Immaculata, M. Uji Aktivitas Imunostimulan daun Ginseng Sumatera (Talinum triangulare Willd) Leaves and Korea Ginseng (Panax ginseng C.A. Mayer) Leaves; Institute Teknologi Bandung: Bandung, Indonesia, 1996. [Google Scholar]
- Liao, D.Y.; Chai, Y.C.; Wang, S.H.; Chen, C.W.; Tsai, M.S. Antioxidant activities and contents of flavonoids and phenolic acids of Talinum triangulare extracts and their immunomodulatory effects. J. Food Drug Anal. 2015, 23, 294–302. [Google Scholar] [CrossRef]
- Wang, L.; Nie, Z.-K.; Zhou, Q.; Zhang, J.-L.; Yin, J.-J.; Xu, W.; Qiu, Y.; Ming, Y.-L.; Liang, S. Antitumor efficacy in H22 tumor bearing mice and immunoregulatory activity on RAW 264.7 macrophages of polysaccharides from Talinum triangulare. Food Funct. 2014, 5, 2183–2193. [Google Scholar] [CrossRef]
- Amorim, A.P.O.; Oliveira, M.C.S.; Amorim, T.A.; Echevarria, A. Antioxidant, iron chelating and tyrosinase inhibitory activities of extracts from Talinum triangulare leach stem. Antioxidants 2013, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Swarna, J.; Lokeswari, T.S.; Smita, M.; Ravindhran, R. Characterization and determination of in vitro antioxidant potential of betalains from Talinum triangulare (Jacq.) Willd. Food Chem. 2013, 141, 4382–4390. [Google Scholar] [CrossRef]
- Afolabi, O.B.; Oloyede, O.I. Antioxidant properties of the extracts of Talinum Triangulare and its effect on antioxidant enzymes in tissue homogenate of Swiss Albino rat. Toxicol. Int. Former. Indian J. Toxicol. 2014, 21, 307–313. [Google Scholar] [CrossRef]
- Liang, D.; Zhou, Q.; Gong, W.; Wang, Y.; Nie, Z.; He, H.; Li, J.; Wu, J.; Wu, C.; Zhang, J. Studies on the antioxidant and hepa-toprotective activities of polysaccharides from Talinum triangulare. J. Ethnopharmacol. 2011, 136, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Olorunnisola, O.S.; Adetutu, A.; Afolayan, A.J.; Owoade, A.O. Effect of methanolic leaf extract of Talinum triangulare (Jacq). Willd. on biochemical parameters in diet induced dyslipidemia Wistar rats. Pharmacogn. Mag. 2016, 12, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, Q.; Yin, J.-J.; Yao, Y.; Zhang, J.-L. Anti-diabetic effects of polysaccharides from Talinum triangulare in streptozotocin (STZ)-induced type 2 diabetic male mice. Int. J. Biol. Macromol. 2015, 72, 575–579. [Google Scholar] [CrossRef]
- Ravindra, B.P.; Rama, R.D.; Prasada, R.M.; Kanth, J.V.K.; Srinivasulu, M.; Hareesh, V. Hypoglycemic activity of methanolic extract of Talinum triangulare leaves in normal and streptozotocin induced diabetic rats. J. Appl. Pharm. Sci. 2012, 2, 197–201. [Google Scholar]
- Nie, Z.K.; Shi, W.J.; He, H. Extraction and antitumor activity of polysaccharides from Talinum triangulare. Food Sci. 2013, 34, 46–49. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Fan, J.-P.; He, C.-H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Hromádková, Z.; Ebringerová, A. Ultrasonic extraction of plant materials––Investigation of hemicellulose release from buckwheat hulls. Ultrason. Sonochemistry 2003, 10, 127–133. [Google Scholar] [CrossRef]
- Huang, L.; Guo, L.; Pan, T.; Zhang, Z.; Yan, Z.; Liu, S.; Huang, S. Optimizing ultrasonic extraction of polysaccharides from Talinum triangulare Willd based on orthogonal design. China Brew. 2012, 31, 105–107. [Google Scholar]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma Lu-cidum terpenoids and polysaccharides: A Review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Role of plant polyphenols in genomic stability. Mutat. Res. Mol. Mech. Mutagen. 2001, 475, 89–111. [Google Scholar] [CrossRef]
- Wang, B.C.; Chen, C.J.; Hua, J. Cultivation and Application of Edible and Medicinal Mushrooms; Food Industry Research and Development Institute: Taipei, Taiwan, 1998; pp. 112–187. [Google Scholar]
- Sun, R.; Fang, J.M.; Goodwin, A.; Lawther, J.M.; Bolton, A.J. Fractionation and characterization of polysaccharides from abaca fibre. Carbohydr. Polym. 1998, 37, 351–359. [Google Scholar] [CrossRef]
- Gomba, G.K.; Synytsya, A.; Švecová, P.; Coimbra, M.A.; Čopíková, J. Distinction of fungal polysaccharides by N/C ratio and mid infrared spectroscopy. Int. J. Biol. Macromol. 2015, 80, 271–281. [Google Scholar] [CrossRef]
- Nycz, J.E.; Malecki, G.; Morag, M.; Nowak, G.; Ponikiewski, L.; Kusz, J.; Świtlicka, A. Arbutin: Isolation, X-ray structure and computional studies. J. Mol. Struct. 2010, 980, 13–17. [Google Scholar] [CrossRef]
- Yang, J.P.; Hsu, T.; Lin, F.; Hsu, W.; Chen, Y. Potential antidiabetic activity of extracellular polysaccharides in submerged fermentation culture of Coriolus versicolor LH. Carbohydr. Polym. 2012, 90, 174–180. [Google Scholar] [CrossRef]
- Ogbonnaya, E.C.; Chinedum, E.K. Vitamin and carotenoid composition of raw and decoctions of water leaf (Talinum trian-gulare). Biochem. Pharmacol. 2013, 2, 121. [Google Scholar] [CrossRef]
- Umeokoli, B.O.; Muharini, R.; Okoye, F.B.; Ajiwe, V.I.; Akpuaka, M.U.; Lin, W.; Liu, Z.; Proksch, P. New C-methylated fla-vonoids and α-pyrone derivative from roots of Talinum triangulare growing in Nigeria. Fitoterapia 2016, 109, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Aja, P.; Okaka, A.; Onu, P.; Ibiam, U.; Urako, A. Phytochemical composition of Talinum triangulare (Water Leaf) leaves. Pak. J. Nutr. 2010, 9, 527–530. [Google Scholar] [CrossRef]
- Ukpabi, C.; Akubugwo, E.I.; Agbafor, K.; Wogu, C.; Chukwu, H.C. Phytochemical and heavy metal composition of Telfairia occidental and Talinum triangulare grown in Aba Nigeria and environmental health implications. Am. J. Biochem. 2013, 3, 67–73. [Google Scholar]
- Swarna, J.; Ravindhran, R. Pharmacognostical and phytochemical evaluation of Talinum triangulare (Jacq.) Willd. Int. J. Pharm. Sci. 2013, 5, 249–256. [Google Scholar]
- Ikewuchi, C.C.; Ikewuchi, J.C.; Ifeanacho, M.O. Bioactive phytochemicals in an aqueous extract of the leaves of Talinum triangulare. Food Sci. Nutr. 2017, 5, 696–701. [Google Scholar] [CrossRef]
- Amorim, A.P.O.; Carvalho, A.R., Jr.; Lopes, N.P.; Castro, R.N.; Oliveira, M.C.C.; Carvalho, M.G. Chemical compounds isolated from Talinum triangulare (Portulacaceae). Food Chem. 2014, 160, 204–208. [Google Scholar] [CrossRef]


| Group | Temperature (°C) | Time (min) | Ratio of Material to Water (g/mL) | Yield (%) a |
|---|---|---|---|---|
| 1 | 75 | 90 | 1/20 | 1.78 ± 0.06 |
| 2 | 75 | 120 | 1/25 | 2.03 ± 0.05 |
| 3 | 75 | 150 | 1/30 | 2.04 ± 0.06 |
| 4 | 85 | 90 | 1/25 | 1.82 ± 0.07 |
| 5 | 85 | 120 | 1/30 | 2.32 ± 0.08 |
| 6 | 85 | 150 | 1/20 | 2.73 ± 0.07 |
| 7 | 95 | 90 | 1/30 | 2.82 ± 0.09 |
| 8 | 95 | 120 | 1/20 | 2.73 ± 0.10 |
| 9 | 95 | 150 | 1/25 | 3.01 ± 0.12 |
| KI | 5.79 | 6.40 | 7.20 | |
| KII | 6.80 | 7.10 | 6.80 | |
| KIII | 8.50 | 7.70 | 7.10 | |
| R | 0.900 | 0.433 | 0.133 |
| Group | Temperature (°C) | Time (min) | Ratio of Material to Water (g/mL) | Yield (%) a |
|---|---|---|---|---|
| 1 | 75 | 45 | 1/15 | 1.78 ± 0.03 |
| 2 | 75 | 60 | 1/20 | 2.27 ± 0.05 |
| 3 | 75 | 75 | 1/25 | 2.32 ± 0.06 |
| 4 | 85 | 60 | 1/15 | 2.41 ± 0.08 |
| 5 | 85 | 75 | 1/20 | 3.14 ± 0.09 |
| 6 | 85 | 45 | 1/25 | 2.78 ± 0.08 |
| 7 | 95 | 75 | 1/15 | 2.23 ± 0.11 |
| 8 | 95 | 45 | 1/20 | 2.64 ± 0.10 |
| 9 | 95 | 60 | 1/25 | 2.82 ± 0.11 |
| KI | 6.43 | 6.41 | 7.25 | |
| KII | 8.30 | 7.99 | 7.44 | |
| KIII | 7.53 | 7.87 | 7.87 | |
| R | 0.633 | 0.533 | 0.133 |
| Sample | Polysaccharides | Triterpenoids | Polyphenols | Proteins | Unknown | pH a |
|---|---|---|---|---|---|---|
| F1 | 12.67 | 8.67 | 15.33 | 0.02 | 63.31 | 6.35 |
| F2 | 21.52 | 7.33 | 2.38 | 0.02 | 68.75 | 6.21 |
| F3 | 25.00 | 41.33 | 13.33 | 0.04 | 20.30 | 6.58 |
| F4 | 5.77 | 38.08 | 18.08 | 0.02 | 38.05 | 6.67 |
| F6 | 0.67 | 9.56 | 8.44 | 0.04 | 81.29 | 6.02 |
| Sample | Retention Time | Log Mw | Calculated Mw (kDa) | Percentage (%) |
|---|---|---|---|---|
| F1 | 10.51 | 5.445269 | 279 | 83.26 |
| F2 | 10.27 | 5.823893 | 667 | 80.72 |
| F3 | 10.54 | 5.397941 | 250 | 93.67 |
| F4 | 10.61 | 5.290664 | 195 | 72.33 |
| F6 | 10.57 | 5.353768 | 226 | 90.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, S.-H.; Hsu, W.-K.; Chang, Z.-Q.; Wang, S.-H.; Hsieh, C.-W.; Liou, G.-G.; Lee, H.-B.; Jiang, B.-H.; Tsou, H.-K.; Tsai, M.-S. Purification and Characterization of Fractions Containing Polysaccharides from Talinum triangulare and Their Immunomodulatory Effects. Processes 2021, 9, 709. https://doi.org/10.3390/pr9040709
Yeh S-H, Hsu W-K, Chang Z-Q, Wang S-H, Hsieh C-W, Liou G-G, Lee H-B, Jiang B-H, Tsou H-K, Tsai M-S. Purification and Characterization of Fractions Containing Polysaccharides from Talinum triangulare and Their Immunomodulatory Effects. Processes. 2021; 9(4):709. https://doi.org/10.3390/pr9040709
Chicago/Turabian StyleYeh, Shu-Hui, Wen-Kuang Hsu, Zi-Qing Chang, Sue-Hong Wang, Chang-Wei Hsieh, Gunn-Guang Liou, Hung-Bin Lee, Bo-Hao Jiang, Hsi-Kai Tsou, and Ming-Shiun Tsai. 2021. "Purification and Characterization of Fractions Containing Polysaccharides from Talinum triangulare and Their Immunomodulatory Effects" Processes 9, no. 4: 709. https://doi.org/10.3390/pr9040709
APA StyleYeh, S.-H., Hsu, W.-K., Chang, Z.-Q., Wang, S.-H., Hsieh, C.-W., Liou, G.-G., Lee, H.-B., Jiang, B.-H., Tsou, H.-K., & Tsai, M.-S. (2021). Purification and Characterization of Fractions Containing Polysaccharides from Talinum triangulare and Their Immunomodulatory Effects. Processes, 9(4), 709. https://doi.org/10.3390/pr9040709








