Iron-Stimulated Production and Antimicrobial Potential of a Novel Biosurfactant Produced by a Drilling Waste-Degrading Pseudomonas citronellolis Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Enrichment Experiments
2.2. Microorganism Identification and Homology
2.3. Bacterial Culture Conditions
2.4. Effect of Iron Forms and Concentration Level on the Isolated Strain
2.5. Biosurfactant Isolation, Extraction and Partial-Purification
2.6. Analytical Methods: Chemical Oxygen Demand (COD)
2.7. Antimicrobial Activity
2.7.1. Bacterial Strains Tested
2.7.2. Preparation of Biosurfactant Solution
2.7.3. Agar Disc Susceptibility Test
2.7.4. Minimum Inhibitory Concentration (MIC) Agar Susceptibility Test
2.8. Emulsification Index (E24)
2.9. Surface Tension
2.10. Fourier Transform Infrared Spectroscopy (FTIR)
2.11. Thermal Gravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
2.12. X-ray Diffraction (XRD) Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Identification of the Microorganism, Production of BS and Determination of Surface Tension and Emulsification Index (E24)
3.2. Microbial Growth and the Effect of Different Iron Forms
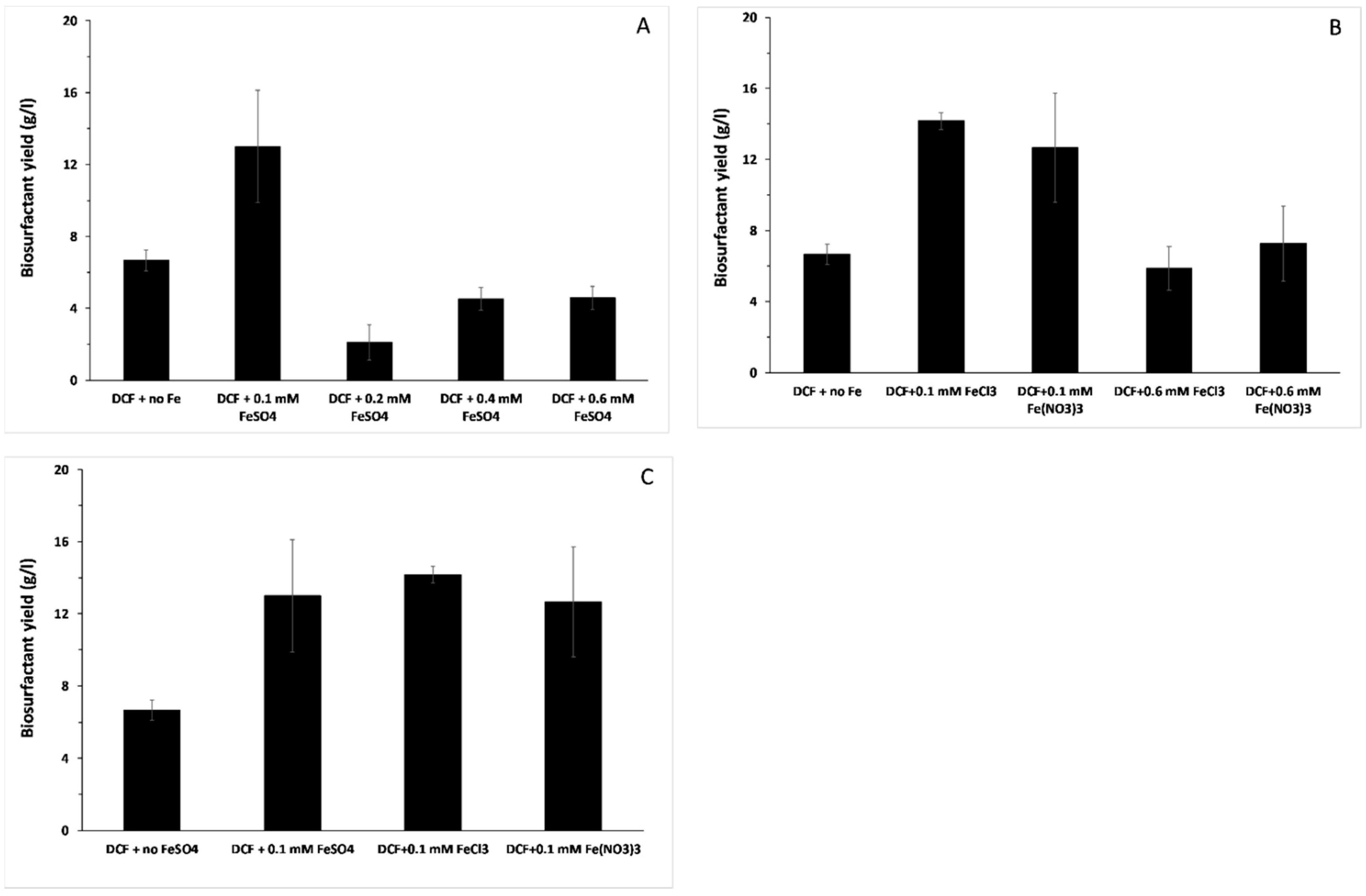
3.3. Biosurfactant Production and the Effect of Different Iron Forms
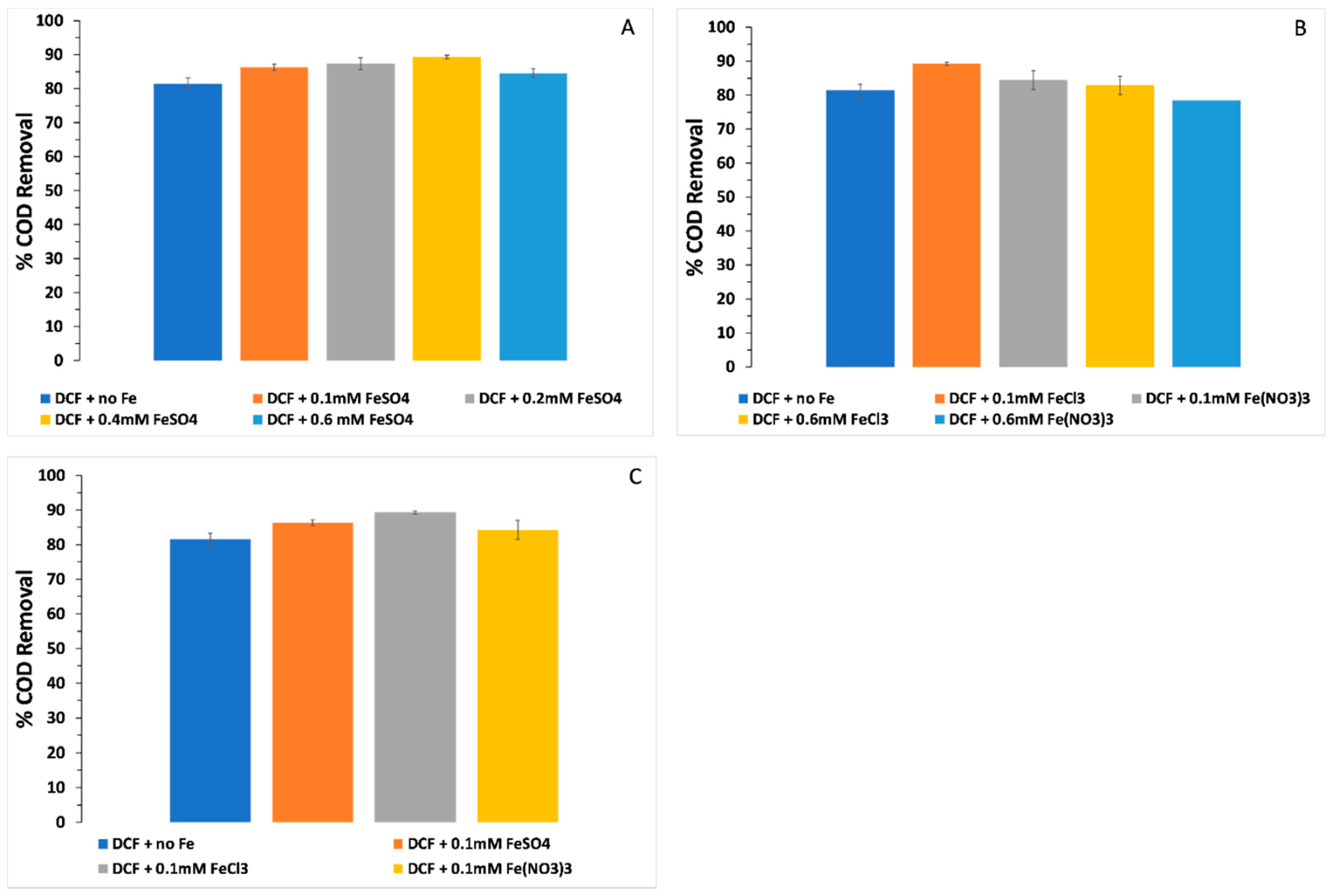
3.4. COD Removal and the Effect of Different Iron Forms
3.5. Biosurfactant Characterization
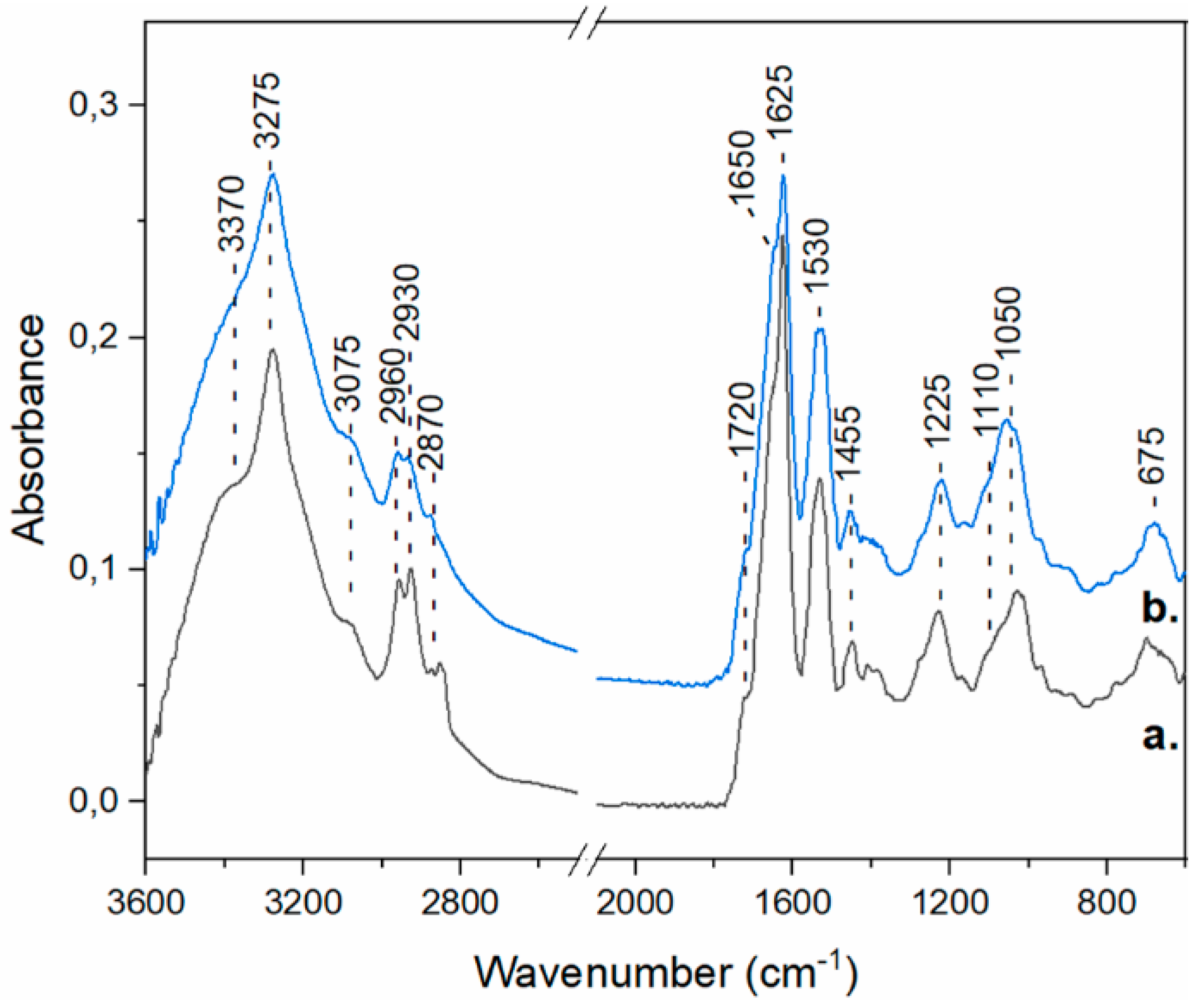
3.5.1. FTIR
3.5.2. TGA, DTA and XRD
3.6. Antimicrobial Activity of the Biosurfactant
3.7. MIC of the Biosurfactant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemlata, B.; Selvin, J.; Tukaram, K. Optimization of iron chelating biosurfactant production by Stenotrophomonas maltophilia NBS-11. Biocatal. Agric. Biotechnol. 2015, 4, 135–143. [Google Scholar] [CrossRef]
- Liu, J.; Vipulanandan, C.; Cooper, T.F.; Vipulanandan, G. Effects of Fe nanoparticles on bacterial growth and biosurfactant production. J. Nanoparticle Res. 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Naughton, P.; Marchant, R.; Naughton, V.; Banat, I. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.; Meena, K.R.; Sharma, A.; Kanwar, S.S. Biosurfactants and their Screening Methods Biosurfactants and their Screening Methods. Res. J. Recent Sci. 2016, 5, 1–6. [Google Scholar]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant Producing Microbes and their Potential Applications: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K.S.M. Biosurfactants: A Covid-19 Perspective. Front. Microbiol. 2020, 11, 1341. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, A.; Gandolfi, I.; Bestetti, G.; Smyth, T.J.P.; Banat, I.M. Production and applications of trehalose lipid biosurfactants. Eur. J. Lipid Sci. Technol. 2010, 112, 617–627. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Yildiz, S. Rhamnolipid Biosurfactants Produced by Pseudomonas Species. Braz. Arch. Biol. Technol. 2016, 59, 1–16. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Onwukwe, S.I.; Nwakaudu, M.S. Drilling Wastes Generation and Management Approach. Int. J. Environ. Sci. Dev. 2012, 3, 252–257. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Dirisala, V.R.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Yan, P.; Lu, M.; Yang, Q.; Zhang, H.-L.; Zhang, Z.-Z.; Chen, R. Oil recovery from refinery oily sludge using a rhamnolipid biosurfactant-producing Pseudomonas. Bioresour. Technol. 2012, 116, 24–28. [Google Scholar] [CrossRef]
- Elshafie, A.E.; Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bemani, A.S.; Al-Bahry, S.N.; Al-Maqbali, D.; Banat, I.M. Sophorolipids Production by Candida bombicola ATCC 22214 and its Potential Application in Microbial Enhanced Oil Recovery. Front. Microbiol. 2015, 6, 1324. [Google Scholar] [CrossRef]
- Datta, P.; Tiwari, P.; Pandey, L.M. Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Bioresour. Technol. 2018, 270, 439–448. [Google Scholar] [CrossRef]
- Kiran, G.S.; Nishanth, L.A.; Priyadharshini, S.; Anitha, K.; Selvin, J. Effect of Fe nanoparticle on growth and glycolipid biosurfactant production under solid state culture by marine Nocardiopsissp. MSA13A. BMC Biotechnol. 2014, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.A.; Lin, J. Enhancement of Paenibacillus sp. D9 Lipopeptide Biosurfactant Production Through the Optimization of Medium Composition and Its Application for Biodegradation of Hydrophobic Pollutants. Appl. Biochem. Biotechnol. 2019, 187, 724–743. [Google Scholar] [CrossRef]
- Haferburg, G.; Kothe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, E. Molecular Cloning: A Laboratory Manual; Cold Spring Harbour Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Suryanti, V.; Marliyana, S.D.; Handayani, D.S.; Ratnaningrum, D. Production and characterization of biosurfactant by pseudomonas fluorescens using cassava flour wastewater as media. Indones. J. Chem. 2013, 13, 229–235. [Google Scholar] [CrossRef]
- De Rienzo, M.A.D.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol. Lett. 2016, 363, fnv224. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef]
- Huck-Iriart, C.; De-Candia, A.; Rodriguez, J.; Rinaldi, C. Determination of Surface Tension of Surfactant Solutions through Capillary Rise Measurements: An Image-Processing Undergraduate Laboratory Experiment. J. Chem. Educ. 2016, 93, 1647–1651. [Google Scholar] [CrossRef]
- Eldin, A.M.; Kamel, Z.; Hossam, N. Isolation and genetic identification of yeast producing biosurfactants, evaluated by different screening methods. Microchem. J. 2019, 146, 309–314. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, X.; Bai, B.; Liang, X.; Shuler, P.J.; Iii, W.A.G.; Tang, Y. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol. Bioeng. 2007, 98, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Remus-Emsermann, M.N.; Schmid, M.; Gekenidis, M.-T.; Pelludat, C.; Frey, J.E.; Ahrens, C.H.; Drissner, D. Complete genome sequence of Pseudomonas citronellolis P3B5, a candidate for microbial phyllo-remediation of hydrocarbon-contaminated sites. Stand. Genom. Sci. 2016, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, X.; Wang, P.; Peng, W.; Ji, N.; Liang, R. Genome Sequence of Pseudomonas citronellolis SJTE-3, an Estrogen- and Polycyclic Aromatic Hydrocarbon-Degrading Bacterium. Genome Announc. 2016, 4, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Oso, S.; Walters, M.; Schlechter, R.O.; Remus-Emsermann, M.N.P. Utilisation of hydrocarbons and production of surfactants by bacteria isolated from plant leaf surfaces. FEMS Microbiol. Lett. 2019, 366, 1–10. [Google Scholar] [CrossRef]
- Singh, P.; Tiwary, B.N. Isolation and characterization of glycolipid biosurfactant produced by a Pseudomonas otitidis strain isolated from Chirimiri coal mines, India. Bioresour. Bioprocess. 2016, 3, 42. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Biosurfactants and oil bioremediation. Curr. Opin. Biotechnol. 2002, 13, 249–252. [Google Scholar] [CrossRef]
- Haidar, C.N.; Pereira, M.M.; Lima, Á.S.; Nerli, B.B.; Malpiedi, L.P. Biosurfactants produced by Pseudomonas syringae pv tabaci: A versatile mixture with interesting emulsifying properties. Process. Biochem. 2020, 97, 121–129. [Google Scholar] [CrossRef]
- Meliani, A. Enhancement of Hydrocarbons Degradation by Use of Pseudomonas Biosurfactants and Biofilms. J. Pet. Environ. Biotechnol. 2014, 05, 1–7. [Google Scholar] [CrossRef]
- Rosenberg, E.; Ron, E.Z. Biosurfactants. In The Prokaryotes: Applied Bacteriology and Biotechnology; Rosenberg, E.F., DeLong, S., Lory, E., Stackebrandt, F., Thompson, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 281–294. [Google Scholar] [CrossRef]
- Abouseoud, M.; Maachi, R.; Amrane, A.; Boudergua, S.; Nabi, A. Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 2008, 223, 143–151. [Google Scholar] [CrossRef]
- Bak, F.; Bonnichsen, L.; Jørgensen, N.O.G.; Nicolaisen, M.H.; Nybroe, O. The biosurfactant viscosin transiently stimulates n-hexadecane mineralization by a bacterial consortium. Appl. Microbiol. Biotechnol. 2015, 99, 1475–1483. [Google Scholar] [CrossRef]
- Ismail Isa, T.; Ngoshe, I.Y.; Gajere, H.M. Biosurfactant Production by Bacteria Isolated from Hydrocarbon-impacted soil. Bioremed. Sci. Technol. Res. 2019, 6, 4–8. [Google Scholar]
- Jacques, R.J.S.; Santos, E.C.; Haddad, R.; Catharino, R.R.; Eberlin, M.N.; Bento, F.M.; Camargo, F.A.D.O. Mass spectrometry analysis of surface tension reducing substances produced by a pah-degrading Pseudomonas citronellolis strain. Braz. J. Microbiol. 2008, 39, 353–356. [Google Scholar] [CrossRef]
- Santos, E.C.; Jacques, R.J.; Bento, F.M.; Peralba, M.D.C.R.; Selbach, P.A.; Sá, E.L.; Camargo, F.A. Anthracene biodegradation and surface activity by an iron-stimulated Pseudomonas sp. Bioresour. Technol. 2008, 99, 2644–2649. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Wang, L.-F.; Changy, J.-S.; Kung, S.-S. Identification of induced acidification in iron-enriched cultures of Bacillus subtilis during biosurfactant fermentation. J. Biosci. Bioeng. 2003, 96, 174–178. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodrı́guez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Dinkla, I.J.T.; Gabor, E.M.; Janssen, D.B. Effects of Iron Limitation on the Degradation of Toluene by Pseudomonas Strains Carrying the TOL (pWWO) Plasmid. Appl. Environ. Microbiol. 2001, 67, 3406–3412. [Google Scholar] [CrossRef]
- Dinkla, I.; Janssen, D. Simultaneous Growth on Citrate Reduces the Effects of Iron Limitation during Toluene Degradation in Pseudomonas. Microb. Ecol. 2003, 45, 97–107. [Google Scholar] [CrossRef][Green Version]
- Wei, Y.-H.; Chu, I.-M. Enhancement of surfactin production in iron-enriched media by bacillus subtilis ATCC 21332. Enzym. Microb. Technol. 1998, 22, 724–728. [Google Scholar] [CrossRef]
- Makkar, S.C.R. An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl. Microbiol. Biotechnol. 2002, 58, 428–434. [Google Scholar] [CrossRef]
- Kiran, G.S.; Thomas, T.A.; Selvin, J. Production of a new glycolipid biosurfactant from marine Nocardiopsis lucentensis MSA04 in solid-state cultivation. Colloids Surf. B Biointerfaces 2010, 78, 8–16. [Google Scholar] [CrossRef]
- Dunham, N.P.; Arnold, F.H. Nature’s Machinery, Repurposed: Expanding the Repertoire of Iron-Dependent Oxygenases. ACS Catal. 2020, 10, 12239–12255. [Google Scholar] [CrossRef]
- Ismail, A.R.; Alias, A.H.; Sulaiman, W.R.W.; Jaafar, M.Z.; Ismail, I. Drilling fluid waste management in drilling for oil and gas wells. Chem. Eng. Trans. 2017, 56, 1351–1356. [Google Scholar] [CrossRef]
- De Almeida, D.G.; Silva, R.D.C.F.S.D.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising Molecules for Petroleum Biotechnology Advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Moldes, A.B.; Paradelo, R.; Vecino, X.; Cruz, J.M.; Gudiña, E.; Rodrigues, L.; Teixeira, J.A.; Domínguez, J.M.; Barral, M.T. Partial Characterization of Biosurfactant fromLactobacillus pentosusand Comparison with Sodium Dodecyl Sulphate for the Bioremediation of Hydrocarbon Contaminated Soil. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Derenne, A.; Derfoufi, K.-M.; Cowper, B.; Delporte, C.; Goormaghtigh, E. FTIR spectroscopy as an analytical tool to compare glycosylation in therapeutic monoclonal antibodies. Anal. Chim. Acta 2020, 1112, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Krilov, D.; Balarin, M.; Kosovic, M.; Gamulin, O.; Brnjas-Kraljević, J. FT-IR spectroscopy of lipoproteins—A comparative study. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2009, 73, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of Lipopeptide Biosurfactant by a Marine Nesterenkonia sp. and Its Application in Food Industry. Front. Microbiol. 2017, 8, 1138. [Google Scholar] [CrossRef] [PubMed]
- Chandankere, R.; Yao, J.; Cai, M.; Masakorala, K.; Jain, A.; Choi, M.M. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 2014, 122, 140–148. [Google Scholar] [CrossRef]
- Kourmentza, C.; Costa, J.; Azevedo, Z.; Servin, C.; Grandfils, C.; De Freitas, V.; Reis, M. Burkholderia thailandensis as a microbial cell factory for the bioconversion of used cooking oil to polyhydroxyalkanoates and rhamnolipids. Bioresour. Technol. 2018, 247, 829–837. [Google Scholar] [CrossRef]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Jen, T.-C.; Lovell, M.R. The influence of fatty acids on tribological and thermal properties of natural oils as sustainable biolubricants. Tribol. Int. 2015, 90, 123–134. [Google Scholar] [CrossRef]
- Saranya, P.; Swarnalatha, S.; Sekaran, G. Lipoprotein biosurfactant production from an extreme acidophile using fish oil and its immobilization in nanoporous activated carbon for the removal of Ca2+and Cr3+in aqueous solution. RSC Adv. 2014, 4, 34144–34155. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Hamley, I.W.; Torras, J.; Alemán, C.; Seitsonen, J.; Ruokolainen, J. Self-Assembly of Lipopeptides Containing Short Peptide Fragments Derived from the Gastrointestinal Hormone PYY3–36: From Micelles to Amyloid Fibrils. J. Phys. Chem. B 2019, 123, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mukherjee, S.; Sen, R. Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J. Appl. Microbiol. 2008, 104, 1675–1684. [Google Scholar] [CrossRef]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 2017, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.D.F.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; T.C., V.; Srirama, K.; Kota, R.K.; Mikkili, I.; Kodali, V.P. Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain. J. King Saud Univ. Sci. 2020, 32, 223–227. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sen, R. Genetic Regulations of the Biosynthesis of Microbial Surfactants: An Overview. Biotechnol. Genet. Eng. Rev. 2008, 25, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Sharma, P. Disinfectant-like activity of lipopeptide biosurfactant produced by Bacillus tequilensis strain SDS21. Colloids Surfaces B: Biointerfaces 2020, 185, 110514. [Google Scholar] [CrossRef]
- Ilori, M.O.; Adebusoye, S.A.; Ojo, A.C. Isolation and characterization of hydrocarbon-degrading and biosurfactant-producing yeast strains obtained from a polluted lagoon water. World J. Microbiol. Biotechnol. 2008, 24, 2539–2545. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, Q.; Du, Y.; Ren, L.; Van Zyl, L.J.; Long, X. Efficient purification of sophorolipids via chemical modifications coupled with extractions and their potential applications as antibacterial agents. Sep. Purif. Technol. 2020, 245, 116897. [Google Scholar] [CrossRef]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef]
- Onbasli, D.; Aslim, B. Determination of antimicrobial activity and production of some metabolites by Pseudomonas aeruginosa B1 and B2 in sugar beet molasses. Afr. J. Biotechnol. 2008, 7, 4614–4619. [Google Scholar]


| Biosurfactant (mg/mL) | Antimicrobial Zone Diameter (mm) | |
|---|---|---|
| Klebsiella pneumoniae | 1 | 9.0 ± 0.7 |
| Pseudomonas aeruginosa | 1 | 8.8 ± 0.8 |
| Escherichia coli resistant | 1 | 10.5 ± 1.5 |
| Escherichia coli | 1 | 8.8 ± 0.4 |
| Enterococcus faecalis resistant | 10 | 9.3 ± 2.2 |
| Enterococcus faecalis | 10 | 8.3 ± 1.9 |
| Acinetobacter baumaniii | 10 | 7.3 ± 5.3 |
| Streptococcus pneumoniae | 10 | 9.8 ± 1.0 |
| Staphylococcus aureus | 10 | 9.0 ± 0.8 |
| Salmonella enterica | 10 | 9.3 ± 0.5 |
| Antimicrobial Zone Diameter (mm) | ||||||
|---|---|---|---|---|---|---|
| Biosurfactant (mg/mL): | 10 | 5 | 1 | 0.5 | 0.25 | 0.1 |
| Klebsiella pneumoniae | - | - | 9.0 ± 0.7 | 8.0 ± 0.0 | 7.0 ± 0.0 | 0.0 ± 0.0 |
| Pseudomonas aeruginosa | - | - | 8.8 ± 0.8 | 8.5 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Escherichia coli resistant | - | - | 10.5 ± 1.5 | 7.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Escherichia coli | - | - | 8.8 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Enterococcus faecalis resistant | 9.3 ± 2.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | - | - |
| Enterococcus faecalis | 8.3 ± 1.9 | 1.8 ± 3.5 | 0.0 ± 0.0 | - | - | - |
| Acinetobacter baumaniii | 7.3 ± 5.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | - | - |
| Streptococcus pneumoniae | 9.8 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | - | - |
| Staphylococcus aureus | 9.0 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | - | - |
| Salmonella enterica | 9.3 ± 0.5 | 8.3 ± 1.9 | 0.0 ± 0.0 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsipa, A.; Stylianou, K.; Papalli, M.; Papageorgiou, E.; Kyriakou, L.; Rigopoulos, I.; Ioannou, I.; Pinakoulaki, E. Iron-Stimulated Production and Antimicrobial Potential of a Novel Biosurfactant Produced by a Drilling Waste-Degrading Pseudomonas citronellolis Strain. Processes 2021, 9, 686. https://doi.org/10.3390/pr9040686
Tsipa A, Stylianou K, Papalli M, Papageorgiou E, Kyriakou L, Rigopoulos I, Ioannou I, Pinakoulaki E. Iron-Stimulated Production and Antimicrobial Potential of a Novel Biosurfactant Produced by a Drilling Waste-Degrading Pseudomonas citronellolis Strain. Processes. 2021; 9(4):686. https://doi.org/10.3390/pr9040686
Chicago/Turabian StyleTsipa, Argyro, Konstantina Stylianou, Maria Papalli, Erato Papageorgiou, Loucas Kyriakou, Ioannis Rigopoulos, Ioannis Ioannou, and Eftychia Pinakoulaki. 2021. "Iron-Stimulated Production and Antimicrobial Potential of a Novel Biosurfactant Produced by a Drilling Waste-Degrading Pseudomonas citronellolis Strain" Processes 9, no. 4: 686. https://doi.org/10.3390/pr9040686
APA StyleTsipa, A., Stylianou, K., Papalli, M., Papageorgiou, E., Kyriakou, L., Rigopoulos, I., Ioannou, I., & Pinakoulaki, E. (2021). Iron-Stimulated Production and Antimicrobial Potential of a Novel Biosurfactant Produced by a Drilling Waste-Degrading Pseudomonas citronellolis Strain. Processes, 9(4), 686. https://doi.org/10.3390/pr9040686








