Adsorption/Desorption Characteristics and Simultaneous Enrichment of Orientin, Isoorientin, Vitexin and Isovitexin from Hydrolyzed Oil Palm Leaf Extract Using Macroporous Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Pretreatment of MARs
2.3. Preparation of Crude and Acid-Hydrolyzed Extracts
2.4. UHPLC Analysis of Orientin, Isoorientin, Vitexin, and Isovitexin
2.5. Preliminary Selection Macroporous Resin as an Effective Adsorbent
2.6. Optimization of Sorption Conditions Using Batch Adsorption Tests
2.7. Dynamic Sorption Experiments on the Chromatography Column
2.8. Adsorption and Desorption Capacity, Kinetics, and Isotherm Model Equations
2.9. Determination of the Total Flavonoid Content and Antioxidant Free Radical Scavenging Activities
2.10. Statistical Analysis
3. Results and Discussion
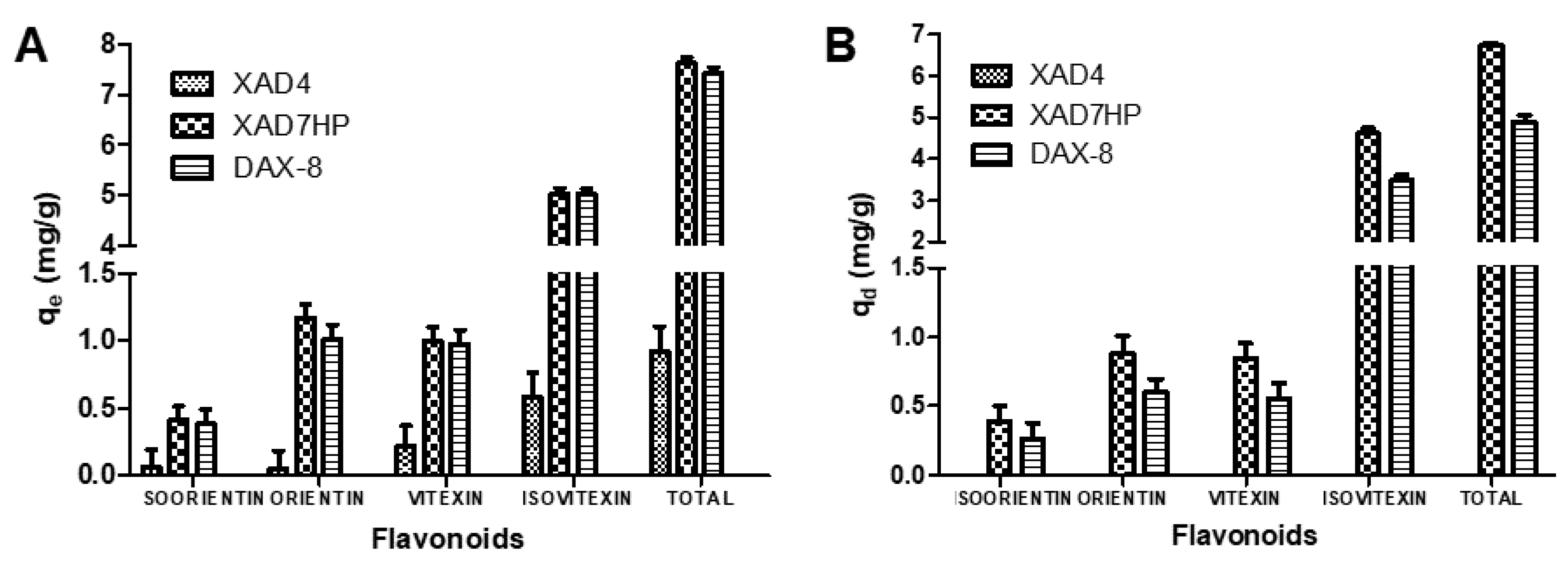
3.1. Adsorption and Desorption Capacities of Selected MARs
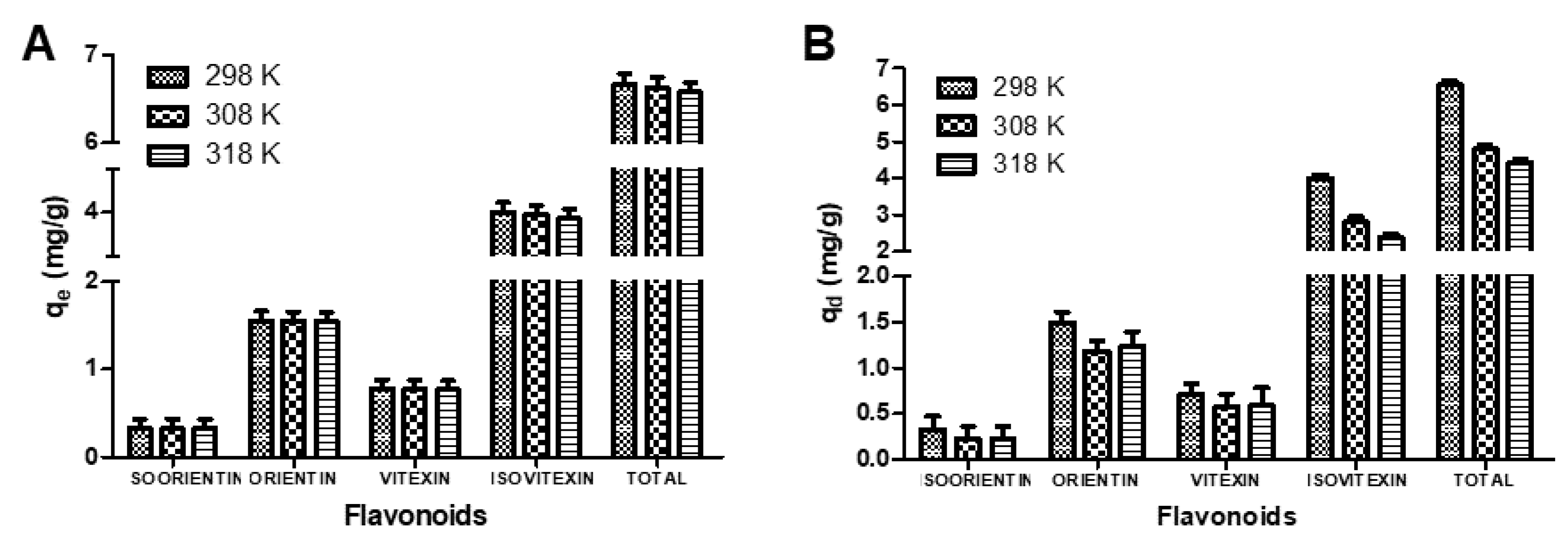
3.2. Effect of Oscillation Temperatures on the Sorption Capacities
3.3. Adsorption Kinetics of the XAD7HP Resin
3.4. Adsorption Isotherms on the XAD7HP Resin
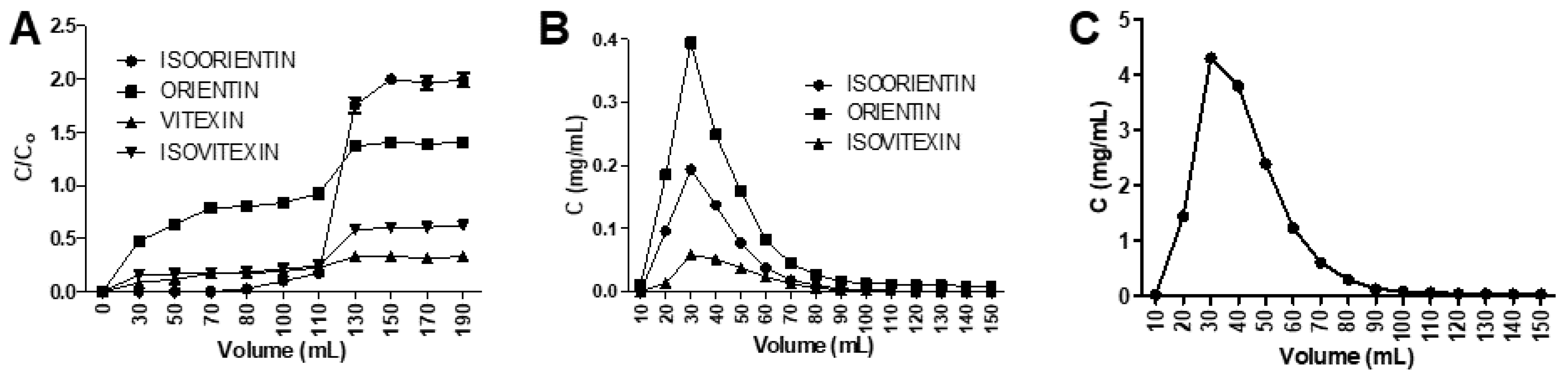
3.5. Dynamic Sorption Properties of the XAD7HP Resin
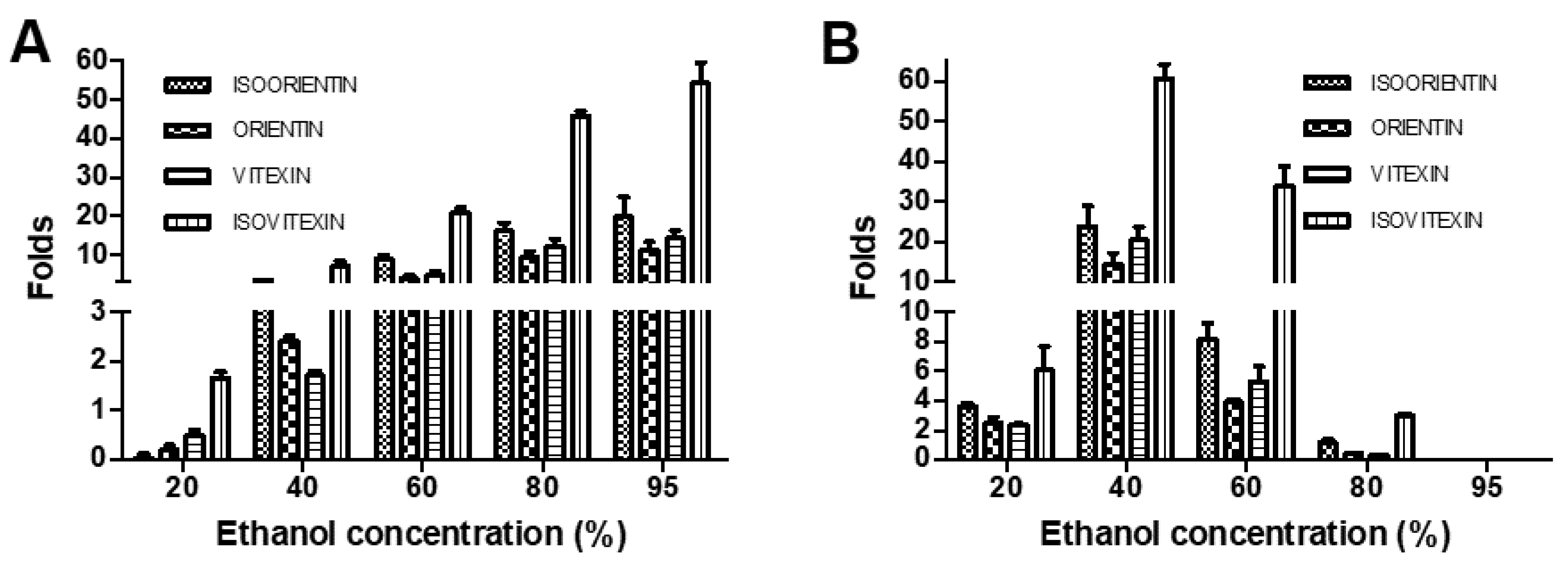
3.6. Comparison between Isocratic and Gradient Elution Modes for Optimal Flavonoid C-Glycoside Enrichment
3.7. Antioxidant DPPH and NO Free Radical Scavenging Activities
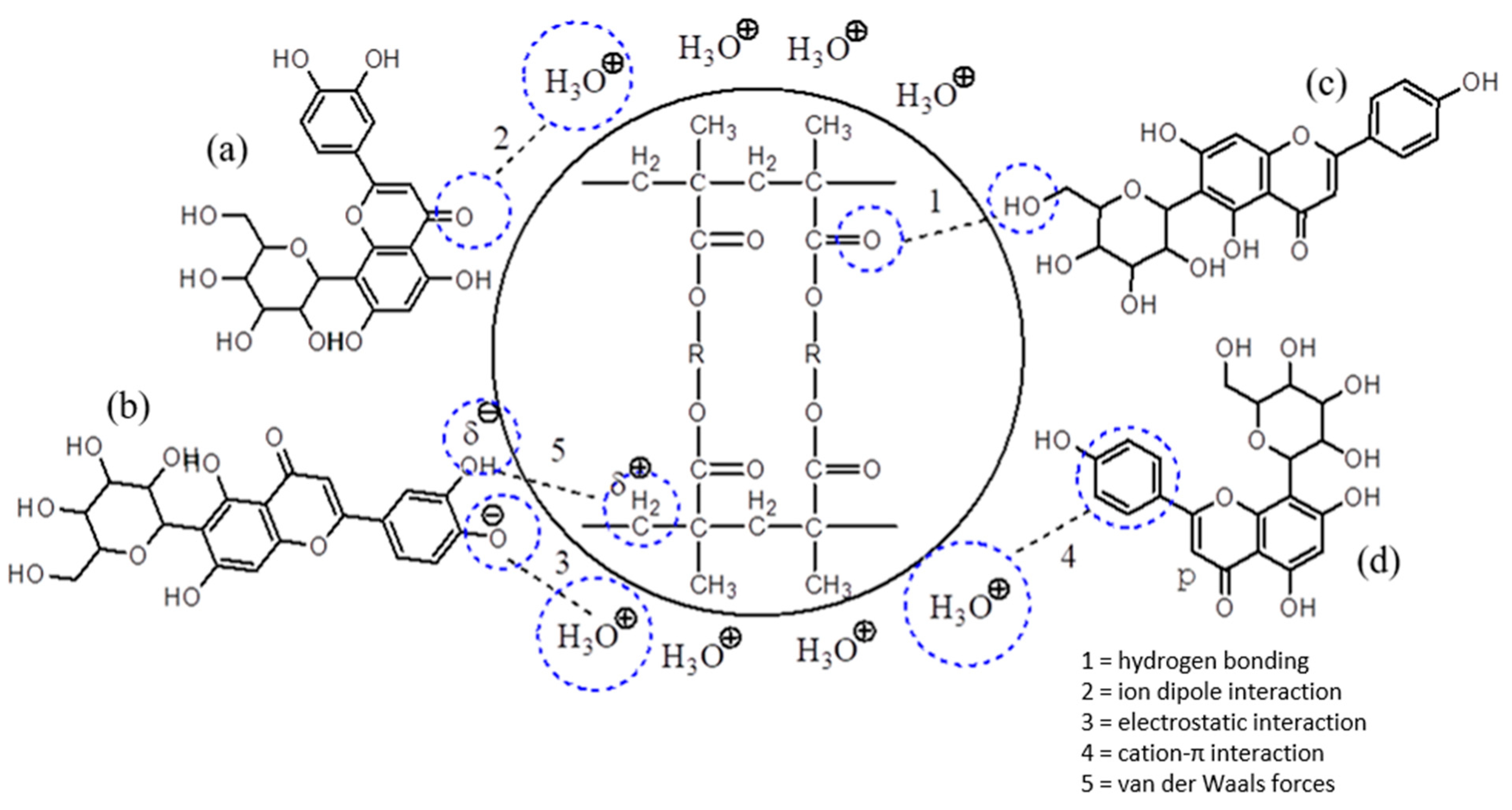
3.8. Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitmore, T.C. The Palms of Malaya; Longmans: Selangor, Malaysia, 1973. [Google Scholar]
- Basiron, Y. Palm oil production through sustainable plantations. Eur. J. Lipid Sci. Technol. 2007, 109, 289–295. [Google Scholar] [CrossRef]
- Malaysian Palm Oil Board (MPOB). Malaysian Oil Palm Industry Performance 2016 and Prospects for 2017. Available online: http://www.mpob.gov.my (accessed on 20 January 2019).
- Malaysian Palm Oil Board (MPOB). Production of Oil Palm Products 2020. Available online: http://bepi.mpob.gov.my (accessed on 27 January 2021).
- Be Sustainable Magazine. Malaysia’s Biomass Potential. Available online: http://www.besustainablemagazine.com (accessed on 13 February 2021).
- Owoyele, B.V.; Owolabi, G.O. Traditional oil palm (Elaeis guineensis Jacq.) and its medicinal uses: A review. Tang Humanit Med. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Tahir, N.I.; Shaari, K.; Abas, F.; Parveez, G.K.A.; Ishak, Z.; Ramli, U.S. Characterization of apigenin and luteolin derivatives from oil palm (Elaeis guineensis Jacq.) leaf using LC-ESI-MS/MS. J. Agric. Food Chem. 2012, 60, 11201–11210. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Zain, M.S.C.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and Desorption properties of total flavonoids from oil palm (Elaeis guineensis Jacq.) mature leaf on macroporous adsorption resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef]
- Zain, M.S.C.; Jakariah, N.A.; Yeoh, J.X.; Lee, S.Y.; Shaari, K. Ultrasound-assisted extraction of polyphenolic contents and acid hydrolysis of flavonoid glycosides from oil palm (Elaeis guineensis Jacq.) leaf: Optimization and correlation with free radical scavenging activity. Processes 2020, 8, 1540. [Google Scholar] [CrossRef]
- Zain, M.S.C.; Lee, S.Y.; Mad Nasir, N.; Fakurazi, S.; Shaari, K. Metabolite characterization and correlations with antioxidant and wound healing properties of oil palm (Elaeis guineensis Jacq.) leaflets via 1H-NMR-based metabolomics approach. Molecules 2020, 25, 5636. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.S.C.; Lee, S.Y.; Sarian, M.N.; Fakurazi, S.; Shaari, K. In vitro wound healing potential of flavonoid c-glycosides from oil palm (Elaeis guineensis Jacq.) leaves on 3t3 fibroblast cells. Antioxidants 2020, 9, 326. [Google Scholar] [CrossRef]
- Mordor Intelligence. Flavonoid Market—Growth, Trends and Forecast (2020–2025). Available online: https://www.mordorintelligence.com (accessed on 20 July 2020).
- Yang, X.; Wei, M.; Tian, H.; Liu, T.; Yang, L. Enrichment and purification of aucubin from Eucommia ulmoides ionic liquid extract using macroporous resins. Materials 2018, 11, 1758. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, D.; Gwon, Y.J.; Lee, K.W.; Lee, T.S. Removal of sodium dodecylbenzenesulfonate by macroporous adsorbent resins. Materials 2018, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in antioxidant flavonoids of stamen extracts from Nymphaea lotus L. using ultrasonic-assisted extraction and macroporous resin adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, H.; Dong, Y.; Yang, B.; Zhao, M. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (MAXIM.) HARA leaf. Food Chem. 2012, 130, 417–424. [Google Scholar] [CrossRef]
- Du, H.; Wang, H.; Yu, J.; Liang, C.; Ye, W.; Li, P. Enrichment and purification of total flavonoid C-glycosides from Abrus mollis extracts with macroporous resins. Ind. Eng. Chem. Res. 2012, 51, 7349–7354. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Zu, Y.G.; Fu, Y.J.; Ma, W.; Zhang, D.Y.; Kong, Y.; Li, X.-J. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresour. Technol. 2010, 101, 4667–4675. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Zhu, L.; Xie, H.; Li, H.; He, J.; Pan, M.; Zhang, T.; Ito, Y. Preparative isolation and purification of flavone C-glycosides from the leaves of Ficus microcarpa L.f by medium-pressure liquid chromatography, high-speed countercurrent chromatography, and preparative liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 462–480. [Google Scholar] [CrossRef]
- International Conference on Harmonization of Technical Requirment for Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedures: Text and Methodology Q2 (R1); ICH: Geneva, Switzerland, 2005; Volume 7, pp. 1–5.
- Zain, M.S.C.; Osman, M.F.; Lee, S.Y.; Shaari, K. UHPLC-UV /PDA method validation for simultaneous quantification of luteolin and apigenin derivatives from Elaeis guineensis leaf extracts: An application for antioxidant herbal preparation. Molecules 2021, 26, 1084. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Venkobachar, C. Removal of Cadmium (II) by low cost adsorbents. J. Environ. Eng. 1984, 110, 110–122. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Tobin, J.M. Metal-anion sorption by chitosan beads: Equilibrium and kinetic studies. Ind. Eng. Chem. Res. 1998, 37, 1454–1463. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Kammerer, J.; Carle, R.; Kammerer, D.R. Adsorption and ion exchange: Basic principles and their application in food processing. J. Agric. Food Chem. 2011, 59, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore and solid diffusion kinetics in fixed-bed adsorption under constant pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Taktak, F.; Ciğeroğlu, Z.; Öğen, Y.; Kirbaşlar, Ş.İ. Resin-loaded cationic hydrogel: A new sorbent for recovering of grapefruit polyphenols. Chem. Eng. Commun. 2018, 205, 1442–1456. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Selective adsorption of oleuropein from olive (Olea europaea) leaf extract using macroporous resin. Chem. Eng. Commun. 2017, 204, 1391–1400. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, H.; Hao, L.; Min, C.; Cai, J.; Liu, J.; Cai, P.; Yang, S. Enrichment and antioxidant properties of flavone C-glycosides from trollflowers using macroporous resin. Food Chem. 2013, 141, 533–541. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Xiao, W.; Wang, Z.; Ding, G.; Zhao, L. Enrichment and purification of total Ginkgo flavonoid O-glycosides from Ginkgo Biloba extract with macroporous resin and evaluation of anti-inflammation activities in vitro. Molecules 2018, 23, 1167. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Huang, K.L.; Wang, A.T.; Yang, Q. Adsorption characteristics of poly(styrene-co-divinylbenzene) resin functionalized with methoxy and phenoxy groups for phenol. J. Colloid Interface Sci. 2008, 327, 302–307. [Google Scholar] [CrossRef]
- Wan, P.; Sheng, Z.; Han, Q.; Zhao, Y.; Cheng, G.; Li, Y. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 945–946, 68–74. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G. Adsorption of lignite-derived humic acids on coal-based mesoporous activated carbons. J. Colloid Interface Sci. 2005, 284, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, E.; Hoda, N. Adsorption kinetics and isotherms of pesticides onto activated carbon-cloth. Chemosphere 2005, 60, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Hong, Z.; Li, Z.; Gang, Z. Study on adsorption characteristic of macroporous resin to phenol in wastewater. Can. J. Chem. Eng. 2010, 88, 417–424. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, Q.; Lou, S.; Di, D.; Li, J.; Guo, M. Adsorption characteristics of (−)-epigallocatechin gallate and caffeine in the extract of waste tea on macroporous adsorption resins functionalized with chloromethyl, amino, and phenylamino groups. J. Agric. Food Chem. 2012, 60, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Chen, Z.; Liu, Y.; Ye, H.; Di, D. Synthesis of functional adsorption resin and its adsorption properties in purification of flavonoids from Hippophae rhamnoides L. leaves. Ind. Eng. Chem. Res. 2012, 51, 2682–2696. [Google Scholar] [CrossRef]
- Wang, R.; Peng, X.; Wang, L.; Tan, B.; Liu, J.; Feng, Y.; Yang, S. Preparative purification of peoniflorin and albiflorin from peony rhizome using macroporous resin and medium-pressure liquid chromatography. J. Sep. Sci. 2012, 35, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Q.; Pan, S.Y.; Yao, X.L.; Fu, H.F. Isolation and Purification of Anthocyanins from Blood Oranges by Column Chromatography. Agric. Sci. China 2010, 9, 207–215. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Gong, G.; Li, F.; Ren, H.; Liu, Y. Adsorption and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.). Food Bioprod. Process. 2015, 93, 148–155. [Google Scholar] [CrossRef]
- Koch, W.; Kukuła-Koch, W.; Czop, M.; Helon, P.; Gumbarewicz, E. The role of extracting solvents in the recovery of polyphenols from green tea and its antiradical activity supported by principal component analysis. Molecules 2020, 25, 2173. [Google Scholar] [CrossRef]
- Abdul-Hamid, N.A.; Mustaffer, N.H.; Maulidiani, M.; Mediani, A.; Ismail, I.S.; Tham, C.L.; Shadid, K.; Abas, F. Quality evaluation of the physical properties, phytochemicals, biological activities and proximate analysis of nine Saudi date palm fruit varieties. J. Saudi Soc. Agric. Sci. 2010, 19, 151–160. [Google Scholar] [CrossRef]
- Sinjman, P.W.; Joubert, E.; Ferreira, D.; Li, X.C.; Ding, Y.; Green, I.R.; Gelderblom, W.C. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef]
- Gerhäuser, C. Phenolic Beer Compounds to Prevent Cancer. Beer in Health and Disease Prevention; Elsevier: London, UK, 2009; pp. 669–684. [Google Scholar]
- Khole, S.; Panat, N.A.; Suryawanshi, P.; Chatterjee, S.; Devasagayam, T.; Ghaskadbi, S. Comprehensive assessment of antioxidant activities of apigenin isomers: Vitexin and isovitexin. Free Radic. Antioxid. 2016, 6, 155–166. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Huo, T.; Chen, Z.; Liu, Y.; Di, D.; Guo, M.; Zhao, L. Multiple interactions on macroporous adsorption resins modified with ionic liquid. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 487, 35–41. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry & medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [PubMed]
- Li, J.; Chase, H.A. Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat. Prod. Rep. 2010, 27, 1493. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X. Structures required of flavonoids for inhibiting digestive enzymes. Anticancer Agents Med. Chem. 2012, 12, 929–939. [Google Scholar] [CrossRef]
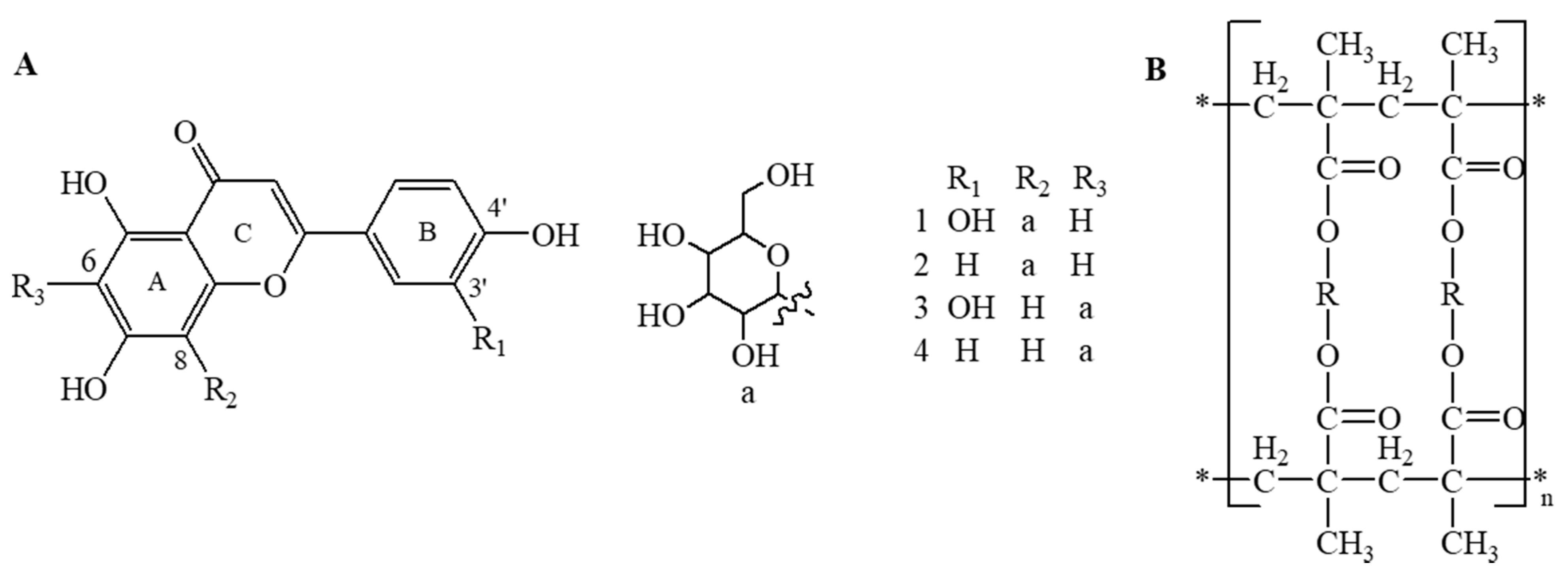

| XAD7HP | DAX-8 | XAD4 | |
|---|---|---|---|
| Functional group | Acrylic | Acrylic ester | Styrene-divinylbenzene |
| Particle diameter (mm) | 0.250–0.841 | 0.250–0.420 | 0.250–0.841 |
| Surface area (m2/g) | 380 | 140 | 750 |
| Pore size (Å) | 300–400 | 225 | 100 |
| Polarity | Moderate | Moderate | Polar |
| Compound | pH | qe (exp) (mg/g) | Pseudo-First Order | Pseudo-Second Order | IntraParticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | qe (mg/g) | k1 (1/min) | R2 | qe (mg/g) | k2 (g/mg.min) | R2 | C (mg/g) | kp (mg/g.min1/2) | |||
| Isoorientin | 5 | 2.5668 Aa | 0.7935 Aa | 2.0738 Aa | 0.0063 Aa | 0.9995 Aa | 2.5813 Aa | 0.0416 Aa | 0.5133 Aa | 1.6715 Aa | 0.0485 Aa |
| 7 | 0.5916 Ba | 0.8134 Ba | 3.1817 Ba | 0.0050 Ba | 0.9971 Ba | 0.6007 Ba | 0.0403 Ba | 0.8506 Ba | 0.1468 Ba | 0.0218 Ba | |
| 9 | 0.1866 Ca | 0.9322 Ca | 8.6789 Ca | 0.0048 Ba | 0.9640 Ca | 0.1851 Ca | 0.1102 Ca | 0.9196 Ca | 0.0701 Ca | 0.0048 Ca | |
| Orientin | 5 | 5.2571 Ab | 0.8178 Ab | 1.2216 Aa | 0.0085 Ab | 0.9995 Aa | 5.3362 Ab | 0.0238 Ab | 0.5008 Ab | 3.5373 Ab | 0.0978 Ab |
| 7 | 1.8269 Bb | 0.7892 Bb | 1.3399 Bb | 0.0059 Ba | 0.9980 Bb | 1.8643 Bb | 0.0195 Bb | 0.7597 Bb | 0.6503 Bb | 0.0613 Bb | |
| 9 | 0.2947 Cb | 0.8829 Cb | 4.9644 Cb | 0.0014 Cb | 0.9641 Ca | 0.2024 Cb | 0.0881 Cb | 0.8223 Cb | 0.0703 Ca | 0.0054 Ca | |
| Vitexin | 5 | 1.6622 Ac | 0.9158 Ac | 3.7371 Ac | 0.0090 Ab | 0.9999 Aa | 1.6793 Ac | 0.0967 Ac | 0.5653 Ac | 1.2447 Ac | 0.0233 Ac |
| 7 | 0.8356 Bc | 0.8718 Bc | 5.0799 Bc | 0.0075 Bb | 0.9999 Ac | 0.8457 Bc | 0.1137 Bc | 0.8157 Bc | 0.3242 Bc | 0.0235 Ac | |
| 9 | 0.2481 Cc | 0.9252 Cc | 6.8244 Cc | 0.0064 Cc | 0.9959 Bb | 0.2683 Cc | 0.0703 Cc | 0.8229 Cb | 0.0518 Cb | 0.0101 Bb | |
| Isovitexin | 5 | 9.5296 Ad | 0.8763 Ad | 1.9927 Ad | 0.0072 Ac | 0.9998 Aa | 9.5877 Ad | 0.0120 Ad | 0.6099 Ad | 6.4869 Ad | 0.1639 Ad |
| 7 | 2.5159 Bd | 0.8952 Ad | 1.1389 Bd | 0.0078 Ab | 0.9983 Bb | 2.6185 Bd | 0.0143 Bd | 0.7582 Bb | 1.0526 Bd | 0.0772 Bd | |
| 9 | 1.4779 Cd | 0.9261 Bc | 1.0830 Cd | 0.0101 Bd | 0.9974 Cc | 1.5564 Cd | 0.0195 Cd | 0.8568 Cc | 0.5758 Cc | 0.0464 Cc | |
| Compound | Langmuir Equation | Freundlich Equation | |||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | R12 | KL (mg/mL) | RL | 1/n | Kf ((mg/g) (mL/mg)1/n) | R22 | |
| Isoorientin | 476.1905 a | 0.9977 a | 0.2381 a | 0.2929 a | 0.3754 a | 366.090 a | 0.9260 a |
| Orientin | 2000.000 b | 0.9519 b | 0.6000 b | 0.0480 b | 0.3757 a | 1075.70 b | 0.9247 a |
| Vitexin | 50000.00 c | 0.9624 c | 30.000 c | 0.0001 c | 0.9538 b | 1422.10 c | 0.9700 b |
| Isovitexin | 204.0816 d | 0.5187 d | 0.2653 d | 0.5120 d | 0.4149 c | 138.930 d | 0.8418 c |
| Extract or Compound | TFC (mg QCE/g) | Flavonoid C-Glycosides (µg/mg) | Antioxidant Activities (IC50, µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| Isoorientin | Orientin | Vitexin | Isovitexin | DPPH | NO | ||
| OPLAH | 88.98 a | 2.34 a | 9.35 a | 84.11 a | 0.25 a | 200.00 a | 44.58 a |
| Enriched OPLAH (Isocratic) | 247.28 b | 46.27 b | 104.88 b | 1197.61 b | 13.03 b | 69.16 b | 6.90 b |
| Enriched OPLAH (Gradient) | 284.18 c | 55.98 c | 136.19 c | 1726.11 c | 14.61 b | 70.63 b | 7.32 b |
| Isoorientin | 14.70 c | 68.19 c | |||||
| Orientin | 57.60 d | 42.72 a | |||||
| Isovitexin | >1000 e | 0.73 d | |||||
| Vitexin | >1000 e | 4.31 e | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption/Desorption Characteristics and Simultaneous Enrichment of Orientin, Isoorientin, Vitexin and Isovitexin from Hydrolyzed Oil Palm Leaf Extract Using Macroporous Resins. Processes 2021, 9, 659. https://doi.org/10.3390/pr9040659
Che Zain MS, Lee SY, Teo CY, Shaari K. Adsorption/Desorption Characteristics and Simultaneous Enrichment of Orientin, Isoorientin, Vitexin and Isovitexin from Hydrolyzed Oil Palm Leaf Extract Using Macroporous Resins. Processes. 2021; 9(4):659. https://doi.org/10.3390/pr9040659
Chicago/Turabian StyleChe Zain, Mohamad Shazeli, Soo Yee Lee, Chian Ying Teo, and Khozirah Shaari. 2021. "Adsorption/Desorption Characteristics and Simultaneous Enrichment of Orientin, Isoorientin, Vitexin and Isovitexin from Hydrolyzed Oil Palm Leaf Extract Using Macroporous Resins" Processes 9, no. 4: 659. https://doi.org/10.3390/pr9040659
APA StyleChe Zain, M. S., Lee, S. Y., Teo, C. Y., & Shaari, K. (2021). Adsorption/Desorption Characteristics and Simultaneous Enrichment of Orientin, Isoorientin, Vitexin and Isovitexin from Hydrolyzed Oil Palm Leaf Extract Using Macroporous Resins. Processes, 9(4), 659. https://doi.org/10.3390/pr9040659








