Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases
Abstract
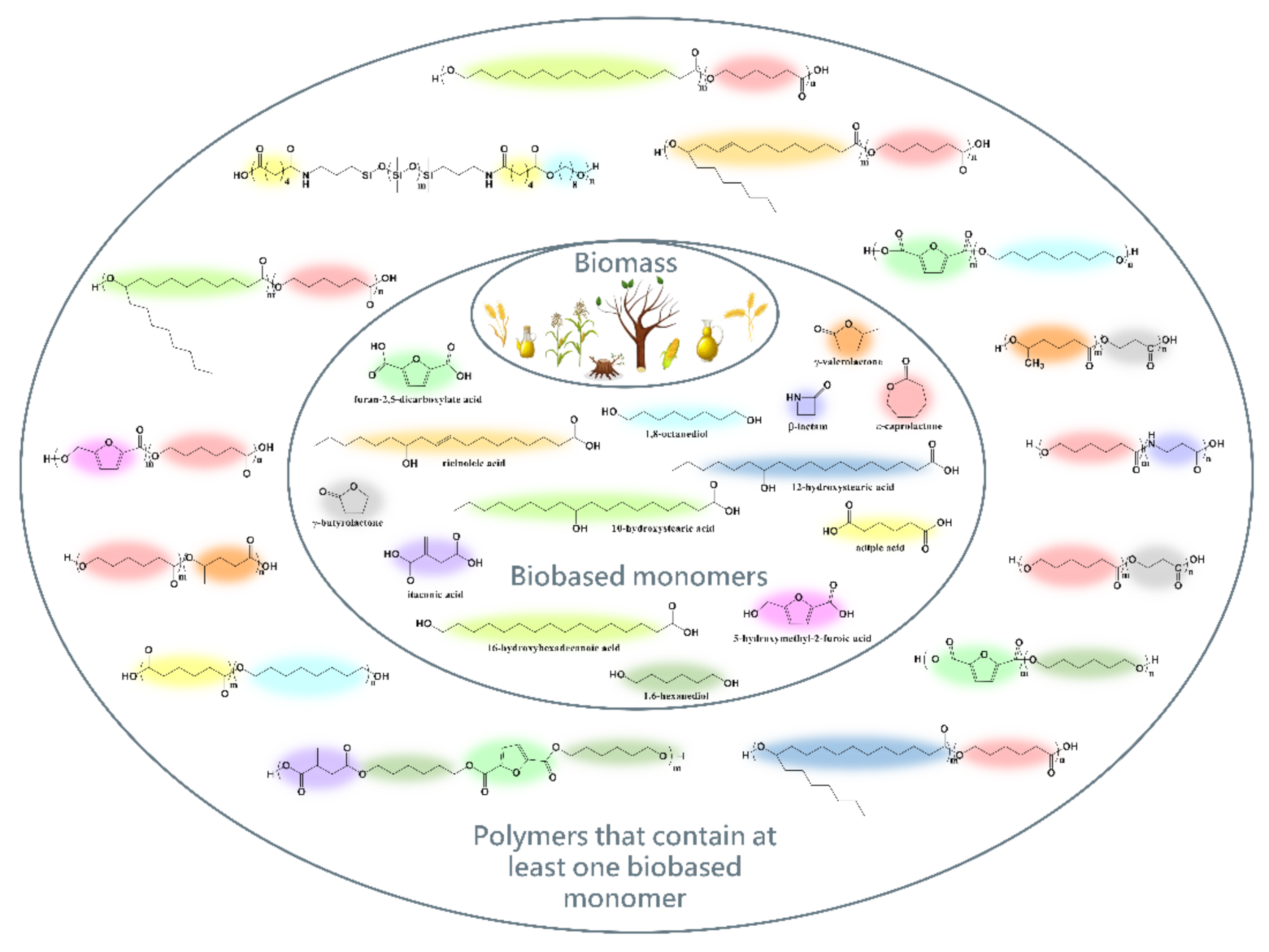
1. Introduction
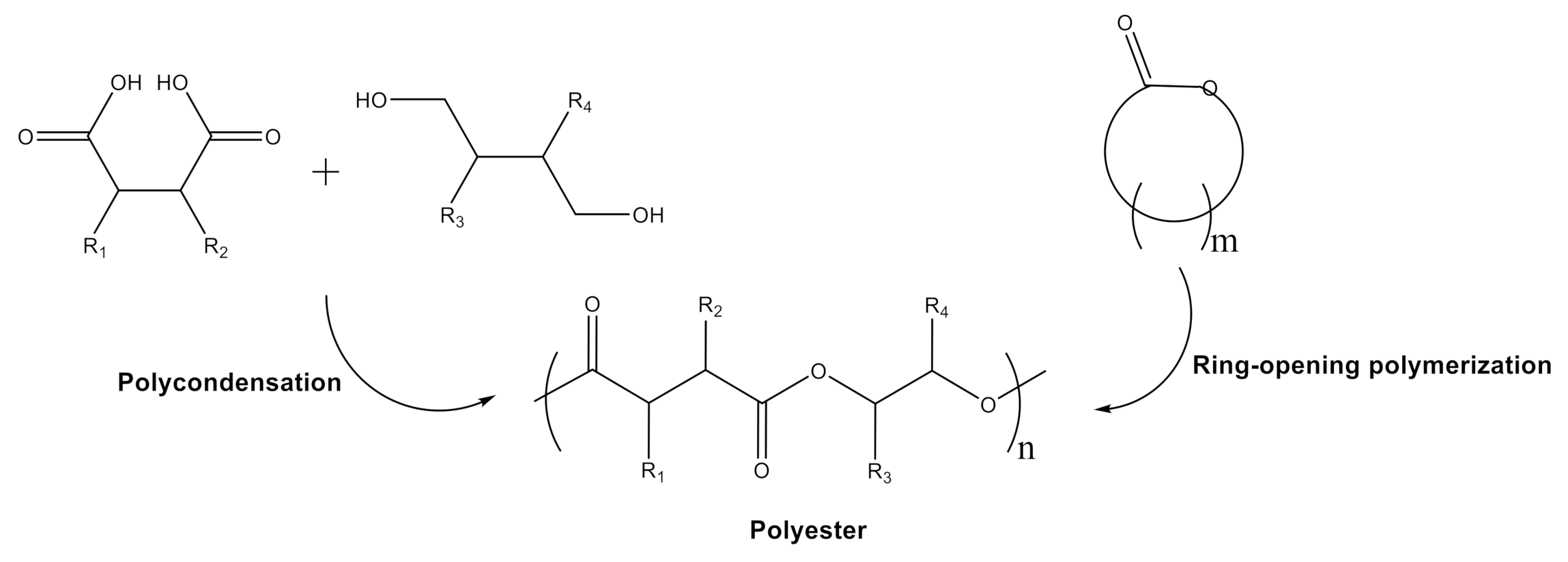
2. Synthesis Opportunities and Importance of Polyesters and Polyesteramides Obtained from Biomass-Based Monomers
- (1)
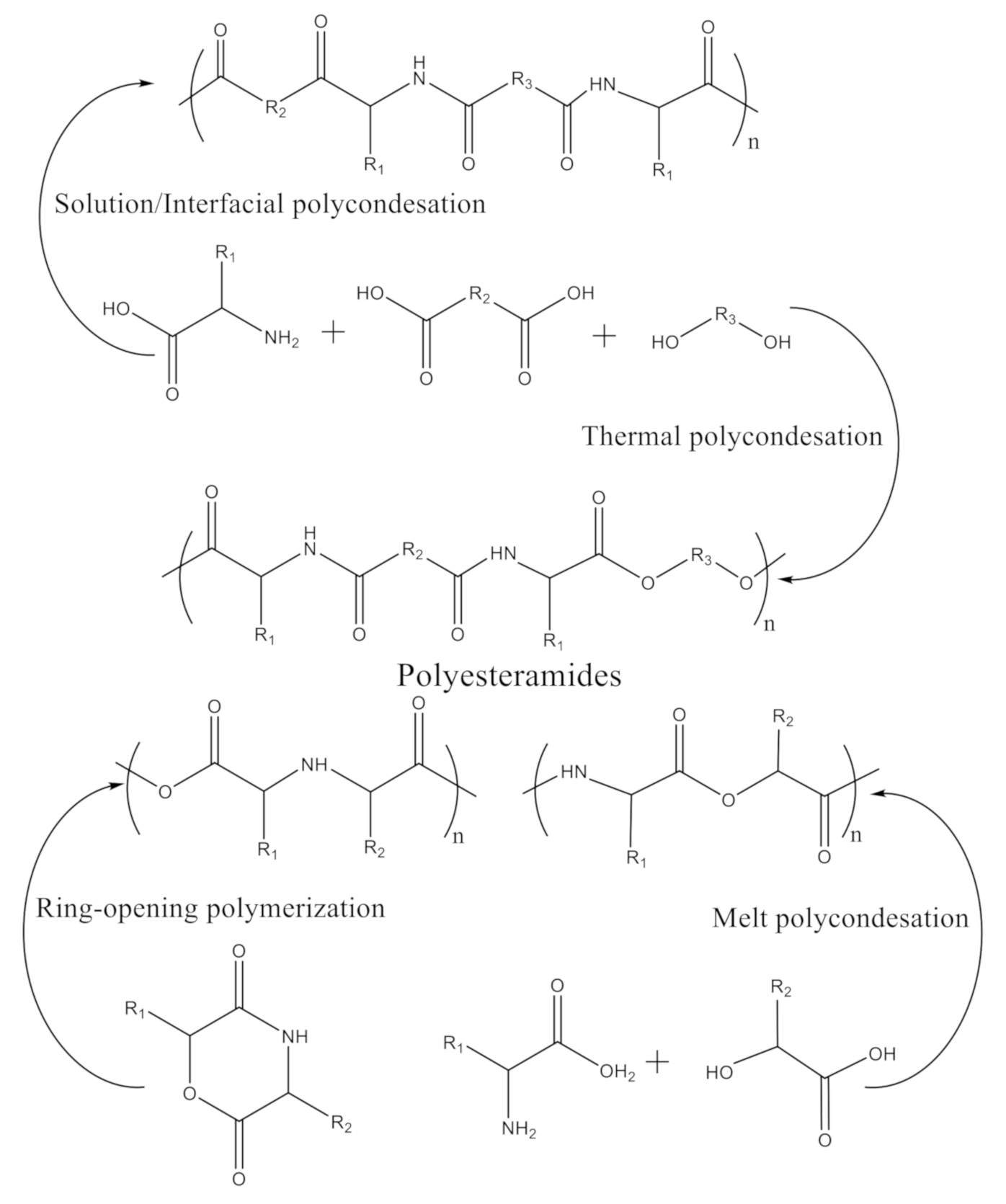
- Polycondensation of α-amino acids, fatty dicarboxylic acids, and diols. In this case, a diamide-diester is first synthesized by condensation of a diacyl chloride with the methyl ester of an α-amino acid, followed by polycondensation with a diol. The reactions can be accomplished by different pathways: (i) thermal polycondensation [74]; (ii) solution polycondensation [75]; (iii) interfacial polycondensation [76].
- (2)
- Polymerization of α-amino acids with α-hydroxy acids, using two main techniques: (i) ring-opening-polymerization (ROP) of cyclic depsipeptides (peptides in which one or more of amide groups are replaced by ester), particularly derivatives of morpholine-2,5-dione [77]; (ii) direct melt polycondensation of α-amino acids with α-hydroxy acids in the presence of metal catalysts [78].
3. Trends in the Lipase-Catalyzed Synthesis of Polyesters and Co-Polyesters of ε-Caprolactone
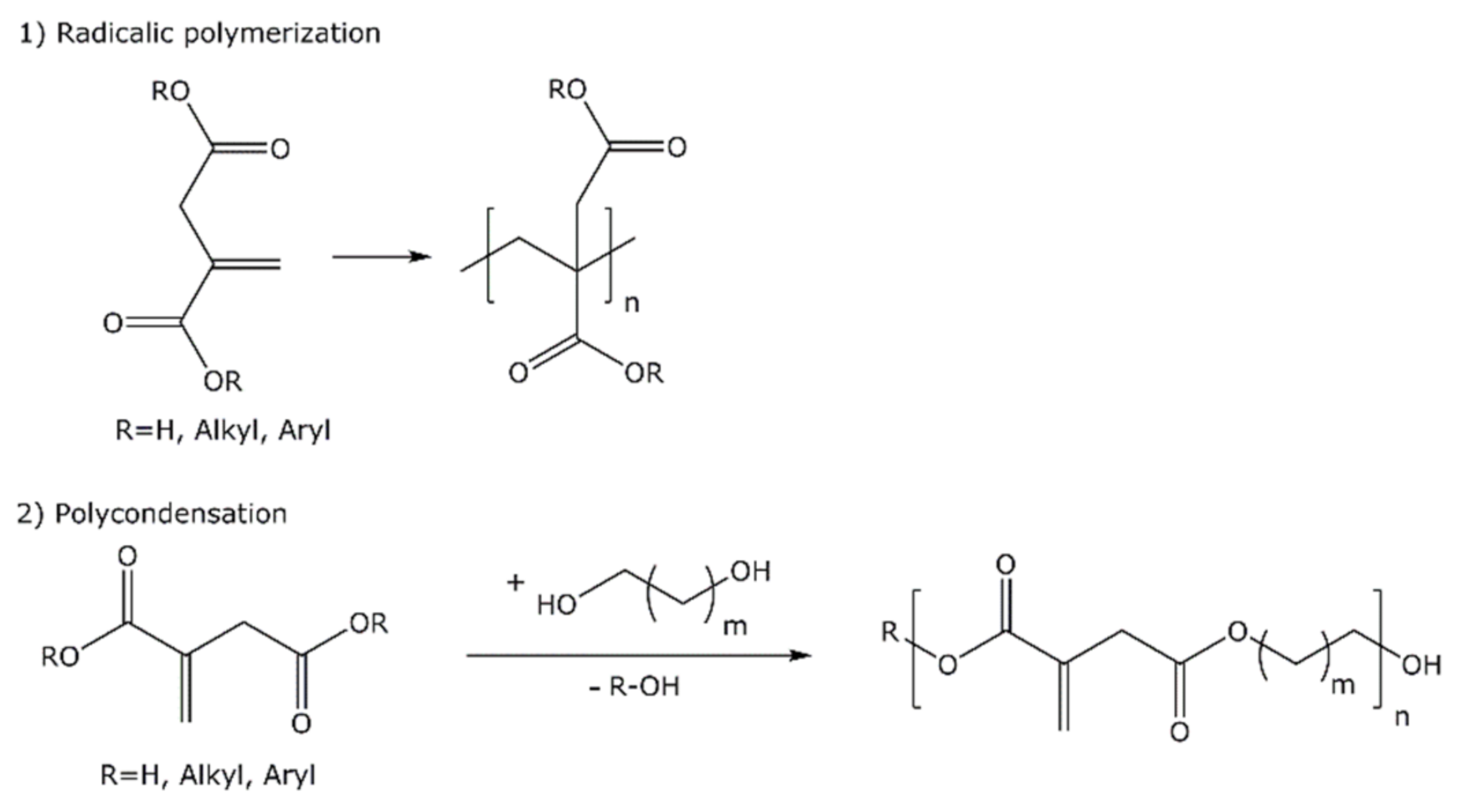
4. Developments in the Enzymatic Synthesis of Itaconic Acid Based Polyesters
5. Hydroxy Fatty Acids as Source for Enzymatic Synthesis of Bio-Based Estolides
6. Lipase-Catalyzed Synthesis of Polyesters Containing Furan Rings
7. Tendencies in Enzymatic Synthesis of Polyesteramides
8. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brämer, C.O.; Steinbüchel, A. Occurrence, functions and biosynthesis of non-carbohydrate biopolymers. In Renewable Bioresources: Scope and Modification for Non-Food Applications; Stevens, C.V., Verhé, R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2004; pp. 189–207. [Google Scholar]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of chitin and chitosan and their isolation. In Chitin and Chitosan: Properties and Applications; Van den Broek, L.A.M., Boeriu, C.G., Eds.; John Wiley & Sons Ltd.: Weinheim, Germany, 2019; pp. 1–34. [Google Scholar]
- MacGregor, E.A. Biopolymers. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 207–245. [Google Scholar]
- Hearle, J.W.S. Fiber production. In UNESCO-Encyclopedia Life Support Systems (EOLSS), 7. Physical Sciences, Engineering and Technology Resources, Materials Science and Engineering; Rawlings, R.D., Ed.; UNESCO: Paris, France, 2019; Volume 2, Available online: https://www.eolss.net/Sample-Chapters/C05/E6-36-03-05.pdf (accessed on 30 March 2021).
- Miller, S.A. Sustainable polymers: Replacing polymers derived from fossil fuels. Polym. Chem. 2014, 5, 3117–3118. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing biobased recyclable polymers for plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Aeschelmann, F.; Carus, M. Bio-based building blocks and polymers in the world: Capacities, production, and applications: Status quo and trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Leferink, N.G.H.; Scrutton, N.S. Chemo-enzymatic routes towards the synthesis of bio-based monomers and polymers. Mol. Cat. 2019, 467, 95–110. [Google Scholar] [CrossRef]
- Shen., L.; Haufe, J.; Patel, M.K. Product Overview and Market Projection of Emerging Bio-Based Plastics: European Polysaccharide Network of Excellence, Utrecht, The Netherlands. 2009. Available online: https://www.uu.nl/sites/default/files/copernicus_probip2009_final_june_2009_revised_in_november_09.pdf (accessed on 25 February 2021).
- Thoen, J.; Busch, R. Industrial chemicals from biomass—Industrial concepts. In Biorefineries—Industrial Processes and Products: Status Quo and Future Directions; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005; pp. 347–365. [Google Scholar]
- Kawaguchi, H.; Ogino, C.; Kondo, A. Microbial conversion of biomass into bio-based polymers. Bioresour. Technol. 2017, 245, 1664–1673. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Gregory, G.L.; Lopez-Vidal, E.M.; Buchard, A. Polymers from sugars: Cyclic monomer synthesis, ring-opening polymerisation, material properties and applications. Chem. Commun. 2017, 53, 2198–2217. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Bampouli, A. Potential of commodity chemicals to become bio-based according to maximum yields and petrochemical prices. Biofuels Bioprod. Bioref. 2017, 11, 798–810. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Busch, H.; Hanefeld, U.; Tonin, F. Biocatalysis explained: From pharmaceutical to bulk chemical production. React. Chem. Eng. 2019, 4, 1878–1894. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Feng, N.; Jiang, W.; Jin, Y.; Li, X. Recent advances in the synthesis of biodegradable polyesters by sustainable polymerization: Lipase-catalyzed polymerization. RSC Adv. 2020, 10, 36230–36240. [Google Scholar] [CrossRef]
- Swainson, S.M.E.; Styliari, I.D.; Taresco, V.; Garnett, M.C. Poly (glycerol adipate) (PGA), an enzymatically synthesized functionalizable polyester and versatile drug delivery carrier: A literature update. Polymers 2019, 11, 1561. [Google Scholar] [CrossRef]
- Urbánek, T.; Jäger, E.; Jäger, A.; Hrubý, M. Selectively biodegradable polyesters: Nature-inspired construction materials for future biomedical applications. Polymers 2019, 11, 1061. [Google Scholar] [CrossRef]
- Ludwicka, K.; Jedrzejczak-Krzepkowska, M.; Kubiak, K.; Kolodziejczyk, M.; Pankiewicz, T.; Bielecki, S. Medical and cosmetic applications of bacterial nanocellulose. In Bacterial Nanocellulose: From Biotechnology to Bio-Economy; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–165. [Google Scholar]
- Cinelli, P.; Coltelli, M.B.; Signori, F.; Morganti, P.; Lazzeri, A. Cosmetic packaging to save the environment: Future perspectives. Cosmetics 2019, 6, 26. [Google Scholar] [CrossRef]
- Tripathi, P.; Sinha, S. Industrial biocatalysis: An insight into trends and future directions. Curr. Sustain. Renew. Energy Rep. 2020, 7, 66–72. [Google Scholar] [CrossRef]
- Marion, P.; Bernela, B.; Piccirilli, A.; Estrine, B.; Patouillard, N.; Guilbot, J.; Jérôme, F. Sustainable chemistry: How to produce better and more from less? Green Chem. 2017, 19, 4973–4989. [Google Scholar] [CrossRef]
- Pellis, A.; Cantone, S.; Ebert, C.; Gardossi, L. Evolving biocatalysis to meet bioeconomy challenges and opportunities. New Biotechnol. 2018, 40, 154–169. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef]
- Kobayashi, S. Lipase-catalyzed polyester synthesis—A green polymer chemistry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 338–365. [Google Scholar] [CrossRef]
- Kobayashi, S. Enzymatic polymerization. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, pp. 217–237. [Google Scholar]
- Kobayashi, S. Enzymatic ring-opening polymerization and polycondensation for the green synthesis of polyesters. Polym. Adv. Technol. 2015, 26, 677–686. [Google Scholar] [CrossRef]
- Kobayashi, S. Green polymer chemistry: New methods of polymer synthesis using renewable starting materials. Struct. Chem. 2017, 28, 461–474. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H. Synthesis of polyesters I: Hydrolase as catalyst for polycondensation (condensation polymerization). In Enzymatic Polymerization Towards Green Polymer Chemistry: Green Chemistry and Sustainable Technology; Kobayashi, S., Uyama, H., Kadokawa, J., Eds.; Springer: Singapore, 2019; pp. 105–163. [Google Scholar]
- Uyama, H.; Kobayashi, S. Synthesis of polyesters II: Hydrolase as catalyst for ring-opening polymerization. In Enzymatic Polymerization Towards Green Polymer Chemistry: Green Chemistry and Sustainable Technology; Kobayashi, S., Uyama, H., Kadokawa, J., Eds.; Springer: Singapore, 2019; pp. 165–197. [Google Scholar]
- Cheng, H.N.; Gross, R.A. Green polymer chemistry: Biocatalysis and biomaterials. In ACS Symposium Series; Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1043, pp. 1–14. [Google Scholar]
- Jiang, Y.; Loos, K. Enzymatic synthesis of biobased polyesters and polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Acero, E.H.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The closure of the cycle: Enzymatic synthesis and functionalization of bio-based polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef]
- Lu, Y.; Lv, Q.; Liu, B.; Liu, J. Immobilized Candida antarctica lipase B catalyzed synthesis of biodegradable polymers for biomedical applications. Biomater. Sci. 2019, 7, 4963–4983. [Google Scholar] [CrossRef]
- Pellis, A.; Silvestrini, L.; Scaini, D.; Coburn, J.M.; Gardossi, L.; Kaplan, D.L.; Acero, E.H.; Guebitz, G.M. Enzyme-catalyzed functionalization of poly(L-lactic acid) for drug delivery applications. Process Biochem. 2017, 59, 77–83. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pagaduan, J.N.M.; Melgar, Z.K.A.; Seitz, S.; Kan, K.; Ajiro, H. Glycerol-modified poly(ε-caprolactone): An biocatalytic approach to improve the hydrophilicity of poly(ε-caprolactone). Polym. Bull. 2019, 76, 1915–1928. [Google Scholar] [CrossRef]
- Kántor, I.; Aparaschivei, D.; Todea, A.; Biró, E.; Babos, G.; Szerényi, D.; Kakasi, B.; Péter, F.; Șișu, E.; Feczkó, T. Biocatalytic synthesis of poly[ε-caprolactone-co-(12-hydroxystearate)] copolymer for sorafenib nanoformulation useful in drug delivery. Cat. Today 2021, 366, 195–201. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freirea, C.S.R.; Silvestrea, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Taresco, V.; Creasey, R.G.; Kennon, J.; Mantovani, G.; Alexander, C.; Burley, J.C.; Garnett, M.C. Variation in structure and properties of poly(glycerol adipate) viacontrol of chain branching during enzymatic synthesis. Polymer 2016, 89, 41–49. [Google Scholar] [CrossRef]
- Martínez de Ilarduya, A.; Muñoz Guerra, S. Ring opening polymerization of macrocyclic oligoesters derived from renewable resources. Polym. Chem. 2020, 11, 4850–4860. [Google Scholar] [CrossRef]
- Varma, I.K.; Albertsson, A.C.; Rajkhowa, R.; Srivastava, R.K. Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 2005, 30, 949–981. [Google Scholar] [CrossRef]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.M.; Papaspyrides, C.D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Williams, C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Yang, J.; Ameer, G.A. Biodegradable polyester elastomers in tissue engineering. Expert Opin. Biol. Ther. 2004, 4, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Boland, E.D.; Coleman, B.D.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005, 1, 115–123. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable poly(ethylene succinate) (PES): 1. Crystal growth kinetics and morphology. Biomacromolecules 2000, 1, 704–712. [Google Scholar] [CrossRef]
- Natarajan, J.; Movva, S.; Madras, G.; Chatterjee, K. Biodegradable galactitol based crosslinked polyesters for controlled release and bone tissue engineering. Mater. Sci. Eng. C 2017, 77, 534–547. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Y.; Xu, W.; Li, L. Linear–dendritic block copolymer for drug and gene delivery. Mater. Sci. Eng. C 2016, 62, 943–959. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms of drug release in nanotherapeutic delivery systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.H.; Li, Z. Biodegradable polyester shape memory polymers: Recent advances in design, material properties and applications. Mater. Sci. Eng. C 2018, 92, 1061–1074. [Google Scholar] [CrossRef]
- Tomkea, P.D.; Zhaoa, X.; Chiplunkara, P.P.; Xua, B.; Wangc, H.; Silvaf, C.; Rathodb, V.K.; Cavaco-Pauloa, A. Lipase-ultrasound assisted synthesis of polyesters. Ultrason. Sonochem. 2017, 38, 496–502. [Google Scholar] [CrossRef][Green Version]
- Matsumura, S. Enzymatic synthesis of polyesters via ring-opening polymerization. Adv. Polym. Sci. 2006, 194, 95–132. [Google Scholar]
- Balaji, A.B.; Pakalapati, H.; Khalid, M.; Walvekar, R.; Siddiqui, H. Natural and synthetic biocompatible and biodegradable polymers. In Biodegradable and Biocompatible Polymer Composites: Processing, Properties and Applications; Shimpi, N.G., Ed.; Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–32. [Google Scholar]
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Khorasani, S.N.; Khalili, S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym. Adv. Technol. 2020, 31, 2939–2954. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021. [Google Scholar] [CrossRef]
- Gross, R.A.; Ganesh, M.; Lu, W. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Namekawa, S.; Uyama, H.; Kobayashi, S. Enzymatic synthesis of polyesters from lactones, dicarboxylic acid divinyl esters, and glycols through combination of ring-opening polymerization and polycondensation. Biomacromolecules 2000, 1, 335–338. [Google Scholar] [CrossRef]
- Tsui, A.; Wright, Z.C.; Frank, C.W. Biodegradable polyesters from renewable resources. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 143–170. [Google Scholar] [CrossRef]
- Isnard, F.; Mazzeo, M.; Thomas, C.M. Novel polyesters from renewable resources. L’Actualité Chim. 2018, 427–428, 50–53. [Google Scholar]
- Xu, Z.H.; Cheng, A.D.; Xing, X.P.; Zong, M.H.; Bai, Y.P.; Li, N. Improved synthesis of 2,5-bis(hydroxymethyl)furan from 5-hydroxymethylfurfural using acclimatized whole cells entrapped in calcium alginate. Bioresour. Technol. 2018, 262, 177–183. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef]
- Rebouillat, S.; Pla, F. Recent strategies for the development of biosourced-monomers, oligomers and polymers-based materials: A review with an innovation and a bigger data focus. J. Biomater. Nanobiotechnol. 2016, 7, 167–213. [Google Scholar] [CrossRef]
- Harmsen, P.F.H.; Hackmann, M.M.; Bos, H.L. Green building blocks for bio-based plastics. Biofuels Bioprod. Bioref. 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Gubitz, G.M.; Paulo, A.C. New substrates for reliable enzymes: Enzymatic modification of polymers. Curr. Opin. Biotechnol. 2003, 14, 577–582. [Google Scholar] [CrossRef]
- Tschan, M.J.L.; Brulé, E.; Haquettea, P.; Thomas, C.M. Synthesis of biodegradable polymers from renewable resources. Polym. Chem. 2012, 3, 836. [Google Scholar] [CrossRef]
- Caretto, A.; Noè, M.; Selva, M.; Perosa, A. Upgrading of biobased lactones with dialkylcarbonates. ACS Sustain. Chem. Eng. 2014, 2, 2131–2141. [Google Scholar] [CrossRef]
- Naira, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Z.; Yuan, D.; Sun, Y.; Li, Z.; He, C. Novel linear-dendritic-like amphiphilic copolymers: Synthesis and self-assembly characteristics. Polym. Chem. 2014, 5, 4069–4075. [Google Scholar] [CrossRef]
- Fan, X.; Li, Z.; Loh, X.J. Recent development of unimolecular micelles as functional materials and applications. Polym. Chem. 2016, 7, 5898–5919. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Gil, M.H.; Simões, P.N. Biodegradable poly(ester amide)s—A remarkable opportunity for the biomedical area: Review on the synthesis, characterization, and applications. Prog. Polym. Sci. 2014, 39, 1291–1311. [Google Scholar] [CrossRef]
- Asín, L.; Armelin, E.; Montané, J.; Rodríguez-Galan, A.; Puiggalí, J. Sequential poly(ester amide)s based on glycine, diols, and dicarboxylic acids: Thermal polyesterification versus interfacial polyamidation: Characterization of polymers containing stiff units. J. Polym. Sci. A Polym. Chem. 2001, 39, 4283–4293. [Google Scholar] [CrossRef]
- Rodriguez-Galan, A.; Franco, L.; Puiggali, J. Degradable Poly(ester amide)s for Biomedical Applications. Polymers 2011, 3, 65–99. [Google Scholar] [CrossRef]
- Zilinskas, G.J.; Soleimani, A.; Gillies, E.R. Poly(ester amide)-poly(ethylene oxide) graft copolymers: Towards micellar drug delivery vehicles. Int. J. Polym. Sci. 2012, 2012, 564348. [Google Scholar] [CrossRef][Green Version]
- Chisholm, M.H.; Galucci, J.; Krempner, C.; Wiggenhorn, C. Paper Comments on the ring-opening polymerization of morpholine-2,5-dione derivatives by various metal catalysts and characterization of the products formed in the reactions involving R2SnX2, where X = OPri and NMe2 and R = Bun, Ph and p-Me2NC6H4. Dalton Trans. 2006, 6, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.D.; Yuan, J.C.; Lei, Z.Q. High molecular weight biodegraded poly(lactic acid-glycolic acid-ε-caprolactam) copolymer: Direct polycondensation of lactic acid, glycolic acid and ε-caprolactam using Sn(II)-organic anhydride as catalysts. Polym. Adv. Technol. 2009, 20, 536–540. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Balligand, F.; Bormann, A.; Bennevault, V.; Guégan, P. Synthesis of new biobased linear poly(ester amide)s. Eur. Polym. J. 2019, 121, 109314. [Google Scholar] [CrossRef]
- Stavila, E.; Loos, K. Synthesis of polyamides and their copolymers via enzymatic polymerization. J. Renew. Mater. 2015, 3, 268–280. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Poly(ester amide)s: Recent insights into synthesis, stability, and biomedical applications. Polym. Chem. 2016, 7, 7039–7046. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, C.W.; Gong, M.S. Preparation of polyesteramides based on aliphatic amine-containing phenol derivatives via interfacial polymerization. Macromol. Res. 2003, 11, 328–333. [Google Scholar] [CrossRef]
- Jin, S.; Mungara, P.M.; Gonsalves, K.E. Synthesis of polyamides and polyureas containing leucine-tyrosine linkages. J. Polym. Sci. A Polym. Chem. 1997, 35, 499–507. [Google Scholar] [CrossRef]
- Lammens, T.M.; Franssen, M.C.R.; Scott, E.L.; Sanders, J.P.M. Availability of protein-derived amino acids as feedstock for the production of bio-based chemicals. Biomass Bioenergy 2012, 44, 168–181. [Google Scholar] [CrossRef]
- Yan, N.; Wang, Y. Catalyst: Is the amino acid a new frontier for biorefineries? Chem 2019, 5, 739–743. [Google Scholar] [CrossRef]
- Zhou, Y.; Shuke Wu, S.; Li, Z. Cascade biocatalysis for sustainable asymmetric synthesis: From biobased L-phenylalanine to high-value chiral chemicals. Angew. Chem. Int. Ed. 2016, 55, 1–5. [Google Scholar] [CrossRef]
- Michell, R.M.; Muller, A.J.; Castelletto, V.; Hamley, I.; Deshayes, G.; Dubois, P. Effect of sequence distribution on the morphology, crystallization, melting, and biodegradation of poly(ε-caprolactone-co-ε-caprolactam) copolymers. Macromolecules 2009, 42, 6671–6681. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.P.; Boutevin, B. Biobased amines: From synthesis to polymers; present and future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Li, Y.; Zhang, C.-J.; Zhang, Y.-Y.; Cao, X.-H.; Zhang, X.-H. Synthesis of high-molecular-weight poly(ε-caprolactone) via heterogeneous zinc-cobalt(III) double metal cyanide complex. Giant 2020, 3, 100030. [Google Scholar] [CrossRef]
- Poojari, Y.; Beemat, J.S.; Clarson, S.J. Enzymatic synthesis of poly(ε-caprolactone): Thermal properties, recovery, and reuse of lipase B from Candida antarctica immobilized on macroporous acrylic resin particles. Polym. Bull. 2013, 70, 1543–1552. [Google Scholar] [CrossRef]
- Düşkünkorur, H.Ö.; Pollet, E.; Phalip, V.; Güvenilir, Y.; Avérous, L. Lipase catalyzed synthesis of polycaprolactone and clay-based nanohybrids. Polymer 2014, 55, 1648–1655. [Google Scholar] [CrossRef]
- Zhao, H.; Toe, C. “Water-like” ammonium-based ionic liquids for lipase activation and enzymatic polymerization. Process Biochem. 2020, 98, 59–64. [Google Scholar] [CrossRef]
- Sandoval, G.; Rivera, I.; Barrera-Rivera, K.A.; Martínez-Richa, A. Biopolymer synthesis catalyzed by tailored lipases. Macromol. Symp. 2010, 289, 135–139. [Google Scholar] [CrossRef]
- Zhang, Z.; He, F.; Zhuo, R. Immobilized lipase on porous silica particles: Preparation and application for biodegradable polymer syntheses in ionic liquid at higher temperature. J. Mol. Catal. B Enzym. 2013, 94, 129–135. [Google Scholar] [CrossRef]
- Li, G.; Li, Q. Thermophilic esterase from the archaeon Archaeoglobus fulgidus physically immobilized on hydrophobic macroporous resin: A novel biocatalyst for polyester synthesis. Biotechnol. Bioproc. Eng. 2011, 16, 1201–1207. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Marcos-Fernández, Á.; Vera-Graziano, R.; Martínez-Richa, A. Enzymatic ring-opening polymerization of ε-caprolactone by Yarrowia lipolytica lipase in ionic liquids. J. Polym. Sci. A Polym. Chem. 2009, 47, 5792–5805. [Google Scholar] [CrossRef]
- Hou, J.; Guo, S. Lipase-catalyzed synthesis and properties of thiol end-functionalized polycaprolactone and poly(ethylene glycol)-b-polycaprolactone. Acta Polym. Sin. 2009, 9, 796–802. [Google Scholar] [CrossRef]
- Hans, M.; Gasteier, P.; Keul, H.; Moeller, M. Ring-opening polymerization of ε-caprolactone by means of mono- and multifunctional initiators: Comparison of chemical and enzymatic catalysis. Macromolecules 2006, 39, 3184–3193. [Google Scholar] [CrossRef]
- Hedfors, C.; Östmark, E.; Malmström, E.; Hult, K.; Martinelle, M. Thiol end-functionalization of poly(ε-caprolactone), catalyzed by Candida antarctica lipase B. Macromolecules 2005, 38, 647–649. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Heise, A.; Lang, M. Organometallic and enzymatic catalysis for ring opening copolymerization of ε-caprolactone and 4-methyl-ε-caprolactone. J. Polym. Sci. A Polym. Chem. 2011, 49, 5293–5300. [Google Scholar] [CrossRef]
- Kumar, A.; Kalra, B.; Dekhterman, A.; Gross, R.A. Efficient ring-opening polymerization and copolymerization of ε-caprolactone and ω-pentadecalactone catalyzed by Candida antarctica lipase B. Macromolecules 2000, 33, 6303–6309. [Google Scholar] [CrossRef]
- Dreavă, D.M.; Benea, I.C.; Bîtcan, I.; Todea, A.; Șișu, E.; Puiu, M.; Peter, F. Biocatalytic approach for novel functional oligoesters of caprolactone and malic acid. Processes 2021, 9, 232. [Google Scholar] [CrossRef]
- Todea, A.; Bîtcan, I.; Aparaschivei, D.; Păușescu, I.; Badea, V.; Péter, F.; Gherman, V.D.; Rusu, G.; Nagy, L.; Kéki, S. Biodegradable oligoesters of ε-caprolactone and 5-hydroxymethyl-2-furancarboxylic acid synthesized by immobilized lipases. Polymers 2019, 11, 1402. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Martínez-Richa, A. Syntheses and characterization of aliphatic polyesters via Yarrowia lipolytica lipase biocatalysis. In Green Polymer Chemistry: Biocatalysis and Materials II; Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; American Chemical Society: Washington, DC, USA, 2015; Volume 1144, pp. 59–68. [Google Scholar]
- Todea, A.; Aparaschivei, D.; Bîtcan, I.; Ledeți, I.V.; Bandur, G.; Péter, F.; Nagy, L.; Kéki, S.; Biró, E. Thermal behavior of oligo[(ε-caprolactone)-co-δ-gluconolactone] enzymatically synthesized in reaction conditions optimized by experimental design. J. Therm. Anal. Calorim. 2020, 141, 1017–1026. [Google Scholar] [CrossRef]
- Todea, A.; Aparaschivei, D.; Badea, V.; Boeriu, C.G.; Peter, P. Biocatalytic route for the synthesis of oligoesters of hydroxy-fatty acids and ε-caprolactone. Biotechnol. J. 2018, 13, 1700629. [Google Scholar] [CrossRef]
- Aparaschivei, D.; Todea, A.; Păuşescu, I.; Badea, V.; Medeleanu, M.; Şişu, E.; Puiu, M.; Chiriţă-Emandi, A.; Francisc Peter, F. Synthesis, characterization and enzymatic degradation of copolymers of ε-caprolactone and hydroxy-fatty acids. Pure Appl. Chem. 2016, 88, 1191–1201. [Google Scholar] [CrossRef]
- Todea, A.; Biro, E.; Badea, V.; Paul, C.; Cimporescu, A.; Nagy, L.; Kéki, K.; Bandur, G.; Boeriu, C.; Péter, F. Optimization of enzymatic ring-opening copolymerizations involving δ-gluconolactone as monomer by experimental design. Catal. Today 2014, 86, 1781–1792. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-catalyzed ring-opening polymerization of cyclic esters in the presence of poly(ethylene glycol). J. Appl. Polym. Sci. 2012, 125, 3602–3609. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Klement, T.; Büchs, J. Itaconic acid—A biotechnological process in change. Bioresour. Technol. 2013, 135, 422–431. [Google Scholar] [CrossRef]
- Kane, J.H.; Finlay, A.C.; Amann, P.F. Production of Itaconic Acid, Chas. Pfizer & Co. U.S. Patent 2385283A, 18 September 1945. [Google Scholar]
- Kuenz, A.; Gallenmüller, Y.; Willke, T.; Vorlop, K.D. Microbial production of itaconic acid: Developing a stable platform for high product concentrations. Appl. Microbiol. Biotechnol. 2012, 96, 1209–1216. [Google Scholar] [CrossRef]
- Guevarra, E.D.; Tabuchi, T. Accumulation of itaconic, 2-hydroxyparaconic, itatartaric, and malic acids by strains of the genus Ustilago. Agric. Biol. Chem. 1990, 54, 2353–2358. [Google Scholar] [CrossRef][Green Version]
- Tabuchi, T.; Sugisawa, T.; Ishidori, T.; Nakahara, T.; Sugiyama, J. Itaconic acid fermentation by a yeast belonging to the genus Candida. Agric. Biol. Chem. 1981, 45, 475–479. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzym. Microb. Technol. 2006, 39, 824–827. [Google Scholar] [CrossRef]
- Strelko, C.L.; Lu, W.; Dufort, F.J.; Seyfried, T.N.; Chiles, T.C.; Rabinowitz, J.D.; Roberts, M.F. Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 2011, 133, 16386–16389. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M. Mesoporous silica nanoparticles with bilayer coating of poly(acrylic acid-co-itaconic acid) and human serum albumin (HSA): A pH-sensitive carrier for gemcitabine delivery. Mater. Sci. Eng. C 2016, 61, 782–790. [Google Scholar] [CrossRef]
- Milašinović, N.; Knežević-Jugović, Z.; Milosavljević, N.; Filipović, J.; Kalagasidis Krušić, M. Controlled release of lipase from Candida rugosa loaded into hydrogels of N-isopropylacrylamide and itaconic acid. Int. J. Pharm. 2012, 436, 332–340. [Google Scholar] [CrossRef]
- Gonçalves, F.A.M.M.; Fonseca, A.C.; Domingos, M.; Gloria, A.; Serra, A.C.; Coelho, J.F.J. The potential of unsaturated polyesters in biomedicine and tissue engineering: Synthesis, structure-properties relationships and additive manufacturing. Prog. Polym. Sci. 2017, 68, 1–34. [Google Scholar] [CrossRef]
- El-Hamshary, H. Synthesis and water sorption studies of pH sensitive poly(acrylamide-co-itaconic acid) hydrogels. Eur. Polym. J. 2007, 43, 4830–4838. [Google Scholar] [CrossRef]
- Barrett, D.G.; Merkel, T.J.; Luft, J.C.; Yousaf, M.N. One-step syntheses of photocurable polyesters based on a renewable resource. Macromolecules 2010, 43, 9660–9667. [Google Scholar] [CrossRef]
- Guo, B.; Chen, Y.; Lei, Y.; Zhang, L.; Zhou, W.Y.; Rabie, A.B.M.; Zhao, J. Biobased poly(propylene sebacate) as shape memory polymer with tunable switching temperature for potential biomedical applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Liu, X.; Han, L.; Wu, Y.; Dai, X.; Zhu, J. Synthesis of bio-based unsaturated polyester resins and their application in waterborne UV-curable coatings. Prog. Org. Coat. 2015, 78, 49–54. [Google Scholar] [CrossRef]
- Guarneri, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vassallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of enzymatically synthesized rigid poly(itaconate)s via post-polymerization Aza-Michael addition of primary amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar]
- Pellis, A.; Corici, L.; Sinigoi, L.; D’Amelio, N.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Towards feasible and scalable solvent-free enzymatic polycondensations: Integrating robust biocatalysts with thin film reactions. Green Chem. 2015, 17, 1756–1766. [Google Scholar] [CrossRef]
- Corici, L.; Pellis, A.; Ferrario, V.; Ebert, C.; Cantone, S.; Gardossi, L. Understanding potentials and restrictions of solvent-free enzymatic polycondensation of itaconic acid: An experimental and computational analysis. Adv. Synth. Catal. 2015, 357, 1763–1774. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda Van Ekenstein, G.O.R.; Loos, K. Enzyme-catalyzed synthesis of unsaturated aliphatic polyesters based on green monomers from renewable resources. Biomolecules 2013, 3, 461–480. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. Environmentally benign synthesis of saturated and unsaturated aliphatic polyesters via enzymatic polymerization of biobased monomers derived from renewable resources. Polym. Chem. 2015, 6, 5451–5463. [Google Scholar] [CrossRef]
- Aparaschivei, D.; Todea, A.; Frissen, A.E.; Badea, V.; Rusu, G.; Sisu, E.; Puiu, M.; Boeriu, C.G.; Peter, F. Enzymatic synthesis and characterization of novel terpolymers from renewable sources. Pure Appl. Chem. 2019, 91, 397–408. [Google Scholar] [CrossRef]
- Brännström, S.; Finnveden, M.; Johansson, M.; Martinelle, M.; Malmström, E. Itaconate based polyesters: Selectivity and performance of esterification catalysts. Eur. Polym. J. 2018, 103, 370–377. [Google Scholar] [CrossRef]
- Naves, A.F.; Fernandes, H.T.C.; Immich, A.P.S.; Catalani, L.H. Enzymatic syntheses of unsaturated polyesters based on isosorbide and isomannide. J. Polym. Sci. A Polym. Chem. 2013, 51, 3881–3891. [Google Scholar] [CrossRef]
- Jiang, Y.; Alberda van Ekenstein, G.O.R.; Woortman, A.J.J.; Loos, K. Fully biobased unsaturated aliphatic polyesters from renewable resources: Enzymatic synthesis, characterization, and properties. Macromol. Chem. Phys. 2014, 215, 2185–2197. [Google Scholar] [CrossRef]
- Pellis, A.; Hanson, P.A.; Comerford, J.W.; Clark, J.H.; Farmer, T.J. Enzymatic synthesis of unsaturated polyesters: Functionalization and reversibility of the aza-Michael addition of pendants. Polym. Chem. 2019, 10, 843–851. [Google Scholar] [CrossRef]
- Müller, F.; Torger, B.; Allertz, P.J.; Jähnichen, K.; Keßler, S.; Müller, M.; Simon, F.; Salchert, K.; Mäurer, H.; Pospiech, D. Multifunctional crosslinkable itaconic acid copolymers for enzyme immobilization. Eur. Polym. J. 2018, 102, 47–55. [Google Scholar] [CrossRef]
- Hoffmann, C.; Stuparu, M.C.; Daugaard, A.; Khan, A. Aza-Michael addition reaction: Post-polymerization modification and preparation of PEI/PEG-based polyester hydrogels from enzymatically synthesized reactive polymers. J. Polym. Sci. Part A Polym. Chem. 2014, 53, 745–749. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Tanha, M.; Hult, A.; Okuda, T.; Ohara, H.; Kobayashi, S. Green polymer chemistry: Lipase-catalyzed synthesis of bio-based reactive polyesters employing itaconic anhydride as a renewable monomer. Polym. J. 2014, 46, 2–13. [Google Scholar] [CrossRef]
- Isbell, T.A. Chemistry and physical properties of estolides. Grasas y Aceites 2011, 62, 8–20. [Google Scholar] [CrossRef]
- Hayes, D.G. The catalytic activity of lipases toward hydroxy fatty acid—A review. J. Am. Oil Chem. Soc. 1996, 73, 543–549. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A. Synthesis of estolides from oleic and saturated fatty acids. J. Am. Oil Chem. Soc. 2001, 78, 557–565. [Google Scholar] [CrossRef]
- Isbell, T.A.; Edgcomb, M.R.; Lowery, B.A. Physical properties of estolides and their ester derivatives. Ind. Crops Prod. 2001, 13, 11–20. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, D.; Liu, C.; Zhao, Z.; Yang, Y.; Li, Q. Lipase/esterase-catalyzed synthesis of aliphatic polyesters via polycondensation: A review. Process Biochem. 2012, 47, 1027–1036. [Google Scholar] [CrossRef]
- Bodalo, A.; Bastida, J.; Máximo, M.F.; Montiel, M.C.; Gómez, M.; Murcia, M.D. Production of ricinoleic acid estolide with free and immobilized lipase from Candida rugosa. Biochem. Eng. J. 2008, 39, 450–456. [Google Scholar] [CrossRef]
- Horchani, H.; Bouaziz, A.; Gargouri, Y.; Sayari, A. Immobilized Staphylococcus xylosus lipase-catalysed synthesis of ricinoleic acid esters. J. Mol. Catal. B Enzym. 2012, 75, 35–42. [Google Scholar] [CrossRef]
- Todea, A.; Otten, L.G.; Frissen, A.E.; Arends, I.W.C.E.; Peter, F.; Boeriu, C.G. Selectivity of lipases for estolides synthesis. Pure Appl. Chem 2015, 87, 51–58. [Google Scholar] [CrossRef]
- Gandini, A. Progress of polymers from renewable resources: Furans, vegetable oils, and polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furanaromatic polyesters synthesized via direct esterification method based on renewable resources. J. Appl. Polym. Sci. A Polym. Chem. 2011, 50, 1026–1036. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources: Thermal behavior comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef]
- Jianhui, Z.; Jiali, C.; Wenchun, X.; Pin-Hsuan, C.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(butylene 2,5-furandicarboxylate), a biobased alternative to PBT: Synthesis, physical properties, and crystal structure. Macromolecules 2013, 46, 796–804. [Google Scholar]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Papageorgiou, M.; Bikinaris, D.N. Evaluation of polyesters from renewable resources as alternatives to the current fossil-based polymers. Phase transitions of poly(butylene 2,5-furan-dicarboxylate). Polymer 2014, 55, 3846–3858. [Google Scholar] [CrossRef]
- Ma, J.; Yu, X.; Xu, J.; Pang, Y. Synthesis and crystallinity of poly(butylene 2,5-furandicarboxylate). Polymer 2012, 53, 4145–4151. [Google Scholar] [CrossRef]
- Ma, J.; Pang, Y.; Wang, M.; Xu, J.; Ma, H.; Nie, X. The copolymerization reactivity of diols with 2,5-furandicarboxylic acid for furan-based copolyester materials. J. Mater. Chem. 2012, 22, 3457–3461. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the bio-based polyester poly(propylene 2,5-furan dicarboxylate). Comparison of thermal behavior and solid state structure with its terephthalate and naphthalate homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrysafis, K.; Bikinaris, D. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.; Ji, J. Synthesis, physical properties and enzymatic degradation of bio-based poly(butylene adipate-co-butylene furandicarboxylate) copolyesters. Polym. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Appl. Polym. Sci. A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly (ethylene terephthalate): Part 1: Equilibrium sorption. Polymer 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate): Part 2: Kinetic sorption. Polymer 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Petrovic, D.M.; Loos, K. Enzymatic synthesis of biobased polyesters using 2,5-bis(hydroxymethyl)furan as the building block. Biomacromolecules 2014, 15, 2482–2493. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.J. Enzymatic polymerization of furan-2,5-dicarboxylic acid-based furanic-aliphatic polyamides as sustainable alternatives to polyphthalamides. Biomacromolecules 2015, 16, 3674–3685. [Google Scholar] [CrossRef]
- Jiang, Y.; Alberda van Ekenstein, G.O.R.; Woortman, A.J.J.; Loos, K. A biocatalytic approach towards sustainable furanic–aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Cruz-Izquierdo, A.; van den Broek, L.A.M.; Serra, J.L.; Llama, M.J.; Boeriu, C.G. Lipase-catalyzed synthesis of oligoesters of 2,5-furandicarboxylic acid with aliphatic diols. Pure Appl. Chem. 2015, 87, 59–69. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Ciulik, C.B.; de Ilarduya, A.M.; Muñoz-Guerra, S. Fully bio-based aromatic–aliphatic copolyesters: poly(butylene furandicarboxylate-co-succinate)s obtained by ring opening polymerization. Polym. Chem. 2017, 8, 748–760. [Google Scholar] [CrossRef]
- Maniar, D.; Hohmann, K.F.; Woortman, A.J.J.; van Dijken, J.; Loos, K. Enzymatic polymerization of dimethyl 2,5-furandicarboxylate and heteroatom diamines. ACS Omega 2018, 3, 7077–7085. [Google Scholar] [CrossRef]
- Maniar, D.; Jiang, Y.; Woortman, A.; van Dijken, J.; Loos, K. Furan-based copolyesters from renewable resources: Enzymatic synthesis and properties. ChemSusChem 2019, 12, 990–999. [Google Scholar] [CrossRef]
- Pellis, A.; Comerford, J.W.; Weinberger, S.; Guebitz, G.M.; Clark, J.H.; Farmer, T.J. Enzymatic synthesis of lignin derivable pyridine based polyesters for the substitution of petroleum derived plastics. Nat. Commun. 2019, 10, 1762. [Google Scholar] [CrossRef]
- Skoczinski, P.; Espinoza Cangahuala, M.K.; Maniar, D.; Albach, R.W.; Bittner, N.; Loos, K. Biocatalytic synthesis of furan-based oligomer diols with enhanced end-group fidelity. ACS Sustain. Chem. Eng. 2020, 8, 1068–1086. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.; Knoop, R.J.I.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957–1966. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A facile method to synthesize high molecular weight biobased polyesters from 2,5-furandicarboxylic acid and long chain diols. J. Polym. Sci. Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martınez de Ilarduya, A.; Muñoz-Guerra, S. Blocky poly(ε-caprolactone-co-butylene 2,5-furandicarboxylate) copolyesters via enzymatic ring opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 290–299. [Google Scholar] [CrossRef]
- Sharma, B.; Azim, A.; Azim, H.; Gross, R.A.; Zini, E.; Focarete, M.L.; Scandola, M. Enzymatic synthesis and solid-state properties of aliphatic polyesteramides with polydimethylsiloxane blocks. Macromolecules 2007, 40, 7919–7927. [Google Scholar] [CrossRef]
- Palsule, A.S.; Poojari, Y. Enzymatic synthesis of silicone fluorinated aliphatic polyesteramides. Polymer 2010, 51, 6161–6167. [Google Scholar] [CrossRef]
- Ragupathy, L.; Ziener, U.; Dyllick-Brenzinger, R.; von Vacano, B.; Landfester, K. Enzyme-catalyzed polymerizations at higher temperatures: Synthetic methods to produce polyamides and new poly(amide-co-ester)s. J. Mol. Catal. B Enzym. 2012, 76, 94–105. [Google Scholar] [CrossRef]
- Stavila, E.; Alberda van Ekenstein, G.O.R.; Woortman, A.J.J.; Loos, K. Lipase-catalyzed ring-opening copolymerization of ε-caprolactone and β-lactam. Biomacromolecules 2014, 15, 234–241. [Google Scholar] [CrossRef]
- Duchiron, S.W.; Pollet, E.; Givry, S.; Avérous, L. Enzymatic synthesis of amino acids endcapped polycaprolactone: A green route towards functional polyesters. Molecules 2018, 23, 290. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Y.; Hu, Y.; Xia, B.; Lin, X.; Wu, Q. Lipase-catalyzed synthesis of chiral poly(ester amide)s with an alternating sequence of hydroxy acid and L/D-aspartate units. Polym. Chem. 2018, 9, 1412–1420. [Google Scholar] [CrossRef]
- Gross, R.A.; Kumar, A.; Kalra, B. Polymer synthesis by in vitro enzyme catalysis. Chem. Rev. 2001, 101, 2097–2124. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Moore, O.B.; Hanson, P.-A.; Comerford, J.W.; Pellis, A.; Farmer, T.J. Improving the post-polymerization modification of bio-based itaconate unsaturated polyesters: Catalyzing aza-michael additions with reusable iodine on acidic alumina. Front. Chem. 2019, 7, 501. [Google Scholar] [CrossRef]
- Chen, Y.; Biresaw, G.; Cermak, S.C.; Isbell, T.A.; Ngo, H.L.; Chen, L.; Durham, A.L. Fatty acid estolides: A review. J. Am. Oil Chem. Soc. 2020, 97, 231–241. [Google Scholar] [CrossRef]
- Boeriu, G.G.; Todea, A.; Arends, I.W.C.E.; Otten, L.G. Production of Fatty Acid Estolides. U.S. Patent 10920252B2, 16 February 2015. [Google Scholar]
- Toncheva-Moncheva, N.; Jerome, R.; Mateva, R. Impact of the structure of poly(ε-caprolactam) containing polyesteramides on mechanical properties and biodegradation. Polym. Degrad. Stab. 2016, 123, 170–177. [Google Scholar] [CrossRef]
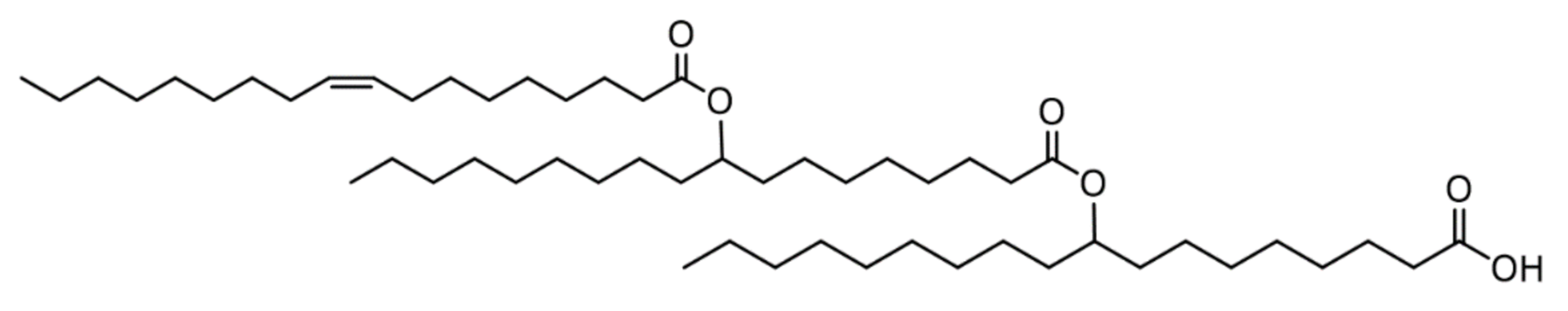
| Source of Lipase | Immobilization Support | Reaction Conditions | Mw/Mn [Da] | Reference |
|---|---|---|---|---|
| C. antarctica B | Acrylic resin Novozyme 435 | toluene, 70 °C, 4 h, 10 reaction cycles | Mn: 50,000 | [90] |
| Montmorillonite and sepiolite clay | toluene, 70 °C, 5–6 h, dry N2 atm | Mn: 3300–8000 | [91] | |
| Acrylic resin Novozyme 435 | Tf2N-based ionic liquids, 70 °C, 48 h | Mn: 12,300–18,000 | [92] | |
| Y. lipolytica | Lewatit Acurel | bulk, 150 °C, 6 h | Mn: 600–1300 | [93] |
| Porcine pancreas | porous silica particles | BMIMPF6, 150 °C, 24 h | Mn: 13,500–17,000 | [94] |
| Archaeoglobus fulgidus | Macroporous hydrophobic resin | 45–80 °C, 72 h | Mn: 850–1400 | [95] |
| Y. lipolytica | [BF4]-based ionic liquids/ [BuPy][CF3COO] [EMIM][NO3] 60–150 °C, 24 h, dry N2 atm | Mn: 1000–8000 | [96] |
| Co-Monomer(s) | Source of Lipase | Immobilization Support | Reaction Conditions | Mw/Mn [Da] | Reference |
|---|---|---|---|---|---|
| glycerol | C. antarcticaB | Acrylic resin (Novozyme 435) | Toluene, 70 °C, 2–8 h, N2 atm | Mn: 1500–3100 Mw: 2400–4300 | [36] |
| 2-mercaptoethanol methoxy poly(ethylene glycol) | C. antarctica B | Acrylic resin (Novozyme 435) | Solvent-less /toluene, 70 ℃, 20 h | Mn: 4600–21,300 | [97] |
| poly(ethylene glycol)methyl ether | C. antarcticaB | Acrylic resin (Novozyme 435) | THF, 70–130 °C, 25–70 h, N2 atm | Mn: 2000–7700 | [98] |
| 2-mercaptoethanol | C. antarctica B | Acrylic resin (Novozyme 435) | Solvent-less, MTBE, 24–72 h | Mn: 2000–6900 | [99] |
| 4-methyl-ε-caprolactone | C. antarctica B | Acrylic resin (Novozyme 435) | Solvent-less, 60 °C, 12 h, Ar atm | Mn: 21,000–23,000 | [100] |
| ω-pentadecalactone | C. antarctica B | Acrylic resin (Novozyme 435) | Solvent-less/toluene, 55–90 °C, 2–24 h | Mn: 22,000–86,000 | [101] |
| malic acid | C. antarctica B | Acrylic resin Novozyme 435 | Toluene/Me-THF/Acetonitrile/solvent-less 70–80 °C, time 2–48 h | Mw: 800–1500 | [102] |
| 5-hydroxymethyl-2-furoic acid | C. antarctica B | Lipozyme CalB | Solvent-less, 40–80 °C, 24 h | Mw: 745–1347/ Mn: 695–1180 | [103] |
| C. antarctica B | GF-CalB-IM | Solvent-less, 40–80 °C, 24 h | Mw: 1085–1404/ Mn: 960–1128 | [103] | |
| C. antarctica B | Acrylic resin Novozyme 435 | Solvent-less, 40–80 °C, 24 h | Mw: 1058–1481/ Mn: 931–1280 | [103] | |
| diethylene glycol, 1,3-propanediol | Y. lipolytica | Lewatit VPOC 1026 | Solvent-less, 120 °C, 6 h | Mn: 300–9000 | [104] |
| δ-gluconolactone | T. lanuginosus | Non-compressible silica gel carrier Lipozyme TL-IM | Solvent-less, 73 °C, 24 h | Mw: 860–893/ Mn: 824–855 | [105] |
| 10HSA | C. antarctica B | Acrylic resin Novozyme 435 | Toluene, 45–85 °C, 24 h | Mw: 1284/ Mn: 1215 | [106] |
| Ps. stutzeri | CLEA | Toluene 45–85 °C, 24 h | Mw: 702/ Mn: 663 | [106] | |
| T. lanuginosus | Silica gel carrier Lipozyme TL-IM | Toluene, 45–85 °C, 24 h | Mw: 1146/ Mn: 1052 | [106] | |
| 12HSA | C. antarctica B | Acrylic resin Novozyme 435 | Toluene, 45–85 °C, 24 h | Mw: 1399/ Mn: 1169 | [106] |
| Ps. stutzeri | CLEA | Toluene, 45–85 °C, 24 h | Mw: 906/ Mn: 806 | [106] | |
| T. lanuginosus | Non-compressible silica gel carrier Lipozyme TL-IM | Toluene, 45–85 °C, 24 h | Mw: 1002/ Mn: 913 | [106] | |
| C. antarctica B | Acrylic resin Novozyme 435 | Toluene, 90–115 °C, 24 h | Mw: 1069–2375/ Mn: 985–2000 | [107] | |
| C. antarctica B | Microporous ion exchange resin GF-CalB-IM | Toluene, 50 °C, 24 h | Mw: 692/ Mn: 689 | [108] | |
| RCA | Ps. stutzeri | CLEA | Toluene, 45–85 °C, 24 h | Mw: 1086/ Mn: 997 | [106] |
| T. lanuginosus | Non-compressible silica gel carrier Lipozyme TL-IM | Toluene, 45–85 °C, 24 h | Mw: 1009/ Mn: 917 | [106] | |
| C. antarctica B | Acrylic resin Novozyme 435 | Toluene, 45–85 °C, 24 h | Mw: 1291/ Mn: 1145 | [106] | |
| 16HHDA | C. antarctica B | Acrylic resin Novozyme 435 | Toluene, 90–115 °C, 400 rpm, 24 h | Mw: 823–1729/ Mn: 742–1334 | [106] |
| Ps. stutzeri | CLEA | Toluene, 45–85 °C, 24 h | Mw: 825/ Mn: 724 | [106] | |
| T. lanuginosus | Non-compressible silica gel carrier Lipozyme TL-IM | Toluene, 45–85 °C, 24 h | Mw: 1232/ Mn: 1101 | [106] | |
| C. antarctica B | Acrylic resin Novozyme 435 | Toluene, 45–85 °C, 24 h | Mw: 1479/ Mn: 1321 | [106] | |
| 10HSA | Ps. fluorescens | - | Toluene, 45–85 °C, 24 h | Mw: 793/ Mn: 727 | [106] |
| 12HSA | Mw: 887/ Mn: 855 | ||||
| RCA | Mw: 1426/ Mn: 1304 | ||||
| 16HHDA | Mw: 1294/ Mn: 1177 | ||||
| glicolide PEG | Ps. cepacia | - | Solvent-less, 80 °C, 14 days, Ar atm | Mn: 2100–3400 | [109] |
| Microorganism | Concentration [g L−1] | Reference |
|---|---|---|
| Aspergillus terreus (initial step) | 24–27 | [113] |
| Aspergillus terreus (optimized steps) | ~86 | [114] |
| Ustilago maydis | 53 | [115] |
| Candida sp. | 35 | [116] |
| Pseudozyma antarctica | 30 | [117] |
| Macrophage | non-specified | [118] |
| Itaconic Monomer | Co-Monomer(s) | Reaction Conditions | Mw/Mn [Da] | Reference |
|---|---|---|---|---|
| DMI | 1,4-cyclohexanedimethanol (PEG; 400 Da) 3-methyl-1,5-pentanediol | 90 °C, inert atm, Low pressure, 48 h | Mn: 2000–11,900 | [123] |
| DMI | 1,4-BDO/1,4-Cyclohexanedimethanol | Solvent-less, 50 °C, 72 h, 70 mbar | Mw: 720–2859 | [126] |
| DMI | 1,4-BDO | Solvent-less, 50 °C, 70 mbar, thin film | n.d. | [127] |
| DMI | 1,4-BDO CHDM | Solvent-less, 50 °C, 70 mbar, thin film | n.d. | [128] |
| IA/DMI/DIE/DBI | Diethyl succinate+1,4-butandiol | Diphenyl ether, 80 °C, 2–94 h | Mn: 730–1403 | [129] |
| DMI | 1,4-butanediol and diethylsuccinate/diethylglutarate/diethyladipate/diethylsebacate | Diphenyl ether I. 80 °C, 2 h, N2 atm II. 80 °C, 2 mmHg, 94 h, N2 atm | Mw: up to 94,000 | [130] |
| DMI | 1,6-HDO+DMFDC | Toluene, 80 °C, 4–72 h | Mn: 836–1205 | [131] |
| DMI | dimethyl succinate +1,4-butanediol | 60 °C, 5h N2-atm 6 h 200 mmbar | Mn: 840 Mw: 1500 | [132] |
| DIE | Isosorbide/Isomannide/Isoidide 1,4-BDO 1,6-HDO | Cyclohexane:toluene 6:1, molecular sieves, magnetically, 90 °C, 168 h/48 h | Mw: 350–27,000 | [133] |
| DMI | Diethyl succinate+1,4-butandiol | Diphenyl ether/toluene/cyclohexane/80 °C, 96 h | Mn: 500–1400, 22,000 | [134] |
| DMI/DMF/DMM/DBI/DBF | 1,4-BDO 1,6-HDO 1,8-ODO | Solvent-less, 85 °C 6 h, 1000 mbar + 20 mbar, 18 h | Mn: up to 3000 | [135] |
| Monomer with Furan Ring | Co-Monomer(s) | Reaction Conditions | Mw/Mn [Da] | Reference |
|---|---|---|---|---|
| 2,5-bis(hydroxymethyl)furan | DES/DEG/DEA/DES | Diphenyl eter 80 °C, 2 h, atm 80 °C, 4 h, 350 mHg 80 °C, 66 h, 2 mHg | Mn: 2100–3000 | [164] |
| DMFDCA | 1,8-ODA | I. Toluene, 60–100 °C for 72 h II. diphenyl ether: (4 steps) different pressures 80 °C, 8 h, atm pressure, N2 atm 80 °C, 16 h, 450 mmHg, N2 atm 90 °C, 36 h, 100 mmHg, N2 atm 140 °C, 12 h, 100 mmHg, N2 atm | I. Mn: 7600–13400 Mw: 11,800–48,300 II. Mn: 4000–11100 Mw: 10,500–54,000 | [165] |
| DMFDCA | 1,3-PDO/1,4-BDO/1,6-HDO/1,8-ODO/1,10-DDO/D-sorbitol/glycerol/ isosorbide | Diphenyl ether, A. I. 80 °C, 2 h, atm, + II. 80 °C, 2 mmHg 72 h. B. I. 80 °C, 2 h atm, + II. 80 °C, 2 mmHg 24 h + III. 95 °C, 2 mmHg 24 h +IV. 95/120/140 °C, 2 mmHg 24 h | Mn: 200–23,700 Mw: 800–48,700 | [166] |
| DMFDCA | C2-C12 diols | Toluene:t-butanol = 70:30% wt., 40 °C, 24 h | n.d. | [167] |
| cyclic butylene 2,5-furandicarboxylate | ε-caprolactone | Solvent-less, 130–150 °C, 24 h, N2 atm | Mw: 22,000–50,000 | [168] |
| DMFDCA | DODA/DETA/EDDA | Solvent-less/Toluene, 90 °C 72 h. | Mn: 6360, 8030 | [169] |
| DMFDCA+ BHMF | aliphatic linear diols/diacid ethyl esters | Diphenyl ether, I. 80 °C, 2 h, N2 atm, + II. 80 °C, 2 mmHg 48 h, + III. 95 °C, full vacuum, 24 h | Mw: up to 35,000 | [170] |
| DET/DEF/DEF | BDO/HDO/ODO | DPE/Bulk-85 °C, 6 h, 1000 mmbar + 90 h, 20 mmbar | Mn: 1500–2000 | [171] |
| DMFDCA | 1,4-BDO/1,4-cyclohexanedimethanol | diphenyl ether/solvent-less 80 to 140 °C for 2 h + 80 to 140 °C, 2 mmHg, 24 to 72 h. | Mn: 695–2648 Mw: 431–8173 | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todea, A.; Dreavă, D.M.; Benea, I.C.; Bîtcan, I.; Peter, F.; Boeriu, C.G. Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases. Processes 2021, 9, 646. https://doi.org/10.3390/pr9040646
Todea A, Dreavă DM, Benea IC, Bîtcan I, Peter F, Boeriu CG. Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases. Processes. 2021; 9(4):646. https://doi.org/10.3390/pr9040646
Chicago/Turabian StyleTodea, Anamaria, Diana Maria Dreavă, Ioana Cristina Benea, Ioan Bîtcan, Francisc Peter, and Carmen G. Boeriu. 2021. "Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases" Processes 9, no. 4: 646. https://doi.org/10.3390/pr9040646
APA StyleTodea, A., Dreavă, D. M., Benea, I. C., Bîtcan, I., Peter, F., & Boeriu, C. G. (2021). Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases. Processes, 9(4), 646. https://doi.org/10.3390/pr9040646








