A Comprehensive Review of HCN-Derived Polymers
Abstract
1. Introduction
2. HCN in Prebiotic Chemistry and Astrobiology
3. Promising Applications in Materials Science
4. Characterization and Kinetic Analyses of HCN-Derived Polymers: Dependence of the Properties on Synthetic Conditions
4.1. Molecular Weight
4.2. Chemical Composition
4.3. Morphology
4.4. Thermal Stability and Fourier Transform Infrared (FT-IR) Spectroscopy
4.5. X-ray Photoelectron Spectroscopy (XPS)
4.6. Nuclear Magnetic Resonance (NMR) and Electron Spin Resonance (ESR)
4.7. X-ray Diffraction (XRD)
4.8. UV-Vis Spectroscopy
4.9. Kinetic Analyses Based on Gravimetric Methods: Aqueous Polymerizations
4.10. Kinetic Analyses Based on DSC Methods: Bulk Polymerizations
5. Structural and Mechanistic Considerations and Approaches
5.1. Ab Initio Studies to Explain the HCN Oligomerization
5.2. Structural Models Based on Spectrometric and Spectroscopic Measures
6. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bacchus-Montabonel, M.-C. Theoretical Investigation of Proton Collisions on Prebiotic Candidates: Hydrogen Cyanide Polymers. Phys. Chem. Chem. Phys. 2017, 19, 19566–19572. [Google Scholar] [CrossRef]
- Rannou, P.; West, R. Supersaturation on Pluto and Elsewhere. Icarus 2018, 312, 36–44. [Google Scholar] [CrossRef]
- Hänni, N.; Altwegg, K.; Pestoni, B.; Rubin, M.; Schroeder, I.; Schuhmann, M.; Wampfler, S. First in Situ Detection of the CN Radical in Comets and Evidence for a Distributed Source. Mon. Not. R. Astron. Soc. 2020, 498, 2239–2248. [Google Scholar] [CrossRef]
- Mandt, K.; Luspay-Kuti, A.; Hamel, M.; Jessup, K.-L.; Hue, V.; Kammer, J.; Filwett, R. Photochemistry on Pluto: Part II HCN and Nitrogen Isotope Fractionation. Mon. Not. R. Astron. Soc. 2017, 472, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Wirström, E.S.; Charnley, S.B. Revised Models of Interstellar Nitrogen Isotopic Fractionation. Mon. Not. R. Astron. Soc. 2018, 474, 3720–3726. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Molaverdikhani, K.; Pudritz, R.E.; Henning, T.; Hébrard, E. HCN Production in Titan’s Atmosphere: Coupling Quantum Chemistry and Disequilibrium Atmospheric Modeling. Astrophys. J. 2020, 901, 110. [Google Scholar] [CrossRef]
- Hill, R.D. An Efficient Lightning Energy Source on the Early Earth. Orig. Life Evol. Biosph. 1992, 22, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.G.; Neubeck, A. Reduction of Nitrogen Compounds in Oceanic Basement and Its Implications for HCN Formation and Abiotic Organic Synthesis. Geochem. Trans. 2009, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Kraus, F.; Hanzlik, M.; Eisenreich, W.; Wächtershäuser, G. Elements of Metabolic Evolution. Chem. Eur. J. 2012, 18, 2063–2080. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Kubelík, P.; Knížek, A.; Pastorek, A.; Sutherland, J.; Civiš, S. High Energy Radical Chemistry Formation of HCN-Rich Atmospheres on Early Earth. Sci. Rep. 2017, 7, 6275. [Google Scholar] [CrossRef] [PubMed]
- Airapetian, V.S.; Glocer, A.; Gronoff, G.; Hébrard, E.; Danchi, W. Prebiotic Chemistry and Atmospheric Warming of Early Earth by an Active Young Sun. Nat. Geosci. 2016, 9, 452–455. [Google Scholar] [CrossRef]
- Matthews, C.N. Dark Matter in the Solar System: Hydrogen Cyanide Polymers. Orig. Life Evol. Biosph. 1991, 21, 421–434. [Google Scholar] [CrossRef]
- Matthews, C.N. Hydrogen Cyanide Polymers: From Laboratory to Space. Planet. Space Sci. 1995, 43, 1365–1370. [Google Scholar] [CrossRef]
- Bauer, H. Die Ersten Organisch-Chemischen Synthesen. Naturwissenschaften 1980, 67, 1–6. [Google Scholar] [CrossRef]
- Proust, J.L. Gehlen’s J. Chem. Physik, 3, 384; 1806. Ann. Chim. Physique 1807, 60, 233. [Google Scholar]
- Wippermann, R. Ueber Tricyanwasserstoff, Eine Der Blausäure Polymere Verbindung. Eur. J. Inorg. Chem. 1874, 7, 767–772. [Google Scholar] [CrossRef]
- Pflüger, E. Nachtrag zu meinem Aufsatz: Ueber die physiologische Verbrennung in den lebendigen Organismen. Pflüger Arch. 1875, 10, 641–644. [Google Scholar] [CrossRef]
- Oró, J. Synthesis of Adenine from Ammonium Cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common Origins of RNA, Protein and Lipid Precursors in a Cyanosulfidic Protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Eschenmoser, A. On a Hypothetical Generational Relationship between HCN and Constituents of the Reductive Citric Acid Cycle. Chem. Biodiver. 2007, 4, 554–573. [Google Scholar] [CrossRef] [PubMed]
- Eschenmoser, A. The Search for the Chemistry of Life’s Origin. Tetrahedron 2007, 63, 12821–12844. [Google Scholar] [CrossRef]
- Das, T.; Ghule, S.; Vanka, K. Insights into the Origin of Life: Did It Begin from HCN and H2O? ACS Cent. Sci. 2019, 5, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bermejo, M.; Zorzano, M.-P.; Osuna-Esteban, S. Simple Organics and Biomonomers Identified in HCN Polymers: An Overview. Life 2013, 3, 421–448. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Kumar, R.; Krishnamurthy, R. Chemistry of Abiotic Nucleotide Synthesis. Chem. Rev. 2020, 120, 4766–4805. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.; Ferris, J.; Orgel, L.E. Conditions for Purine Synthesis: Did Prebiotic Synthesis Occur at Low Temperatures? Science 1966, 153, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in Prebiodc Synthesis: II. Synthesis of Purine Precursors and Amino Acids from Aqueous Hydrogen Cyanide. J. Mol. Biol. 1967, 30, 223–253. [Google Scholar]
- Ferris, J.P.; Donner, D.B.; Lobo, A.P. Possible Role of Hydrogen Cyanide in Chemical Evolution: The Oligomerization and Condensation of Hydrogen Cyanide. J. Mol. Biol. 1973, 74, 511–518. [Google Scholar] [CrossRef]
- Ferris, J.P.; Wos, J.D.; Ryan, T.J.; Lobo, A.P.; Donner, D.B. Biomolecules from HCN. Orig. Life 1974, 5, 153–157. [Google Scholar] [CrossRef]
- Ferris, J.P.; Joshi, P.C.; Lawless, J.G. Chemical Evolution XXIX. Pyrimidines from Hydrogen Cyanide. BioSystems 1977, 9, 81–86. [Google Scholar] [CrossRef]
- Ferris, J.P.; Joshi, P.C.; Edelson, E.H.; Lawless, J.G. HCN: A Plausible Source of Purines, Pyrimidines and Amino Acids on the Primitive Earth. J. Mol. Evol. 1978, 11, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hagan, W.J., Jr. HCN and Chemical Evolution: The Possible Role of Cyano Compounds in Prebiotic Synthesis. Tetrahedron 1984, 40, 1093–1120. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Joosten, H.; Voet, A.B. Prebiotic Adenine Synthesis via HCN Oligomerization in Ice. Biosystems 1982, 15, 191–193. [Google Scholar] [CrossRef]
- Voet, A.B.; Schwartz, A.W. Uracil Synthesis via HCN Oligomerization. Orig. Life 1982, 12, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W.; Goverde, M. Acceleration of HCN Oligomerization by Formaldehyde and Related Compounds: Implications for Prebiotic Syntheses. J. Mol. Evol. 1982, 18, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Voet, A.B.; Schwartz, A.W. Prebiotic Adenine Synthesis from HCN—Evidence for a Newly Discovered Major Pathway. Bioorg. Chem. 1983, 12, 8–17. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Voet, A.B.; Van der Veen, M. Recent Progress in the Prebiotic Chemistry of HCN. Orig. Life 1984, 14, 91–98. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Bakker, C.G. Was Adenine the First Purine? Science 1989, 245, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The Cold Origin of Life: B. Implications Based on Pyrimidines and Purines Produced from Frozen Ammonium Cyanide Solutions. Orig. Life Evol. Biosph. 2002, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Miller, S.L.; Brinton, K.; Bada, J.L. Prebiotic Synthesis of Adenine and Amino Acids under Europa-like Conditions. Icarus 2000, 145, 609–613. [Google Scholar] [CrossRef]
- da Silva, J.B.P.; de Araujo, A.P.M. A New Mechanism of Guanine-Isomer Formation from Species Previously Observed in the Interstellar Medium. ACS Earth Space Chem. 2017, 1, 376–383. [Google Scholar] [CrossRef]
- Gupta, V.P.; Tandon, P.; Rawat, P.; Singh, R.N.; Singh, A. Quantum Chemical Study of a New Reaction Pathway for the Adenine Formation in the Interstellar Space. Astron. Astrophys. 2011, 528, A129. [Google Scholar] [CrossRef]
- Jung, S.H.; Choe, J.C. Mechanisms of Prebiotic Adenine Synthesis from HCN by Oligomerization in the Gas Phase. Astrobiology 2013, 13, 465–475. [Google Scholar] [CrossRef]
- Oró, J.; Kimball, A.P. Synthesis of Purines under Possible Primitive Earth Conditions. I. Adenine from Hydrogen Cyanide. Arch. Biochem. Biophys. 1961, 94, 217–227. [Google Scholar] [CrossRef]
- Oro, J.; Kamat, S.S. Amino-Acid Synthesis from Hydrogen Cyanide under Possible Primitive Earth Conditions. Nature 1961, 190, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.U.; Rees, M.W.; Markham, R. Synthesis of Complex Organic Compounds from Simple Precursors: Formation of Amino-Acids, Amino-Acid Polymers, Fatty Acids and Purines from Ammonium Cyanide. Nature 1963, 199, 219–222. [Google Scholar] [CrossRef]
- Abelson, P.H. Chemical Events on the Primitive Earth. Proc. Natl. Acad. Sci. USA 1966, 55, 1365–1372. [Google Scholar] [CrossRef]
- Matthews, C.N.; Moser, R.E. Peptide Synthesis from Hydrogen Cyanide and Water. Nature 1967, 215, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Wos, J.D.; Nooner, D.W.; Oró, J. Chemical Evolution. J. Mol. Evol. 1974, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Wos, J.D.; Lobo, A.P. Chemical Evolution. J. Mol. Evol. 1974, 3, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Marín-Yaseli, M.R.; Mompeán, C.; Ruiz-Bermejo, M. A Prebiotic Synthesis of Pterins. Chem. Eur. J. 2015, 21, 13531–13534. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; González-Toril, E.; Mompeán, C.; Ruiz-Bermejo, M. The Role of Aqueous Aerosols in the “Glyoxylate Scenario”: An Experimental Approach. Chem. Eur. J. 2016, 22, 12785–12799. [Google Scholar] [CrossRef]
- Hortal, L.; Pérez-Fernández, C.; de la Fuente, J.L.; Valles, P.; Mateo-Martí, E.; Ruiz-Bermejo, M. A Dual Perspective on the Microwave-Assisted Synthesis of HCN Polymers towards the Chemical Evolution and Design of Functional Materials. Sci. Rep. 2020, 10, 22350. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Mendoza, A.; Draganić, Z.D.; Navarro-González, R.; Draganić, I.G. Aldehydes, Ketones, and Carboxylic Acids Formed Radiolytically in Aqueous Solutions of Cyanides and Simple Nitriles. Radiat. Res. 1983, 95, 248–261. [Google Scholar] [CrossRef]
- Negrón-Mendoza, A.; Draganić, Z.D. Search for Heterocyclic Radiolytic Products in Aqueous Solutions of Cyanides. Adv. Space Res. 1984, 4, 121–124. [Google Scholar] [CrossRef]
- Negrón-Mendoza, A.; Ramos-Bernal, S.; Cruz, E.; Juárez, J.M. Radiolysis of HCN in Heterogeneous Phase. Radiat. Phys. Chem. 2001, 61, 771–772. [Google Scholar] [CrossRef]
- Yi, R.; Tran, Q.P.; Ali, S.; Yoda, I.; Adam, Z.R.; Cleaves, H.J.; Fahrenbach, A.C. A Continuous Reaction Network That Produces RNA Precursors. Proc. Natl. Acad. Sci. USA 2020, 117, 13267–13274. [Google Scholar] [CrossRef]
- Yi, R.; Hongo, Y.; Yoda, I.; Adam, Z.R.; Fahrenbach, A.C. Radiolytic Synthesis of Cyanogen Chloride, Cyanamide and Simple Sugar Precursors. Chem. Sel. 2018, 3, 10169–10174. [Google Scholar] [CrossRef]
- Ritson, D.; Sutherland, J.D. Prebiotic Synthesis of Simple Sugars by Photoredox Systems Chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ritson, D.J.; Ranjan, S.; Todd, Z.R.; Sasselov, D.D.; Sutherland, J.D. Photochemical Reductive Homologation of Hydrogen Cyanide Using Sulfite and Ferrocyanide. Chem. Comm. 2018, 54, 5566–5569. [Google Scholar] [CrossRef]
- Sutherland, J.D. The Origin of Life—Out of the Blue. Angew. Chem. Int. Ed. 2016, 55, 104–121. [Google Scholar] [CrossRef]
- Labadie, M.; Jensen, R.; Neuzil, E. Recherches sur l’évolution pré-biologique III. Les acides azulmiques noirs formés à partir du cyanure d’ammonium. Biochim. et Biophys. Acta Gen. Subj. 1968, 165, 525–533. [Google Scholar] [CrossRef]
- Ghoshal, S.; Pramanik, A.; Sarkar, P. Theoretical Investigations on the Possibility of Prebiotic HCN Formation via O-Addition Reactions. J. Phys. Chem. A 2020, 124, 4782–4792. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, P.B.; Ferus, M.; Waldmann, I.P.; Knížek, A.; Kalvaitis, D.; Ivanek, O.; Kubelík, P.; Yurchenko, S.N.; Burian, T.; Dostál, J.; et al. Identifiable Acetylene Features Predicted for Young Earth-like Exoplanets with Reducing Atmospheres Undergoing Heavy Bombardment. Astrophys. J. 2019, 888, 21. [Google Scholar] [CrossRef]
- Ghoshal, S.; Pramanik, A.; Biswas, S.; Sarkar, P. CH3NO as a Potential Intermediate for Early Atmospheric HCN: A Quantum Chemical Insight. Phys. Chem. Chem. Phys. 2019, 21, 25126–25138. [Google Scholar] [CrossRef]
- Parkos, D.; Pikus, A.; Alexeenko, A.; Melosh, H.J. HCN Production via Impact Ejecta Reentry During the Late Heavy Bombardment. J. Geophys. Res. Planets 2018, 123, 892–909. [Google Scholar] [CrossRef]
- Rimmer, P.B.; Shorttle, O. Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents. Life 2019, 9, 12. [Google Scholar] [CrossRef]
- Maruyama, S.; Kurokawa, K.; Ebisuzaki, T.; Sawaki, Y.; Suda, K.; Santosh, M. Nine Requirements for the Origin of Earth’s Life: Not at the Hydrothermal Vent, but in a Nuclear Geyser System. Geosci. Front. 2019, 10, 1337–1357. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; González-Toril, E.; Ruiz-Bermejo, M. Reactivity of Cyanide at Water-Ice Interfaces: Implications for the Search for Organics on Icy Worlds. Front. Astron. Space Sci. 2020, 7, 519017. [Google Scholar] [CrossRef]
- Kulp, T.R.; Hoeft, S.E.; Miller, L.G.; Saltikov, C.; Murphy, J.N.; Han, S.; Lanoil, B.; Oremland, R.S. Dissimilatory Arsenate and Sulfate Reduction in Sediments of Two Hypersaline, Arsenic-Rich Soda Lakes: Mono and Searles Lakes, California. Appl. Environ. Microbiol. 2006, 72, 6514–6526. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.T.; Gallagher, K.L.; Bouton, A.; Farias, M.E.; Kurth, D.; Sancho-Tomás, M.; Philippot, P.; Somogyi, A.; Medjoubi, K.; Vennin, E.; et al. Modern Arsenotrophic Microbial Mats Provide an Analogue for Life in the Anoxic Archean. Commun. Earth Environ. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Sancho-Tomás, M.; Somogyi, A.; Medjoubi, K.; Bergamaschi, A.; Visscher, P.T.; van Driessche, A.E.S.; Gérard, E.; Farias, M.E.; Contreras, M.; Philippot, P. Geochemical Evidence for Arsenic Cycling in Living Microbialites of a High Altitude Andean Lake (Laguna Diamante, Argentina). Chem. Geol. 2020, 549, 119681. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C. Alkaline Lake Settings for Concentrated Prebiotic Cyanide and the Origin of Life. Geochim. Cosmochim. Acta 2019, 260, 124–132. [Google Scholar] [CrossRef]
- Villafañe-Barajas, S.A.; Colín-García, M.; Negrón-Mendoza, A.; Ruiz-Bermejo, M. An Experimental Study of the Thermolysis of Hydrogen Cyanide: The Role of Hydrothermal Systems in Chemical Evolution. Int. J. Astrobiol. 2020, 19, 369–378. [Google Scholar] [CrossRef]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Colín-García, M. Characterization of HCN-Derived Thermal Polymer: Implications for Chemical Evolution. Processes 2020, 8, 968. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; Menor-Salván, C.; Osuna-Esteban, S.; Veintemillas-Verdaguer, S. The Effects of Ferrous and Other Ions on the Abiotic Formation of Biomolecules Using Aqueous Aerosols and Spark Discharges. Orig. Life Evol. Biosph. 2007, 37, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, L. Evolution of Organic Compounds in Volcanic Regions. Nature 1974, 251, 50–51. [Google Scholar] [CrossRef]
- Mukhin, L.; Bondarev, V.B.; Safanova, E.N. The Role of Volcanic Processes in the Evolution of Organic Compounds on the Primitive Earth. Mod. Geol. 1978, 6, 119–122. [Google Scholar]
- Ruiz-Bermejo, M.; Osuna-Esteban, S.; Zorzano, M.-P. Role of Ferrocyanides in the Prebiotic Synthesis of α-Amino Acids. Orig. Life Evol. Biosph. 2013, 43, 191–206. [Google Scholar] [CrossRef]
- Bolm, C.; Mocci, R.; Schumacher, C.; Turberg, M.; Puccetti, F.; Hernández, J.G. Mechanochemical Activation of Iron Cyano Complexes: A Prebiotic Impact Scenario for the Synthesis of α-Amino Acid Derivatives. Angew. Chem. Int. Ed. 2018, 57, 2423–2426. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; Rogero, C.; Menor-Salván, C.; Osuna-Esteban, S.; Martín-Gago, J.Á.; Veintemillas-Verdaguer, S. Thermal Wet Decomposition of Prussian Blue: Implications for Prebiotic Chemistry. Chem. Biodiver. 2009, 6, 1309–1322. [Google Scholar] [CrossRef]
- Sharma, R.; Iqubal, M.A.; Kamaluddin. Possible Role of Prussian Blue Nanoparticles in Chemical Evolution: Interaction with Ribose Nucleotides. Int. J. Astrobiol. 2016, 15, 17–25. [Google Scholar] [CrossRef]
- Burcar, B.; Castañeda, A.; Lago, J.; Daniel, M.; Pasek, M.A.; Hud, N.V.; Orlando, T.M.; Menor-Salván, C. A Stark Contrast to Modern Earth: Phosphate Mineral Transformation and Nucleoside Phosphorylation in an Iron- and Cyanide-Rich Early Earth Scenario. Angew. Chem. Int. Ed. 2019, 58, 16981–16987. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S.; Brister, B.D.; Jiang, J.; Lou, S.; Wang, Z.; Yarberry, F. Cyanide Self-Addition, Controlled Adsorption, and Other Processes at Layered Double Hydroxides. Orig. Life Evol. Biosph. 2001, 31, 53–69. [Google Scholar] [CrossRef]
- Colin-Garcia, M.; Heredia, A.; Negron-Mendoza, A.; Ortega, F.; Pi, T.; Ramos-Bernal, S. Adsorption of HCN onto Sodium Montmorillonite Dependent on the PH as a Component to Chemical Evolution. Int. J. Astrobiol. 2014, 13, 310–318. [Google Scholar] [CrossRef]
- Ferris, J.P.; Edelson, E.H.; Mount, N.M.; Sullivan, A.E. The Effect of Clays on the Oligomerization of HCN. J. Mol. Evol. 1979, 13, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hagan, W.J.; Alwis, K.W.; McCrea, J. Chemical Evolution Clay-Mediated Oxidation of Diaminomaleonitrile. J. Mol. Evol. 1982, 18, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bermejo, M.; Menor-Salván, C.; Zorzano, M.-P.; El-Hachemi, Z.; Osuna-Esteban, S.; Veintemillas-Verdaguer, S. Water interfacial processes in prebiotic chemistry. In Astrobiology: Physical Origin, Biological Evolution and Spatial Distribution; Hegedus, S., Csonka, J., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2009. [Google Scholar]
- Griffith, E.C.; Tuck, A.F.; Vaida, V. Ocean–Atmosphere Interactions in the Emergence of Complexity in Simple Chemical Systems. Acc. Chem. Res. 2012, 45, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Fábián, B.; Szőri, M.; Jedlovszky, P. Floating Patches of HCN at the Surface of Their Aqueous Solutions—Can They Make “HCN World” Plausible? J. Phys. Chem. C 2014, 118, 21469–21482. [Google Scholar] [CrossRef]
- Matthews, C.N.; Minard, R.D. Hydrogen Cyanide Polymers, Comets and the Origin of Life. Faraday Discuss. 2006, 133, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Quirico, E.; Szopa, C.; Cernogora, G.; Lees, V.; Derenne, S.; McMillan, P.F.; Montagnac, G.; Reynard, B.; Rouzaud, J.-N.; Fray, N.; et al. Tholins and Their Relevance for Astrophysical Issues. Proc. Int. Astron. Union 2008, 4, 409–416. [Google Scholar] [CrossRef][Green Version]
- Matthews, C.N.; Minard, R.D. Hydrogen Cyanide Polymers Connect Cosmochemistry and Biochemistry. Proc. Int. Astron. Union 2008, 4, 453–458. [Google Scholar] [CrossRef]
- Fray, N.; Bénilan, Y.; Cottin, H.; Gazeau, M.-C.; Minard, R.D.; Raulin, F. Experimental Study of the Degradation of Polymers: Application to the Origin of Extended Sources in Cometary Atmospheres. Meteorit. Planet. Sci. 2004, 39, 581–587. [Google Scholar] [CrossRef]
- Bonnet, J.-Y.; Quirico, E.; Buch, A.; Thissen, R.; Szopa, C.; Carrasco, N.; Cernogora, G.; Fray, N.; Cottin, H.; Roy, L.L.; et al. Formation of Analogs of Cometary Nitrogen-Rich Refractory Organics from Thermal Degradation of Tholin and HCN Polymer. Icarus 2015, 250, 53–63. [Google Scholar] [CrossRef]
- Todd, Z.R.; Öberg, K.I. Cometary Delivery of Hydrogen Cyanide to the Early Earth. Astrobiology 2020, 20, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; House, C.H.; Arevalo, R.D.; Dworkin, J.P.; Callahan, M.P. Organometallic Compounds as Carriers of Extraterrestrial Cyanide in Primitive Meteorites. Nat. Commun. 2019, 10, 2777. [Google Scholar] [CrossRef]
- Ferus, M.; Rimmer, P.; Cassone, G.; Knížek, A.; Civiš, S.; Šponer, J.E.; Ivanek, O.; Šponer, J.; Saeidfirozeh, H.; Kubelík, P.; et al. One-Pot Hydrogen Cyanide-Based Prebiotic Synthesis of Canonical Nucleobases and Glycine Initiated by High-Velocity Impacts on Early Earth. Astrobiology 2020, 20, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, B.M.; Puertas, M.L.; Fabiano, F.; Adriani, A.; Moriconi, M.L.; Funke, B.; García-Comas, M.; Oliva, F.; D’Aversa, E.; Filacchione, G. Climatology of CH4, HCN and C2H2 in Titan’s Upper Atmosphere from Cassini/VIMS Observations. Icarus 2019, 331, 83–97. [Google Scholar] [CrossRef]
- Lebonnois, S.; Bakes, E.L.O.; McKay, C.P. Transition from Gaseous Compounds to Aerosols in Titan’s Atmosphere. Icarus 2002, 159, 505–517. [Google Scholar] [CrossRef]
- Khare, B.N.; Sagan, C.; Thompson, W.R.; Arakawa, E.T.; Meisse, C.; Tuminello, P.S. Optical Properties of Poly-HCN and Their Astronomical Applications. Can. J. Chem. 1994, 72, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Budil, D.E.; Roebber, J.L.; Liebman, S.A.; Matthews, C.N. Multifrequency Electron Spin Resonance Detection of Solid-State Organic Free Radicals in HCN Polymer and a Titan Tholin. Astrobiology 2003, 3, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Vuitton, V.; Bonnet, J.-Y.; Frisari, M.; Thissen, R.; Quirico, E.; Dutuit, O.; Schmitt, B.; Roy, L.L.; Fray, N.; Cottin, H.; et al. Very High Resolution Mass Spectrometry of HCN Polymers and Tholins. Faraday Discuss. 2010, 147, 495–508. [Google Scholar] [CrossRef]
- Rahm, M.; Lunine, J.I.; Usher, D.A.; Shalloway, D. Polymorphism and Electronic Structure of Polyimine and Its Potential Significance for Prebiotic Chemistry on Titan. Proc. Natl. Acad. Sci. USA 2016, 113, 8121–8126. [Google Scholar] [CrossRef]
- Ennis, C.; Cable, M.L.; Hodyss, R.; Maynard-Casely, H.E. Mixed Hydrocarbon and Cyanide Ice Compositions for Titan’s Atmospheric Aerosols: A Ternary-Phase Co-Crystal Predicted by Density Functional Theory. ACS Earth Space Chem. 2020, 4, 1195–1200. [Google Scholar] [CrossRef]
- Wilson, P.D.; Sagan, C. Spectrophotometry and Organic Matter on Iapetus: Composition Models. J. Geophys. Res. Planets 1995, 100, 7531–7537. [Google Scholar] [CrossRef] [PubMed]
- Brucato, J.R.; Migliorini, A.; Barucci, M.A.; Carvano, J.M.; Dotto, E.; Mennella, V. Reflectance Spectra of Titan Tholin between 7000 and 10 Cm-1—Interpretation of Cassini/CIRS Observation of Saturn’s Satellite Phoebe. Astron. Astrophys. 2010, 516, A92. [Google Scholar] [CrossRef][Green Version]
- Jeilani, Y.A.; Williams, P.N.; Walton, S.; Nguyen, M.T. Unified Reaction Pathways for the Prebiotic Formation of RNA and DNA Nucleobases. Phys. Chem. Chem. Phys. 2016, 18, 20177–20188. [Google Scholar] [CrossRef]
- Rimmer, P.B.; Rugheimer, S. Hydrogen Cyanide in Nitrogen-Rich Atmospheres of Rocky Exoplanets. Icarus 2019, 329, 124–131. [Google Scholar] [CrossRef]
- Gerakines, P.A.; Moore, M.H.; Hudson, R.L. Ultraviolet Photolysis and Proton Irradiation of Astrophysical Ice Analogs Containing Hydrogen Cyanide. Icarus 2004, 170, 202–213. [Google Scholar] [CrossRef]
- Saladino, R.; Di Mauro, E.; García-Ruiz, J.M. A Universal Geochemical Scenario for Formamide Condensation and Prebiotic Chemistry. Chem. Eur. J. 2019, 25, 3181–3189. [Google Scholar] [CrossRef]
- Enchev, V.; Angelov, I.; Dincheva, I.; Stoyanova, N.; Slavova, S.; Rangelov, M.; Markova, N. Chemical Evolution: From Formamide to Nucleobases and Amino Acids without the Presence of Catalyst. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef]
- Menor-Salván, C. From the Dawn of Organic Chemistry to Astrobiology: Urea as a Foundational Component in the Origin of Nucleobases and Nucleotides. In Prebiotic Chemistry and Chemical Evolution of Nucleic Acids; Springer International Publishing: Cham, Switzerland, 2018; pp. 85–142. [Google Scholar] [CrossRef]
- Menor-Salván, C.; Ruiz-Bermejo, D.M.; Guzmán, M.I.; Osuna-Esteban, S.; Veintemillas-Verdaguer, S. Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases. Chem. Eur. J. 2009, 15, 4411–4418. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Manini, P.; Moracci, M.; Saladino, R.; Ball, V.; Thissen, H.; Evans, R.A.; Puzzarini, C.; Barone, V. Astrochemistry and Astrobiology: Materials Science in Wonderland? Int. J. Mol. Sci. 2019, 20, 4079. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lin, G.; Upton, K.T.; Imanaka, H.; Smith, M.A. Structural Investigation of HCN Polymer Isotopomers by Solution-State Multidimensional NMR. J. Phys. Chem. A 2012, 116, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, Y.; Su, Y.; Ge, C.; Jin, B.; Li, Z.; Wu, S. Preparation and Characterization of Poly-Hydrogen Cyanide Nanofibers with High Visible Light Photocatalytic Activity. Catal. Commun. 2014, 46, 197–200. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Carretero-González, J.; García-Fernández, L.; Aguilar, M.R. A Comparative Study on HCN Polymers Synthesized by Polymerization of NH4CN or Diaminomaleonitrile in Aqueous Media: New Perspectives for Prebiotic Chemistry and Materials Science. Chem. Eur. J. 2019, 25, 11437–11455. [Google Scholar] [CrossRef]
- Mas, I.; de la Fuente, J.L.; Ruiz-Bermejo, M. Temperature Effect on Aqueous NH4CN Polymerization: Relationship between Kinetic Behaviour and Structural Properties. Eur. Polym. J. 2020, 132, 109719. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Thissen, H.; Evans, R.A.; Ball, V. Films and Materials Derived from Aminomalononitrile. Processes 2021, 9, 82. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Thissen, H.; Koegler, A.; Salwiczek, M.; Easton, C.D.; Qu, Y.; Lithgow, T.; Evans, R.A. Prebiotic-Chemistry Inspired Polymer Coatings for Biomedical and Material Science Applications. NPG Asia Mater. 2015, 7, e225. [Google Scholar] [CrossRef]
- Ball, V.; Toh, R.J.; Voelcker, N.H.; Thissen, H.; Evans, R.A. Electrochemical Deposition of Aminomalonitrile Based Films. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 124–129. [Google Scholar] [CrossRef]
- Menzies, D.J.; Ang, A.; Thissen, H.; Evans, R.A. Adhesive Prebiotic Chemistry Inspired Coatings for Bone Contacting Applications. ACS Biomater. Sci. Eng. 2017, 3, 793–806. [Google Scholar] [CrossRef]
- Chen, W.-H.; Liao, T.-Y.; Thissen, H.; Tsai, W.-B. One-Step Aminomalononitrile-Based Coatings Containing Zwitterionic Copolymers for the Reduction of Biofouling and the Foreign Body Response. ACS Biomater. Sci. Eng. 2019, 5, 6454–6462. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-Y.; Easton, C.D.; Thissen, H.; Tsai, W.-B. Aminomalononitrile-Assisted Multifunctional Antibacterial Coatings. ACS Biomater. Sci. Eng. 2020, 6, 3349–3360. [Google Scholar] [CrossRef]
- Jung, J.; Menzies, D.J.; Thissen, H.; Easton, C.D.; Evans, R.A.; Henry, R.; Deletic, A.; McCarthy, D.T. New Prebiotic Chemistry Inspired Filter Media for Stormwater/Greywater Disinfection. J. Hazard. Mater. 2019, 378, 120749. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Antioxidant Activity of Films Inspired by Prebiotic Chemistry. Mater. Lett. 2021, 285, 129050. [Google Scholar] [CrossRef]
- Minard, R.D.; Hatcher, P.G.; Gourley, R.C.; Matthews, C.N. Structural Investigations of Hydrogen Cyanide Polymers: New Insights Using TMAH Thermochemolysis/GC-MS. Orig. Life Evol. Biosph. 1998, 28, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Donner, D.B.; Lobo, A.P. Possible Role of Hydrogen Cyanide in Chemical Evolution: Investigation of the Proposed Direct Synthesis of Peptides from Hydrogen Cyanide. J. Mol. Biol. 1973, 74, 499–510. [Google Scholar] [CrossRef]
- Mizutani, H.; Mikuni, H.; Takahasi, M.; Noda, H. Study on the Photochemical Reaction of HCN and Its Polymer Products Relating to Primary Chemical Evolution. Orig. Life Evol. Biosph. 1975, 6, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Draganić, Z.D.; Niketić, V.; Jovanović, S.; Draganić, I.G. The Radiolysis of Aqueous Ammonium Cyanide: Compounds of Interest to Chemical Evolution Studies. J. Mol. Evol. 1980, 15, 239–260. [Google Scholar] [CrossRef]
- Niketić, V.; Draganić, Z.D.; Nešković, S.; Jovanović, S.; Draganić, I.G. Radiolysis of Aqueous Solutions of Hydrogen Cyanide (pH∼6): Compounds of Interest in Chemical Evolution Studies. J. Mol. Evol. 1983, 19, 184–191. [Google Scholar] [CrossRef]
- Vujošević, S.I.; Negrón-Mendoza, A.; Draganić, Z.D. Radiation-Induced Polymerization in Dilute Aqueous Solutions of Cyanides. Orig. Life Evol. Biosph. 1990, 20, 49–54. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; Cid, C.; Yagüe, A.I.; Ruiz-Bermejo, M. Detection of Macromolecular Fractions in HCN Polymers Using Electrophoretic and Ultrafiltration Techniques. Chem. Biodiver. 2017, 14, e1600241. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.-Y.; Thissen, R.; Frisari, M.; Vuitton, V.; Quirico, É.; Orthous-Daunay, F.-R.; Dutuit, O.; Le Roy, L.; Fray, N.; Cottin, H.; et al. Compositional and Structural Investigation of HCN Polymer through High Resolution Mass Spectrometry. Int. J. Mass Spectrom. 2013, 354–355, 193–203. [Google Scholar] [CrossRef]
- Fernández, A.; Ruiz-Bermejo, M.; De La Fuente, J.L. Modelling the Kinetics and Structural Property Evolution of a Versatile Reaction: Aqueous HCN Polymerization. Phys. Chem. Chem. Phys. 2018, 20, 17353–17366. [Google Scholar] [CrossRef] [PubMed]
- Toh, R.J.; Evans, R.; Thissen, H.; Voelcker, N.H.; d’Ischia, M.; Ball, V. Deposition of Aminomalononitrile-Based Films: Kinetics, Chemistry, and Morphology. Langmuir 2019, 35, 9896–9903. [Google Scholar] [CrossRef]
- de la Fuente, J.L.; Ruiz-Bermejo, M.; Nna-Mvondo, D.; Minard, R.D. Further Progress into the Thermal Characterization of HCN Polymers. Polym. Degrad. Stab. 2014, 110, 241–251. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Marín-Yaseli, M.R. The Influence of Reaction Conditions in Aqueous HCN Polymerization on the Polymer Thermal Degradation Properties. J. Anal. Appl. Pyrolysis 2017, 124, 103–112. [Google Scholar] [CrossRef]
- de la Fuente, J.L.; Ruiz-Bermejo, M.; Menor-Salván, C.; Osuna-Esteban, S. Thermal Characterization of HCN Polymers by TG–MS, TG, DTA and DSC Methods. Polym. Degrad. Stab. 2011, 96, 943–948. [Google Scholar] [CrossRef]
- Cataldo, F.; Lilla, E.; Ursini, O.; Angelini, G. TGA–FT-IR Study of Pyrolysis of Poly(Hydrogen Cyanide) Synthesized from Thermal Decomposition of Formamide. Implications in Cometary Emissions. J. Anal. Appl. Pyrolysis 2010, 87, 34–44. [Google Scholar] [CrossRef]
- Cruikshank, D.P.; Allamandola, L.J.; Hartmann, W.K.; Tholen, D.J.; Brown, R.H.; Matthews, C.N.; Bell, J.F. Solid C≡N Bearing Material on Outer Solar System Bodies. Icarus 1991, 94, 345–353. [Google Scholar] [CrossRef]
- Völker, T. Polymere Blausäure. Angew. Chem. 1960, 72, 379–384. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Rogero, C.; Menor-Salván, C.; Osuna-Esteban, S.; Martín-Gago, J.A. New Insights into the Characterization of ‘Insoluble Black HCN Polymers’. Chem. Biodiver. 2012, 9, 25–40. [Google Scholar] [CrossRef]
- Quirico, E.; Montagnac, G.; Lees, V.; McMillan, P.F.; Szopa, C.; Cernogora, G.; Rouzaud, J.-N.; Simon, P.; Bernard, J.-M.; Coll, P.; et al. New Experimental Constraints on the Composition and Structure of Tholins. Icarus 2008, 198, 218–231. [Google Scholar] [CrossRef]
- Liebman, S.A.; Pesce-Rodriguez, R.A.; Matthews, C.N. Organic Analysis of Hydrogen Cyanide Polymers: Prebiotic and Extraterrestrial Chemistry. Adv. Space Res. 1995, 15, 71–80. [Google Scholar] [CrossRef]
- Umemoto, K.; Takahasi, M.; Yokota, K. Studies on the Structure of HCN Oligomers. Orig. Life Evol. Biosph. 1987, 17, 283–293. [Google Scholar] [CrossRef]
- Sagan, C.; Khare, B.N. Tholins: Organic Chemistry of Interstellar Grains and Gas. Nature 1979, 277, 102–107. [Google Scholar] [CrossRef]
- Khare, B.N.; Sagan, C.; Thompson, W.R.; Arakawa, E.T.; Suits, F.; Callcott, T.A.; Williams, M.W.; Shrader, S.; Ogino, H.; Willingham, T.O.; et al. The Organic Aerosols of Titan. Adv. Space Res. 1984, 4, 59–68. [Google Scholar] [CrossRef]
- McDonald, G.D.; Khare, B.N.; Reid Thompson, W.; Sagan, C. CH4/NH3/H2O Spark Tholin: Chemical Analysis and Interaction with Jovian Aqueous Clouds. Icarus 1991, 94, 354–367. [Google Scholar] [CrossRef]
- Matthews, C.N.; Ludicky, R. The Dark Nucleus of Comet Halley: Hydrogen Cyanide Polymers. In ESLAB Symposium on the Exploration of Halley’s Comet; European Space Agency: Paris, France, 1986; Volume 250. [Google Scholar]
- Coll, P.; Coscia, D.; Smith, N.; Gazeau, M.-C.; Ramı́rez, S.I.; Cernogora, G.; Israël, G.; Raulin, F. Experimental Laboratory Simulation of Titan’s Atmosphere: Aerosols and Gas Phase. Planet. Space Sci. 1999, 47, 1331–1340. [Google Scholar] [CrossRef]
- Sciamma-O’Brien, E.; Carrasco, N.; Szopa, C.; Buch, A.; Cernogora, G. Titan’s Atmosphere: An Optimal Gas Mixture for Aerosol Production? Icarus 2010, 209, 704–714. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; Menor-Salván, C.; de la Fuente, J.L.; Mateo-Martí, E.; Osuna-Esteban, S.; Martín-Gago, J.Á.; Veintemillas-Verdaguer, S. CH4/N2/H2-Spark Hydrophobic Tholins: A Systematic Approach to the Characterisation of Tholins. Part II. Icarus 2009, 204, 672–680. [Google Scholar] [CrossRef]
- Cataldo, F.; Patanè, G.; Compagnini, G. Synthesis of HCN Polymer from Thermal Decomposition of Formamide. J. Macromol. Sci. Part A 2009, 46, 1039–1048. [Google Scholar] [CrossRef]
- Ferris, J.P.; Edelson, E.H.; Auyeung, J.M.; Joshi, P.C. Structural Studies on HCN Oligomers. J. Mol. Evol. 1981, 17, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Garbow, J.R.; Schaefer, J.; Ludicky, R.; Matthews, C.N. Detection of Secondary Amides in Hydrogen Cyanide Polymers by Dipolar Rotational Spin-Echo Nitrogen-15NMR. Macromolecules 1987, 20, 305–309. [Google Scholar] [CrossRef]
- Matthews, C.N.; Ludicky, R.; Schaefer, J.; Stejskal, E.O.; McKay, R.A. Heteropolypeptides from Hydrogen Cyanide and Water? Solid State 15N NMR Investigations. Orig. Life Evol. Biosph. 1984, 14, 243–250. [Google Scholar] [CrossRef]
- McKay, R.A.; Schaefer, J.; Stejskal, E.O.; Ludicky, R.; Matthews, C.N. Double-Cross-Polarization Detection of Labeled Chemical Bonds in Hydrogen Cyanide Polymerization. Macromolecules 1984, 17, 1124–1130. [Google Scholar] [CrossRef]
- Mamajanov, I.; Herzfeld, J. HCN Polymers Characterized by Solid State NMR: Chains and Sheets Formed in the Neat Liquid. J. Chem. Phys. 2009, 130, 134503. [Google Scholar] [CrossRef]
- Mamajanov, I.; Herzfeld, J. HCN Polymers Characterized by SSNMR: Solid State Reaction of Crystalline Tetramer (Diaminomaleonitrile). J. Chem. Phys. 2009, 130, 134504. [Google Scholar] [CrossRef]
- Eastman, M.P.; Helfrich, F.S.E.; Umantsev, A.; Porter, T.L.; Weber, R. Exploring the Structure of a Hydrogen Cyanide Polymer by Electron Spin Resonance and Scanning Force Microscopy. Scanning 2003, 25, 19–24. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; Moreno, M.; de la Fuente, J.L.; Briones, C.; Ruiz-Bermejo, M. Experimental Conditions Affecting the Kinetics of Aqueous HCN Polymerization as Revealed by UV–Vis Spectroscopy. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Edelson, E.H. Chemical Evolution. Mechanism of the Condensation of Cyanide to Hydrogen Cyanide Oligomers. J. Org. Chem. 1978, 43, 3989–3995. [Google Scholar] [CrossRef]
- Cataldo, F. Concerning the Chemical Structure of the Dicyanogen Photopolymer and Its Presence in Certain Bodies of the Solar System. Implications on the Synthesis of Some Prebiotic Molecules. Int. J. Astrobiol. 2002, 1, 25–30. [Google Scholar] [CrossRef]
- Mas, I.; Hortelano, C.; Ruiz-Bermejo, M.; de la Fuente, J.L. Highly Efficient Melt Polymerization of Diaminomaleonitrile. Eur. Polym. J. 2021, 143, 110185. [Google Scholar] [CrossRef]
- Nandi, S.; Bhattacharyya, D.; Anoop, A. Prebiotic Chemistry of HCN Tetramerization by Automated Reaction Search. Chem. Eur. J. 2018, 24, 4885–4894. [Google Scholar] [CrossRef]
- Andersen, J.L.; Andersen, T.; Flamm, C.; Hanczyc, M.M.; Merkle, D.; Stadler, P.F. Navigating the Chemical Space of HCN Polymerization and Hydrolysis: Guiding Graph Grammars by Mass Spectrometry Data. Entropy 2013, 15, 4066–4083. [Google Scholar] [CrossRef]
- Choe, J.C. Water-Assisted Dimerization of Hydrogen Cyanide: A Computational Study. Bull. Korean Chem. Soc. 2017, 38, 1531–1533. [Google Scholar] [CrossRef]
- Kua, J.; Thrush, K.L. HCN, Formamidic Acid, and Formamide in Aqueous Solution: A Free-Energy Map. J. Phys. Chem. B 2016, 120, 8175–8185. [Google Scholar] [CrossRef]
- Choe, J.C. Dimerization of HCN in Interstellar Icy Grain Mantles: A DFT Study. Bull. Korean Chem. Soc. 2019, 40, 205–206. [Google Scholar] [CrossRef]
- Glaser, R.; Hodgen, B.; Farrelly, D.; McKee, E. Adenine Synthesis in Interstellar Space: Mechanisms of Prebiotic Pyrimidine-Ring Formation of Monocyclic HCN-Pentamers. Astrobiology 2007, 7, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.W.M.; Talbi, D.; Herbst, E. The Production of HCN Dimer and More Complex Oligomers in Dense Interstellar Clouds. Astron. Astrophys. 2001, 369, 611–615. [Google Scholar] [CrossRef]
- Kikuchi, O.; Watanabe, T.; Satoh, Y.; Inadomi, Y. Ab Initio GB Study of Prebiotic Synthesis of Purine Precursors from Aqueous Hydrogen Cyanide: Dimerization Reaction of HCN in Aqueous Solution. J. Mol. Struct. THEOCHEM 2000, 507, 53–62. [Google Scholar] [CrossRef]
- Yim, M.K.; Choe, J.C. Dimerization of HCN in the Gas Phase: A Theoretical Mechanistic Study. Chem. Phys. Lett. 2012, 538, 24–28. [Google Scholar] [CrossRef]
- Lee, H.M.; Choe, J.C. Formation of Glycine from HCN and H2O: A Computational Mechanistic Study. Chem. Phys. Lett. 2017, 675, 6–10. [Google Scholar] [CrossRef]
- Roy, D.; Najafian, K.; von R. Schleyer, P. Chemical Evolution: The Mechanism of the Formation of Adenine under Prebiotic Conditions. Proc. Natl. Acad. Sci. USA 2007, 104, 17272–17277. [Google Scholar] [CrossRef]
- Guo Hong, S.; Qu, X.; Yuan Fu, X. A Quantum Chemical Study on the Mechanism of HCN Oligomerization. J. Mol. Struct. THEOCHEM 1992, 257, 25–32. [Google Scholar] [CrossRef]
- Kofranek, M.; Karpfen, A.; Lischka, H. Ab Initio Studies on Hydrogen-Bonded Clusters. I. Linear and Cyclic Oligomers of Hydrogen Cyanide. Chem. Phys. 1987, 113, 53–64. [Google Scholar] [CrossRef]
- Bochvar, D.A.; Nikerov, M.V.; Stankevich, I.V. A CNDO/2 Study of the Relative Stability of Several Hydrogen Cyanide Oligomers (HCN)x. J. Struct. Chem. 1984, 24, 621–622. [Google Scholar] [CrossRef]
- Hall, H.K.; Padias, A.B.; Kamachi, M.; Hashidzume, A. Synthesis and Polymerizability of C≡N Monomers. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3467–3474. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal Stabilization of Polyacrylonitrile Fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- De Vries, L. Stable Glycinonitrile Radical. Evidence Suggesting Generation of Aminocyanocarbenes from Aminomalonitriles in Basic Media. J. Org. Chem. 1973, 38, 2604–2613. [Google Scholar] [CrossRef]
- De Vries, L. Thermal Transformations of an Aminomalononitrile and of an Aminocyanoketenimine. Evidence for Homolysis and Heterolysis and for Aminocyanocarbenes. J. Org. Chem. 1973, 38, 4357–4362. [Google Scholar] [CrossRef]
- Sruthi, P.R.; Anas, S. An Overview of Synthetic Modification of Nitrile Group in Polymers and Applications. J. Polym. Sci. 2020, 58, 1039–1061. [Google Scholar] [CrossRef]
- Wöuhrle, D.; Knothe, G. Polymers from Nitriles. VII. Polymerization of Fumaronitrile with Triethylamine as Initiator. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 2435–2447. [Google Scholar] [CrossRef]
- Padias, A.B.; Hall, H.K. Semiconducting Polymers via the High Temperature Free Radical Polymerization of Multinitriles. J. Polym. Sci. Part A Polym. Chem. 1986, 24, 1675–1683. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, A.; Tang, B.Z. Polymerizations Based on Triple-Bond Building Blocks. Prog. Polym. Sci. 2018, 78, 92–138. [Google Scholar] [CrossRef]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon Nitrides: Synthesis and Characterization of a New Class of Functional Materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yan, D. Hyperbranched Polymers: From Synthesis to Applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Amal, A.-A. Recent Developments in the Chemistry of Diaminomaleonitrile. Curr. Org. Synth. 2015, 12, 110. [Google Scholar] [CrossRef]
- Johnson, D.M.; Reybuck, S.E.; Lawton, R.G.; Rasmussen, P.G. Condensation of DAMN with Conjugated Aldehydes and Polymerizations of the Corresponding Imines. Macromolecules 2005, 38, 3615–3621. [Google Scholar] [CrossRef]
- Aruna; Rani, B.; Swami, S.; Agarwala, A.; Behera, D.; Shrivastava, R. Recent Progress in Development of 2,3-Diaminomaleonitrile (DAMN) Based Chemosensors for Sensing of Ionic and Reactive Oxygen Species. RSC Adv. 2019, 9, 30599–30614. [Google Scholar] [CrossRef]
- Thissen, H.; Evans, R.A.; Koegler, A. Hydrogen Cyanide-Based Polymer Surface Coatings and Hydrogels. U.S. Patent 2015/0140660 A, 21 May 2015. [Google Scholar]
- Chung, K.K.; Fechler, N.; Patrini, M.; Galinetto, P.; Comoretto, D.; Antonietti, M. High Definition Conductive Carbon Films from Solution Processing of Nitrogen-Containing Oligomers. Carbon 2015, 94, 1044–1051. [Google Scholar] [CrossRef]
- Hall, H.K.; Padias, A.B. Organic and Polymer Chemistry of Electrophilic Tri- and Tetrasubstituted Ethylenes. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2845–2858. [Google Scholar] [CrossRef]
- Ozimiński, W.P.; Dobrowolski, J.C.; Mazurek, A.P. DFT Studies on Tautomerism of C5-Substituted 1,2,4-Triazoles. J. Mol. Struct. THEOCHEM 2004, 680, 107–115. [Google Scholar] [CrossRef]
- Dines, T.J.; Onoh, H. An Infrared and Resonance Raman Spectroscopic Study of Phenylazonaphthol Pigments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Derradji, M.; Wang, J.; Liu, W. 2—Phthalonitrile Resins’ Properties. In Phthalonitrile Resins and Composites; Derradji, M., Wang, J., Liu, W., Eds.; Plastics Design Library; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 55–106. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Xu, T.; Xie, Z.; Liu, J.; Yu, X.; Ma, S.; Qin, T.; Chen, L. De Novo Design and Facile Synthesis of 2D Covalent Organic Frameworks: A Two-in-One Strategy. J. Am. Chem. Soc. 2019, 141, 13822–13828. [Google Scholar] [CrossRef] [PubMed]
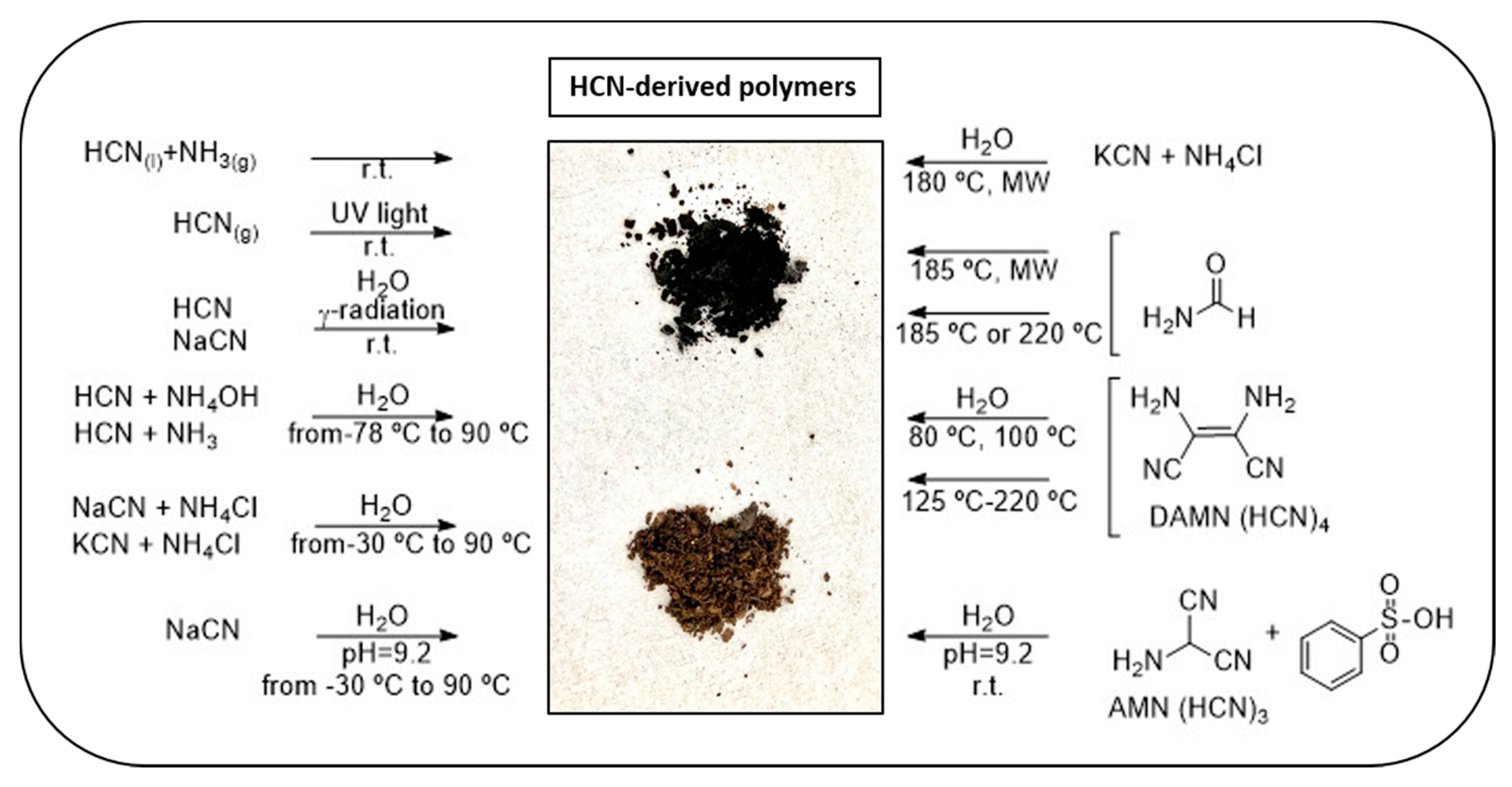
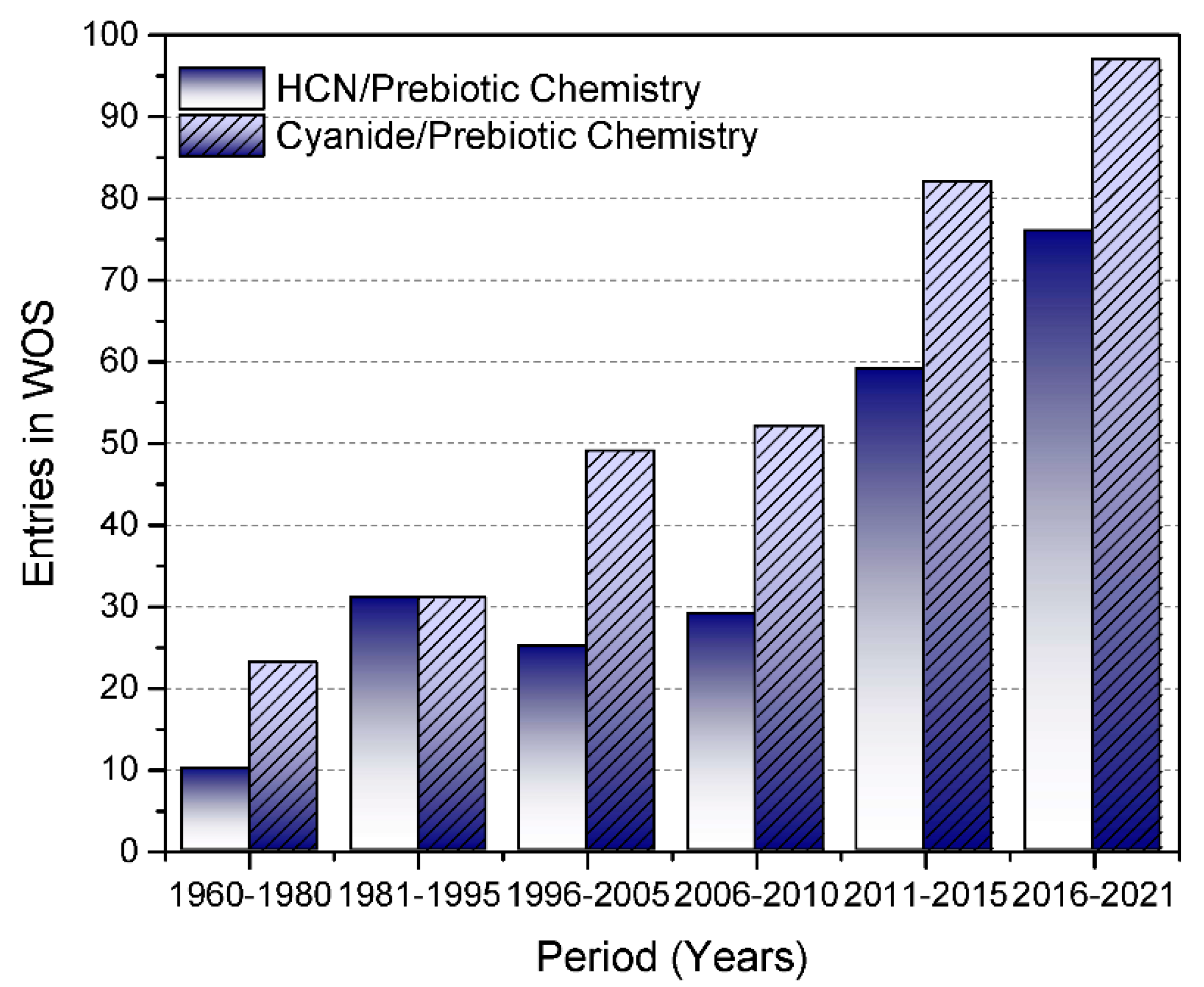
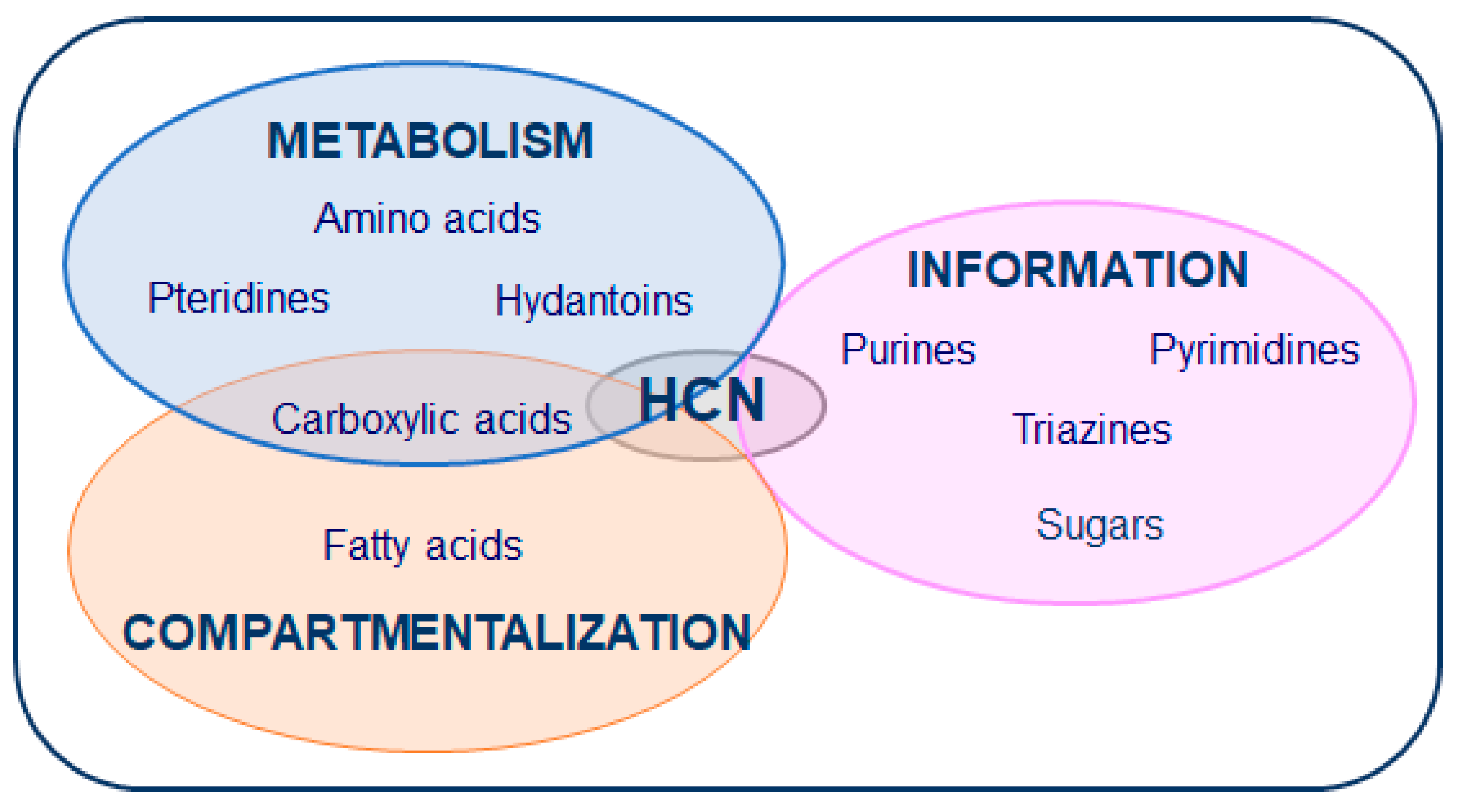
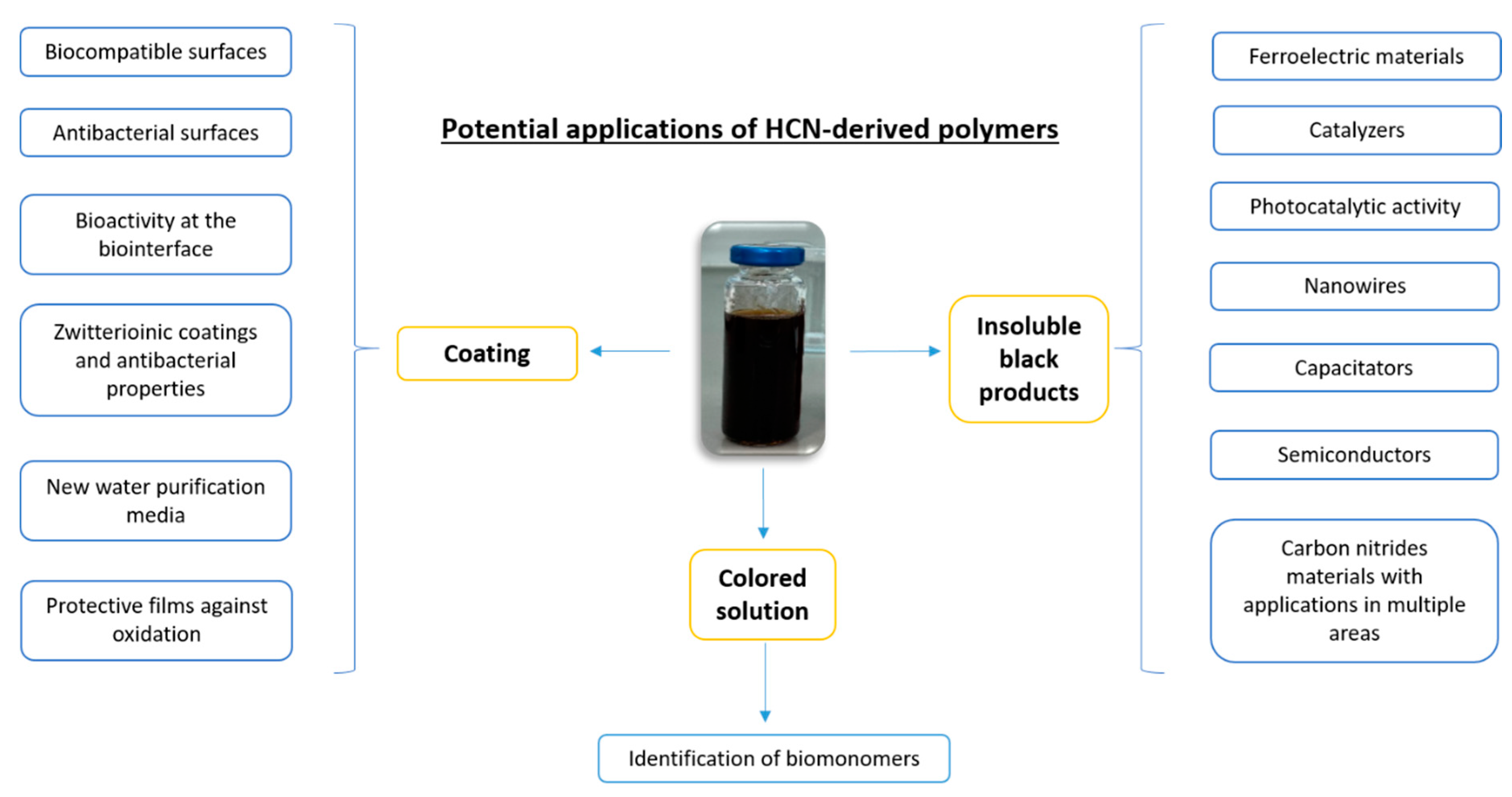

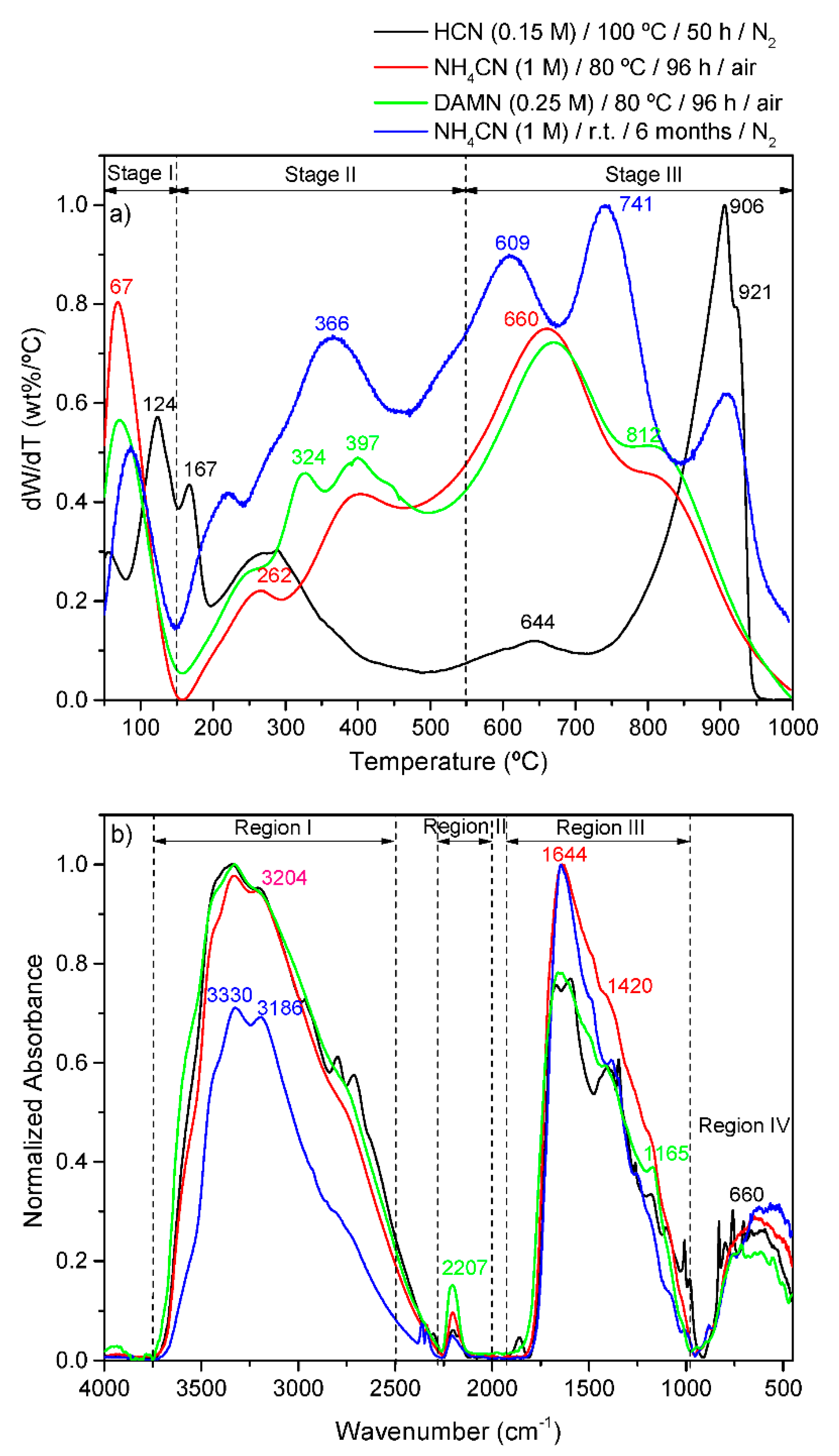
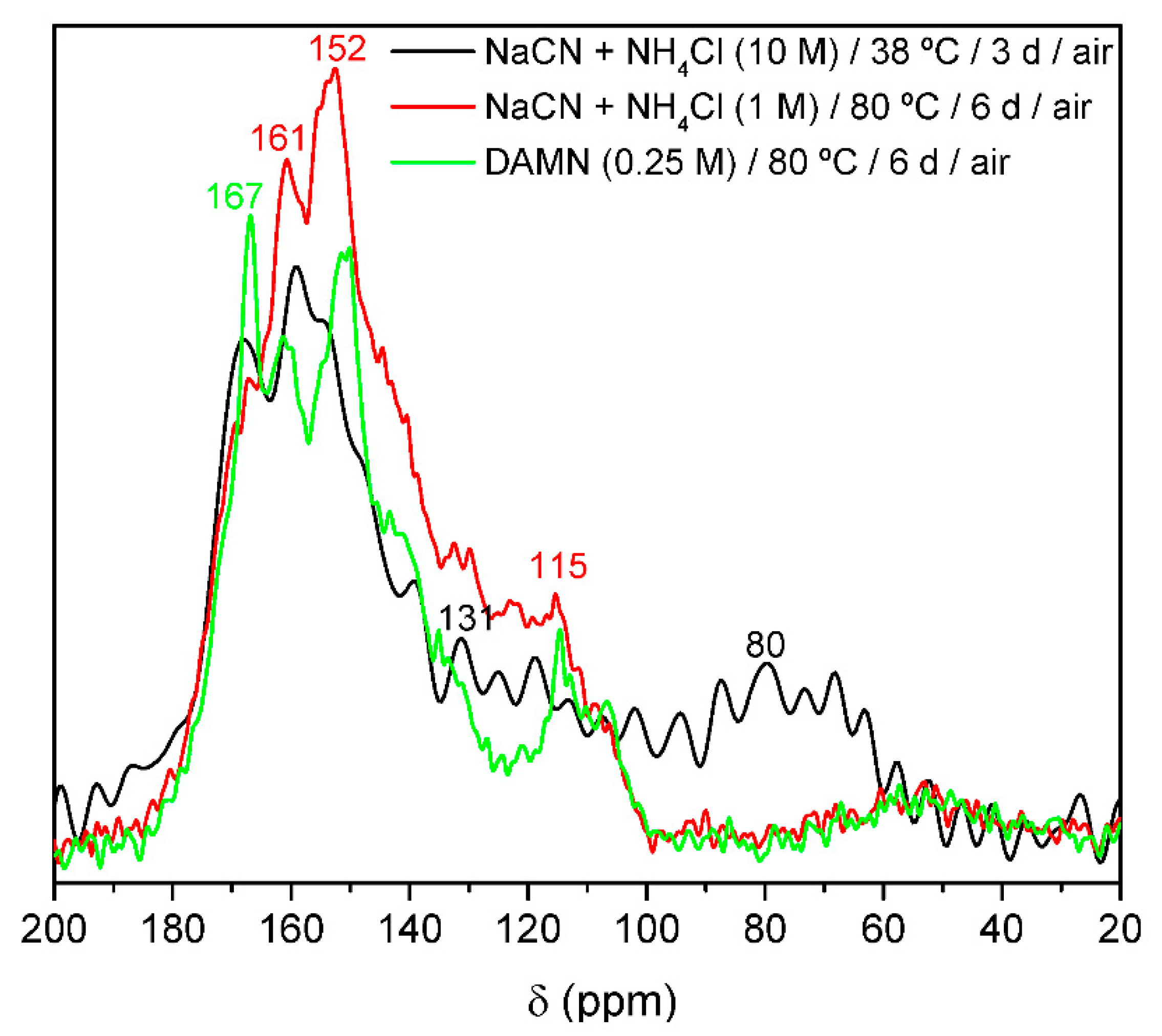
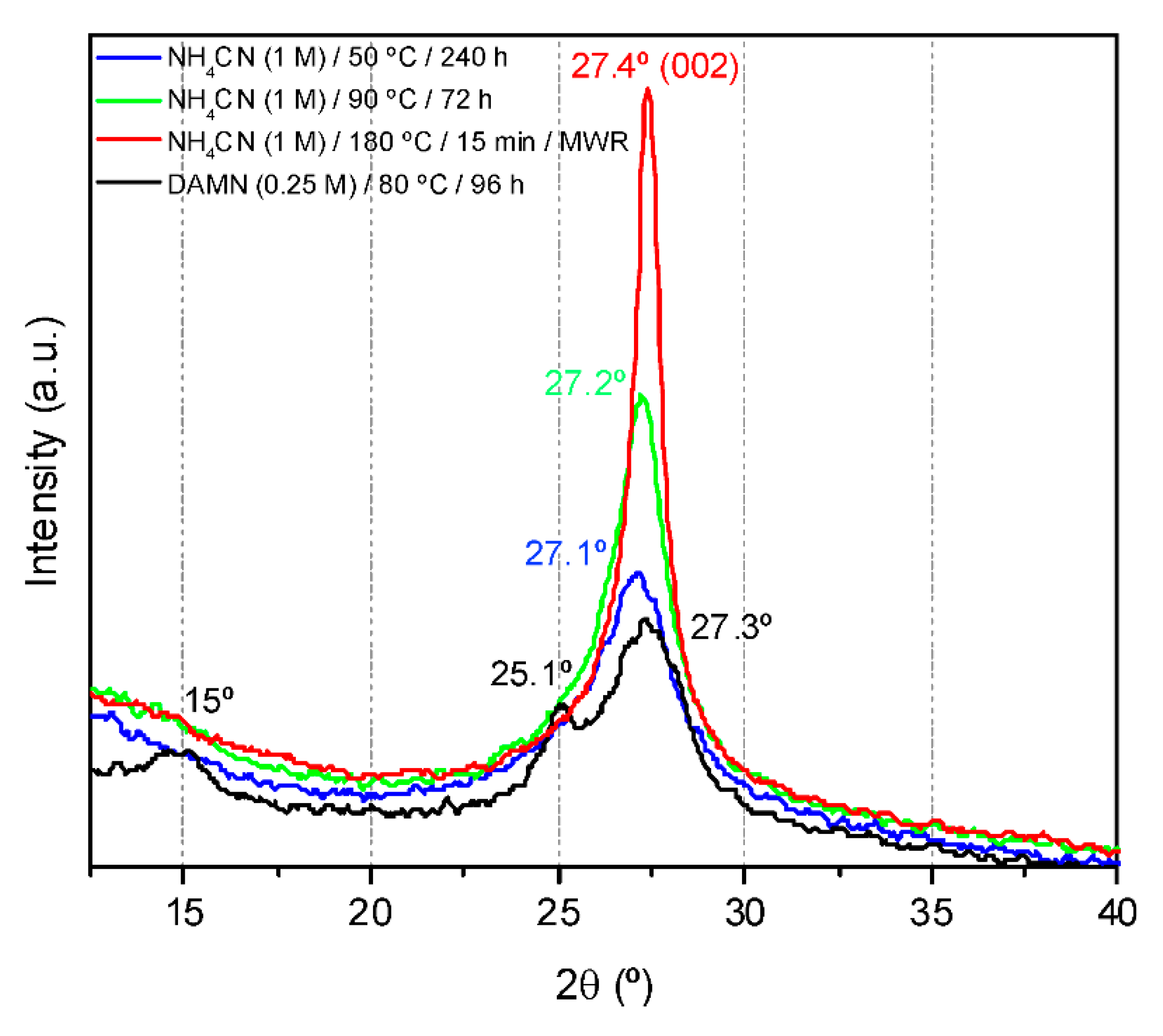
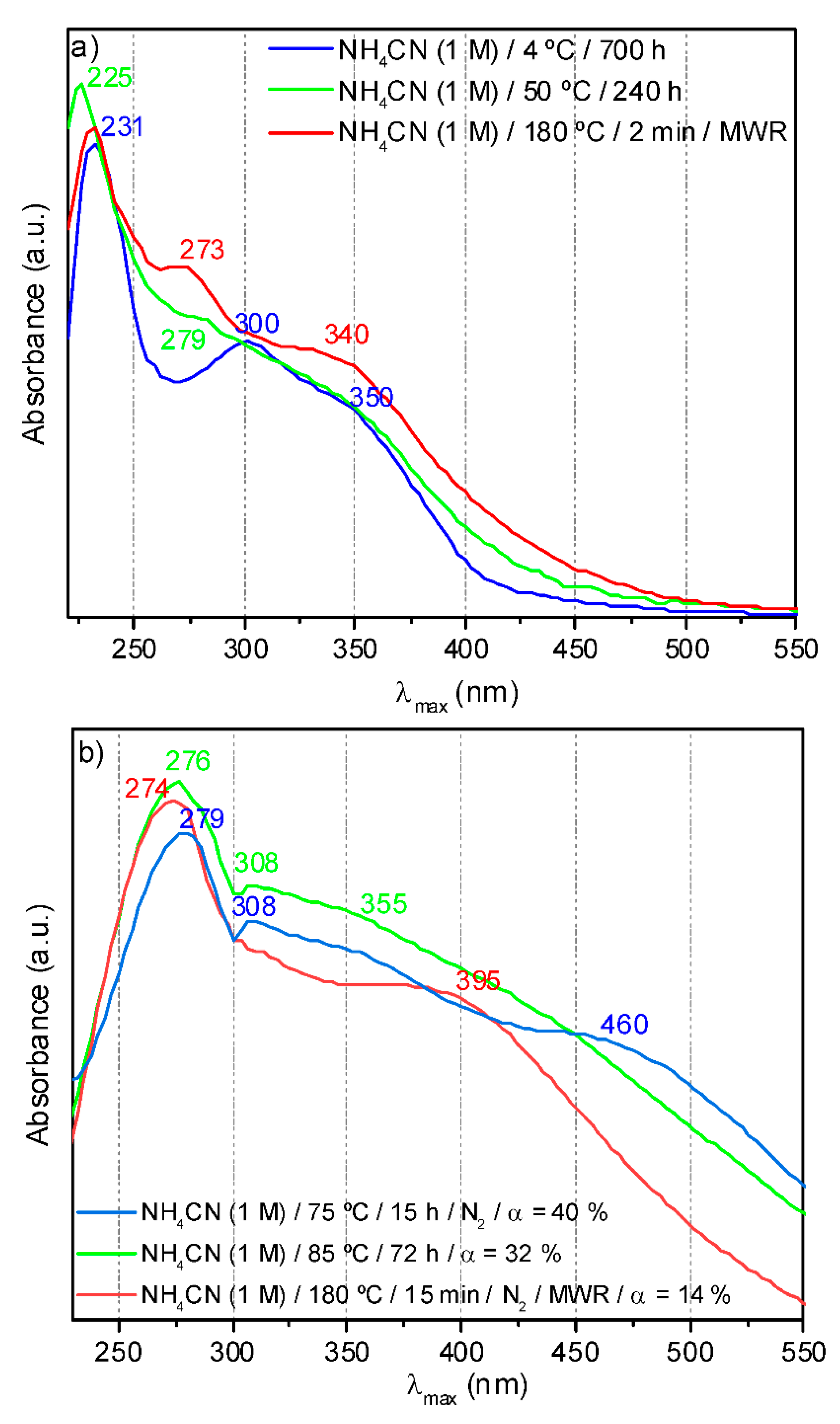
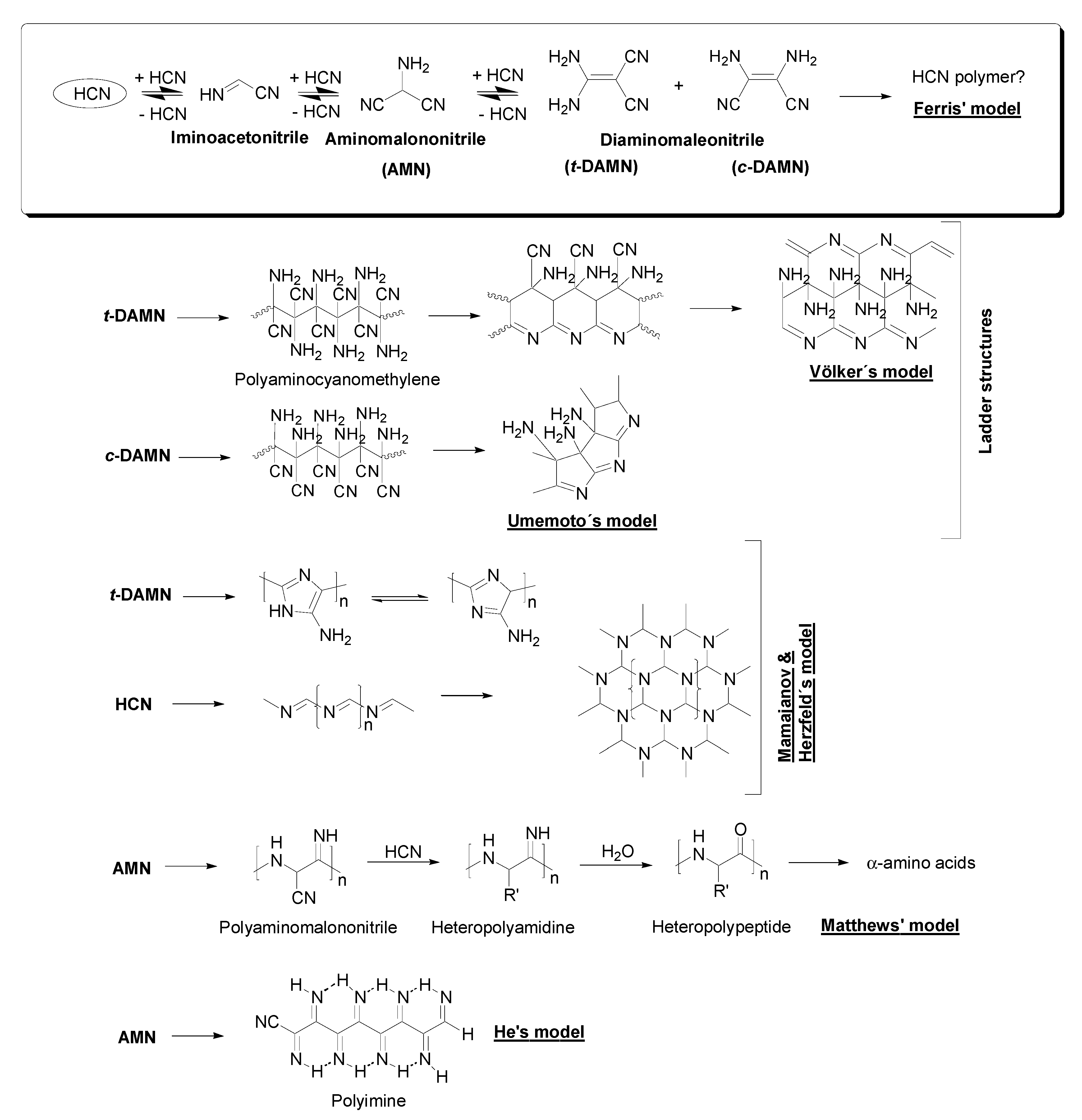
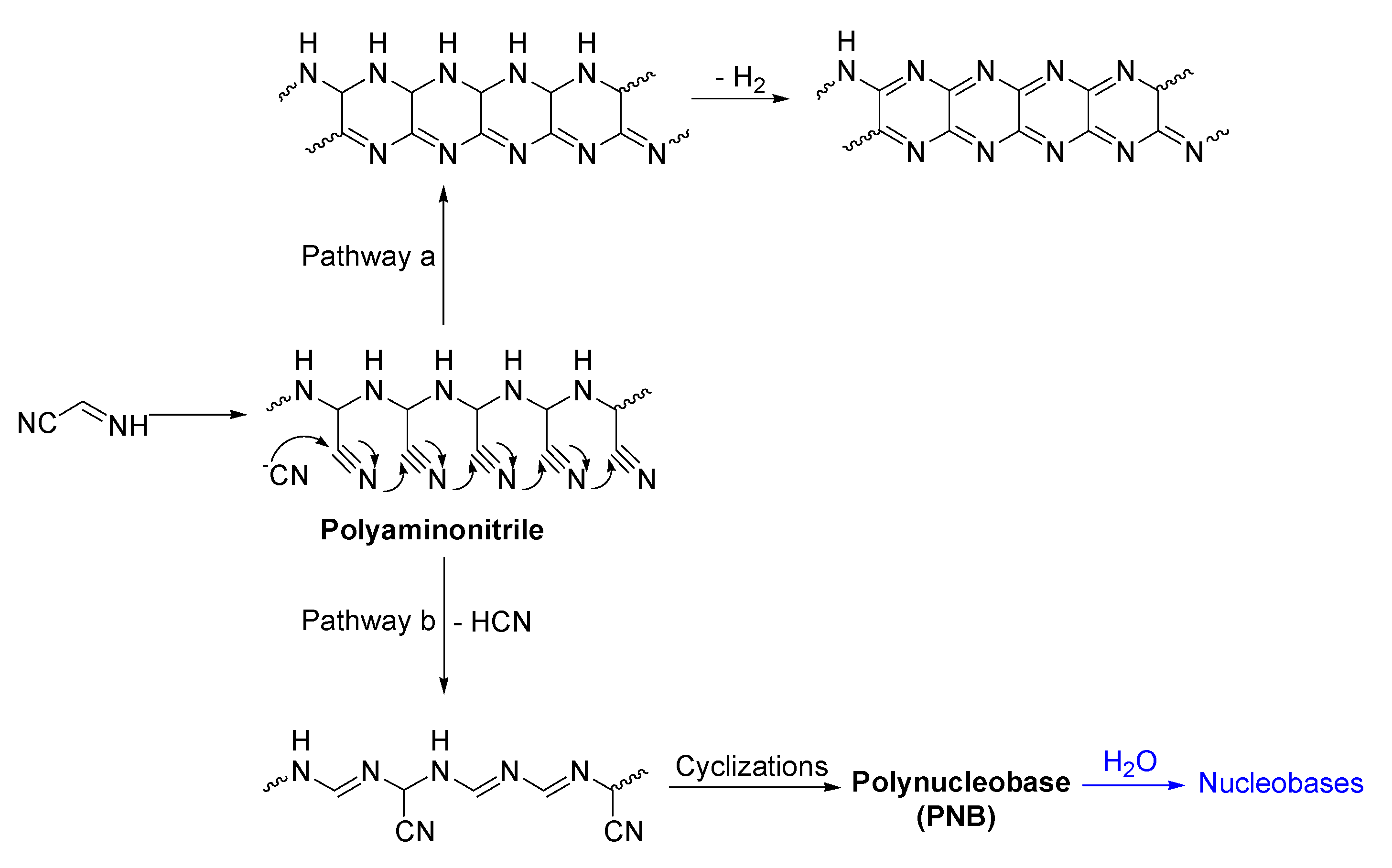

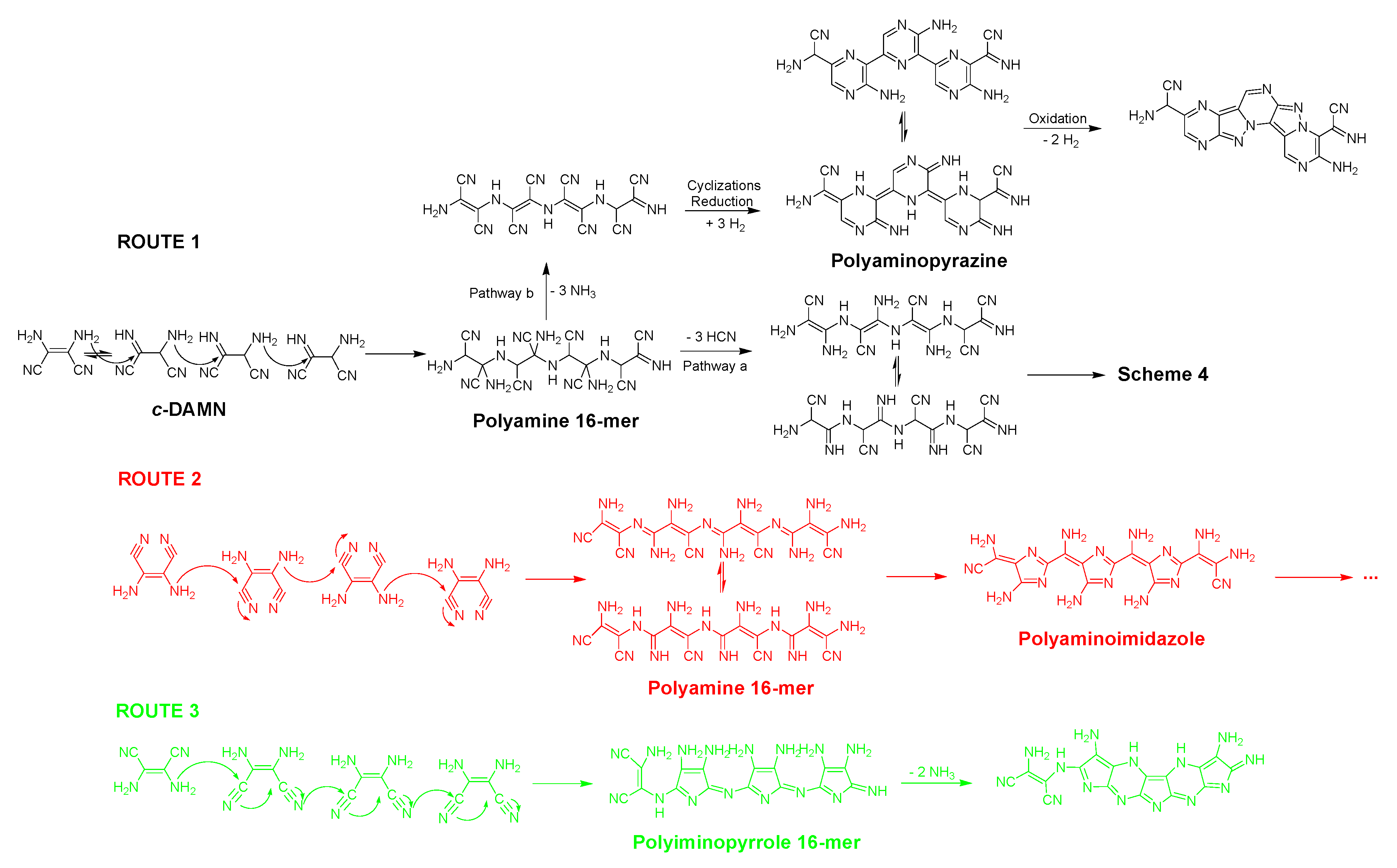
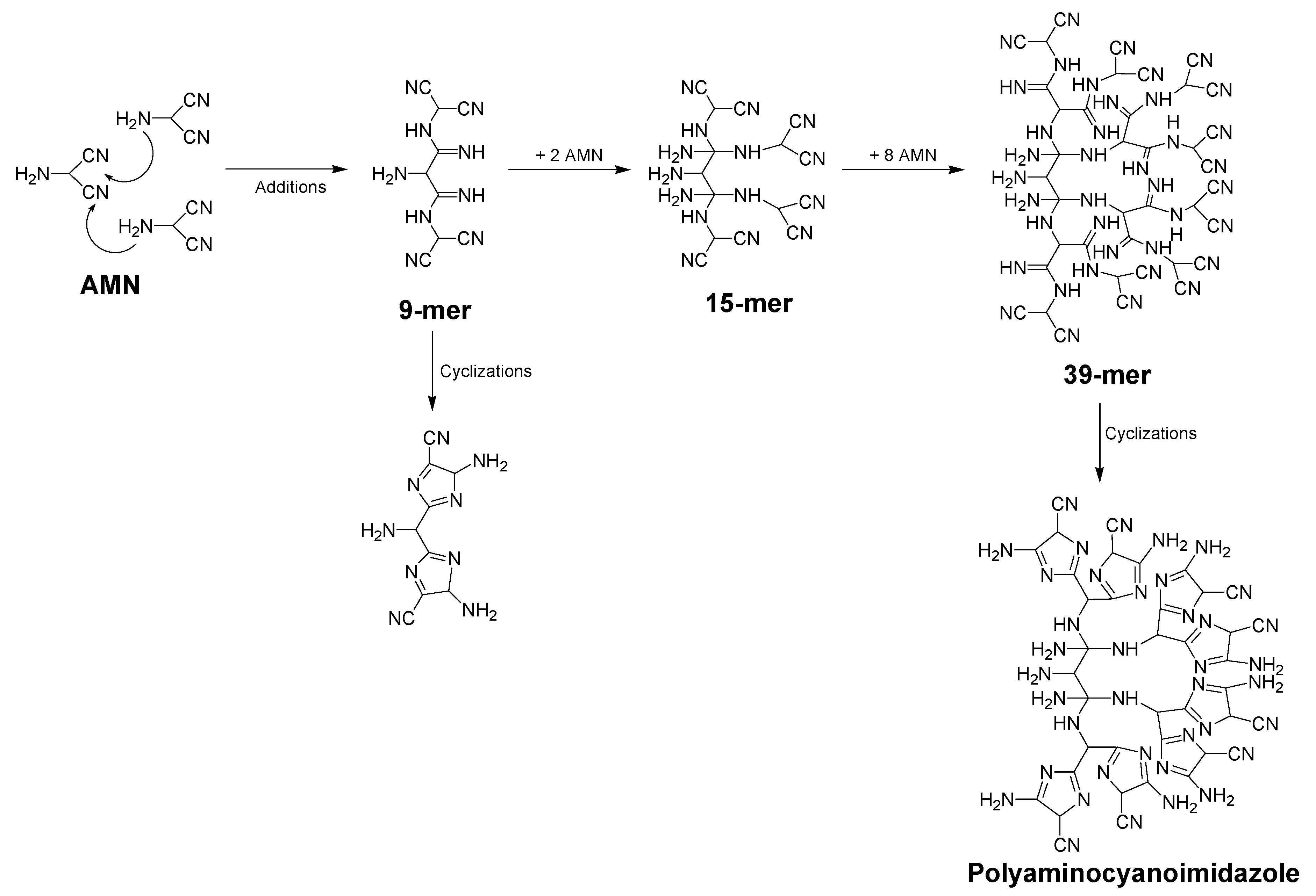
| Entry | Reactants | c (M) | T (°C) | t | Air | C/H | C/N | C/O | N/O | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NaCN + NH4Cl | 1 | 38 | 30 d | + | 0.77 | 1.07 | 2.51 | 2.36 | [139,145] |
| 2 | NaCN + NH4Cl | 1 | 38 | 30 d | - | 0.74 | 1.14 | 2.43 | 2.14 | [135] |
| 3 | NaCN + NH4Cl | 1 | r.t. | 30 d | - | 0.75 | 1.08 | 2.62 | 2.44 | [140] |
| 4 | NaCN + NH4Cl | 1 | 38 | 3 d | + | 0.69 | 1.04 | 2.80 | 2.68 | [141] |
| 5 | NaCN + NH4Cl | 1 | 38 | 10 d | + | 0.69 | 1.02 | 2.83 | 2.78 | [141] |
| 6 | NaCN + NH4Cl | 1 | r.t. | 6 m | - | 0.74 | 1.09 | 1.39 | 1.27 | [140] |
| 7 | NaCN + NH4Cl | 1 | 50 | 10 d | + | 0.82 | 1.13 | 3.54 | 3.14 | [118] |
| 8 | NaCN + NH4Cl | 1 | 60 | 7 d | + | 0.84 | 1.15 | 3.42 | 2.98 | [118] |
| 9 | NaCN + NH4Cl | 1 | 70 | 5 d | + | 0.83 | 1.18 | 3.58 | 3.03 | [118] |
| 10 | NaCN + NH4Cl | 1 | 75 | 7 d | - | 1.29 | 1.21 | 2.66 | 2.19 | [137] |
| 11 | NaCN + NH4Cl | 1 | 90 | 2 d | + | 0.84 | 1.17 | 2.94 | 2.5 | [118] |
| 12 | NaCN + NH4Cl | 1 | 90 | 2 d | - | 1.19 | 1.20 | 3.89 | 3.24 | [137] |
| 13 | NaCN + NH4Cl | 0.5 | 80 | 4 d | + | 1.17 | 1.21 | 3.28 | 2.72 | [117] |
| 14 | NaCN + NH4Cl | 1 | 80 | 4 d | + | 1.39 | 1.20 | 3.91 | 3.25 | [117] |
| 15 | NaCN + NH4Cl | 1 | 80 | 7 d | + | 1.23 | 1.20 | 6.18 | 5.14 | [117] |
| 16 | Diamino-maleo-nitirile (DAMN) | 0.5 | 80 | 4 d | + | 1.28 | 1.22 | 4.42 | 3.62 | [117] |
| 17 | DAMN | 1 | 80 | 4 d | + | 1.29 | 1.18 | 4.37 | 3.71 | [117] |
| 18 | DAMN | 1 | 80 | 7 d | + | 1.31 | 1.18 | 2.56 | 2.17 | [117] |
| 19 | NaCN (pH = 9.2) | 1 | 38 | 30 d | + | 0.77 | 1.16 | 2.00 | 1.72 | [135] |
| 20 | NaCN (pH = 9.2) | 1 | 38 | 30 d | - | 0.77 | 1.19 | 1.96 | 1.64 | [135] |
| 21 | NaCN (pH = 9.2) | 1 | r.t. | 30 d | - | 1.74 | 1.64 | 1.74 | 1.64 | [140] |
| 22 | HCN(g) + H2O (l) | NR | >60 | NR | + | 0.64 | 0.77 | 2.37 | 3.07 | [139] |
| 23 | HCN(l) + NH3(aq) | NR | 0 | 4 d | - | 0.70 | 1.14 | 1.84 | 1.62 | [139] |
| 24 | HCN(g) + NH3(aq) | 12.5 | r.t. | 4 d | - | 0.83 | 1.00 | 5.08 | 5.07 | [148] |
| 25 | HCN(l) + NH3(aq) + NEt3 | NR | r.t. | 3 d | - | NR | 1.07 | 3.89 | 3.62 | [158] |
| 26 | HCN(aq)+ NH4OH | 1 | 90 | 16 h | - | 0.82 | 1.11 | 3.15 | 2.84 | [61] |
| 27 | HCN(aq) + NH3(aq) | NR | 40–50 | 5 h | - | 0.88 | 1.12 | 3.77 | 3.36 | [144] |
| 28 | KCN + NH4Cl | 1–1.5 | 70 | 80 h | + | 0.72 | 1.09 | 3.10 | 2.85 | [163] |
| 29 | HCN (pH < 10) | 0.15 | 100 | 50 h | - | 0.62 | 1.36 | 0.57 | 0.42 | [74] |
| 30 | KCN + NH4Cl | 1 | 180 * | 0.25 h | - | 1.13 | 1.3 | 4.14 | 3.2 | [52] |
| 31 | HCN(l) + NH3 | NR | r.t. | 30 d | - | 0.93 | 0.99 | - | - | [47] |
| 32 | HCN(l) + NH3(g) | 10:1 | r.t. | 51 d | - | 0.84 | 0.96 | - | - | [94,136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Bermejo, M.; de la Fuente, J.L.; Pérez-Fernández, C.; Mateo-Martí, E. A Comprehensive Review of HCN-Derived Polymers. Processes 2021, 9, 597. https://doi.org/10.3390/pr9040597
Ruiz-Bermejo M, de la Fuente JL, Pérez-Fernández C, Mateo-Martí E. A Comprehensive Review of HCN-Derived Polymers. Processes. 2021; 9(4):597. https://doi.org/10.3390/pr9040597
Chicago/Turabian StyleRuiz-Bermejo, Marta, José Luis de la Fuente, Cristina Pérez-Fernández, and Eva Mateo-Martí. 2021. "A Comprehensive Review of HCN-Derived Polymers" Processes 9, no. 4: 597. https://doi.org/10.3390/pr9040597
APA StyleRuiz-Bermejo, M., de la Fuente, J. L., Pérez-Fernández, C., & Mateo-Martí, E. (2021). A Comprehensive Review of HCN-Derived Polymers. Processes, 9(4), 597. https://doi.org/10.3390/pr9040597









