Study of the Physical and Mechanical Properties of Thermoplastic Starch/Poly(Lactic Acid) Blends Modified with Acid Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Blends TPS
2.2. Rheological Analysis
2.3. Fourier Transformed Infrared Spectroscopy (FTIR)
2.4. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
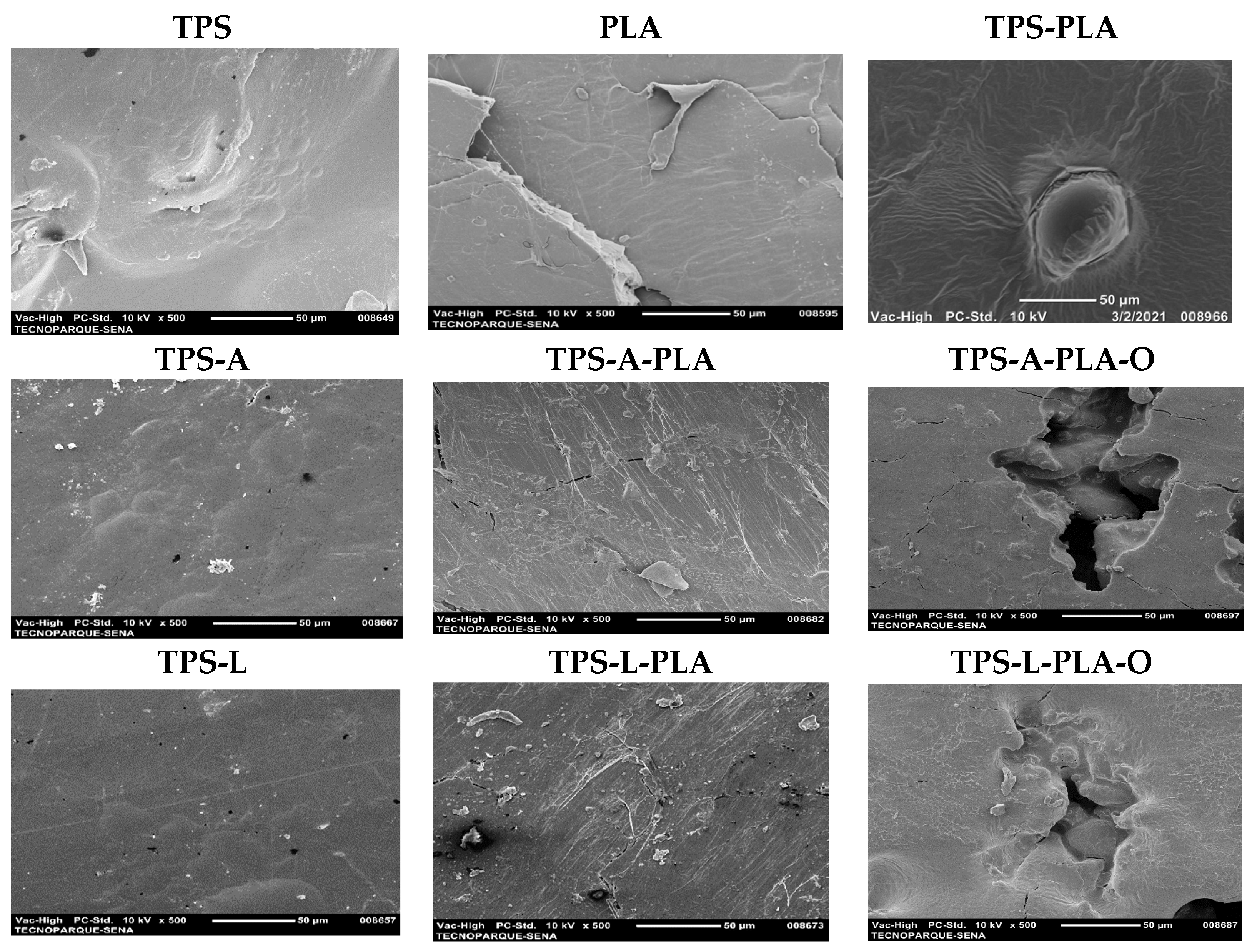
2.5. Scanning Electron Microscopy Analysis
2.6. Contact Angle Analysis
2.7. Tensile Test
3. Results
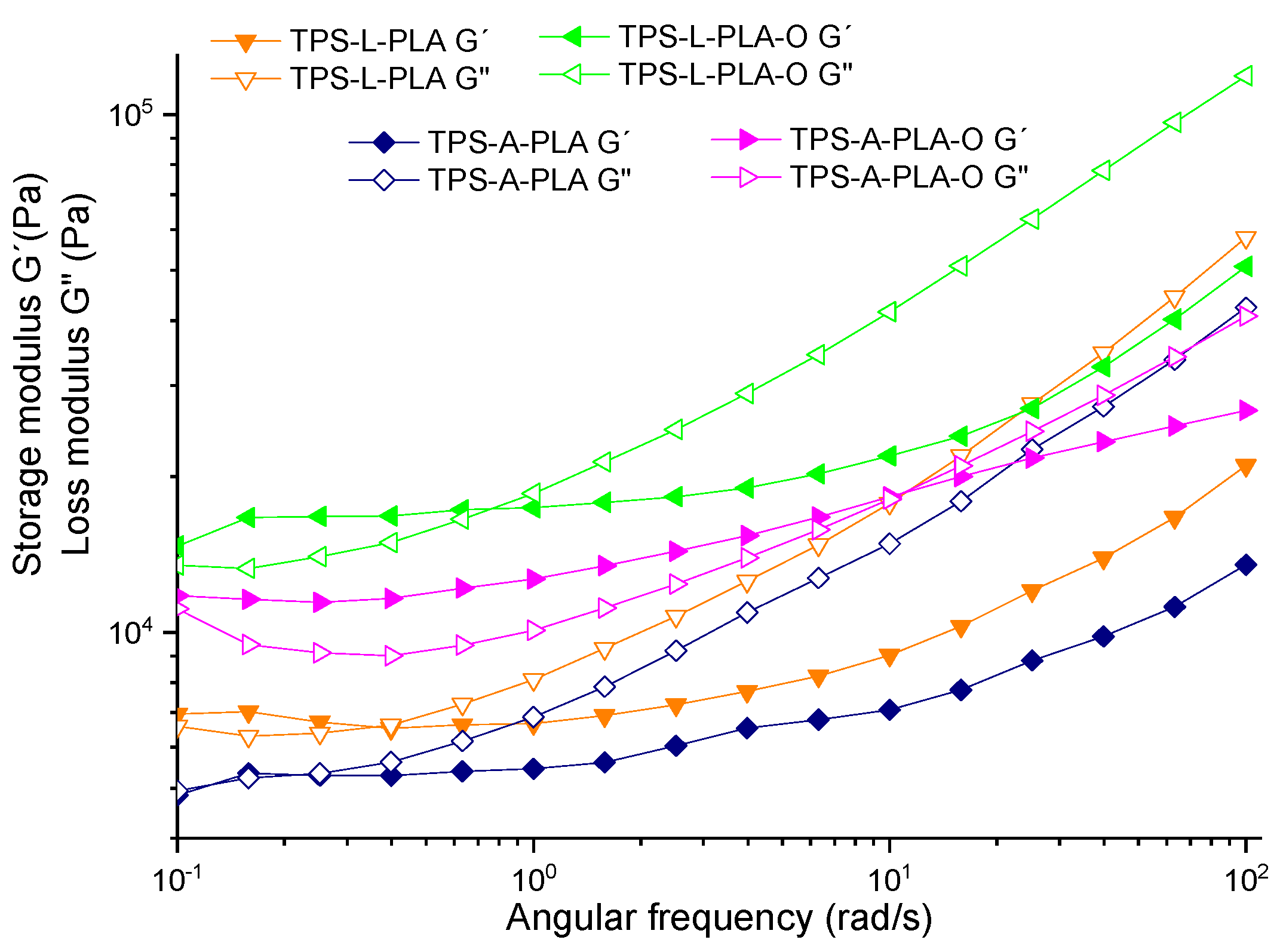
3.1. Rheological Analysis
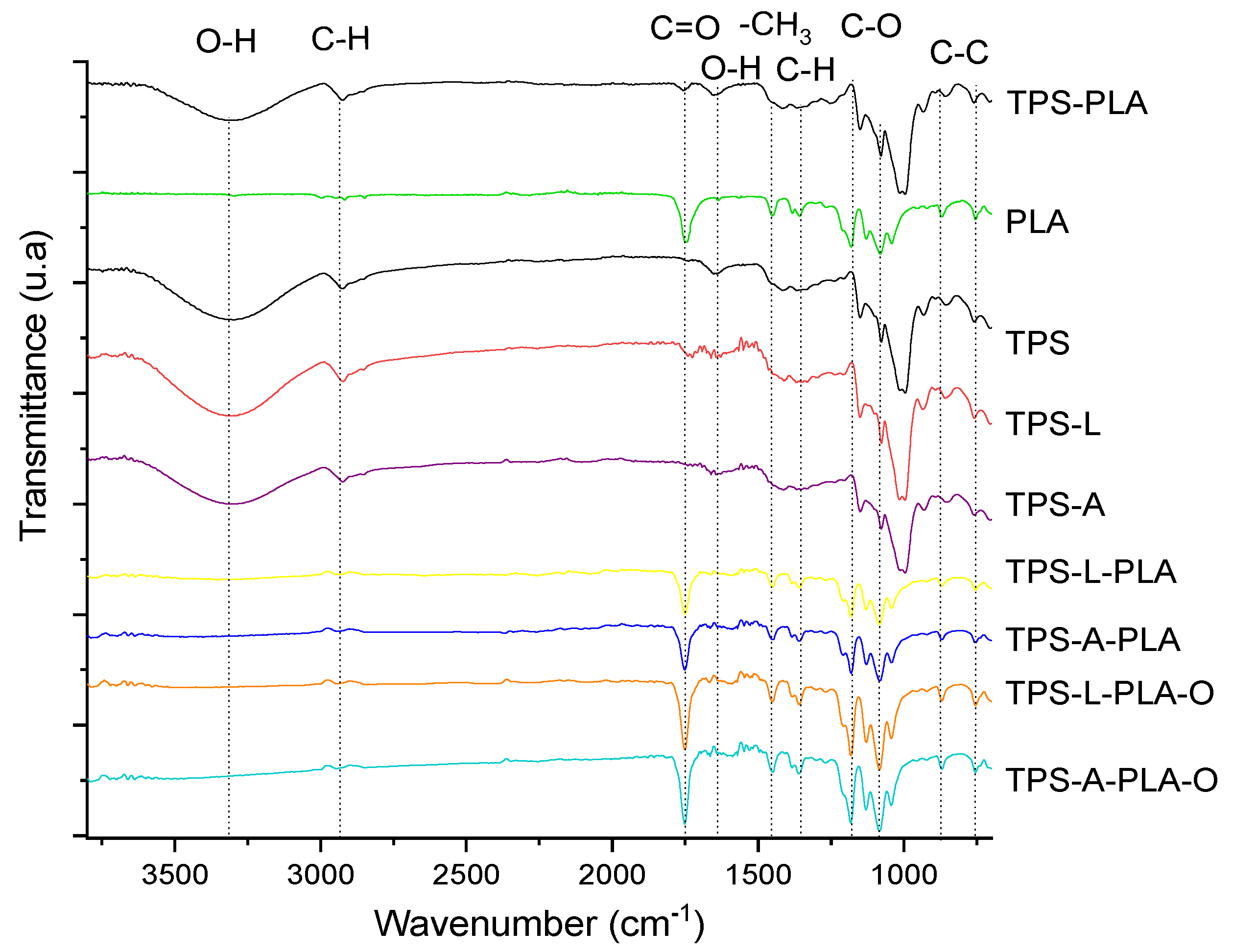
3.2. IR Analysis
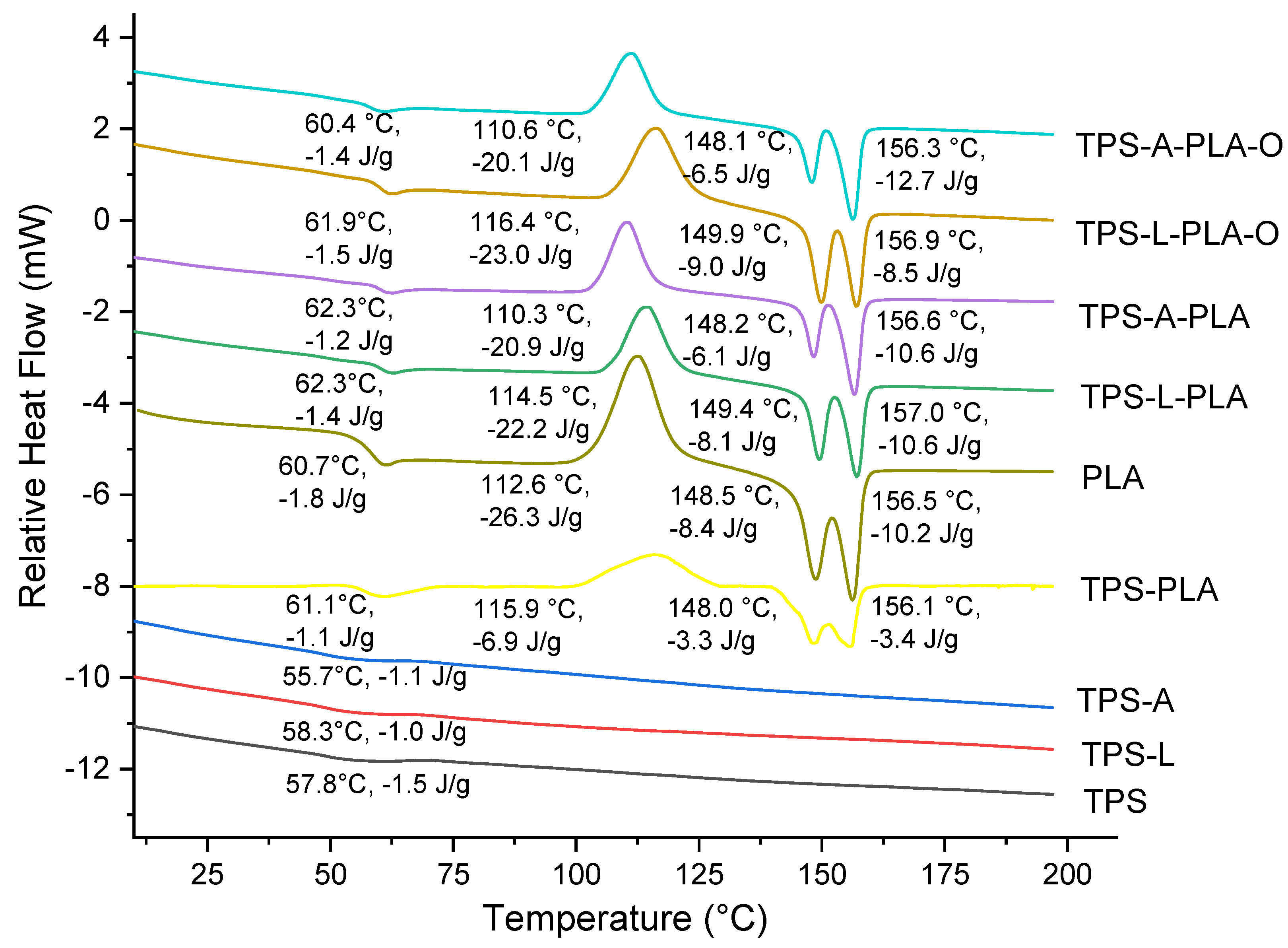
3.3. Thermal Analysis—TGA, DSC
3.4. Contact Angle Analysis
3.5. Tensile Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Acid acetic |
| C | Carbon |
| CP | Cassava pulp |
| DSC | Differential scanning calorimetry |
| DTG | Derivative thermogravimetric |
| ECO | Epoxidized cardoon oil |
| FTIR | Fourier Transformed Infrared Spectroscopy |
| G’ | Modulus storage |
| G” | Modulus loss |
| H | Hydrogen |
| L | Acid lactic |
| MDI | Methylenediphenyl diisocyanate |
| O | Acid oleic |
| O | Oxygen |
| PLA | Poly(lactic acid) |
| SEM | Scanning electron microscope |
| TGA | Thermogravimetric analysis |
| TPS | Thermoplastic starch |
| TPS/PLA | Thermoplastic starch/poly(lactic acid) |
| η´ | Real viscosity component |
| η” | Imaginary viscosity component |
References
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Palai, B.; Biswal, M.; Mohanty, S.; Nayak, S.K. In Situ Reactive Compatibilization of Polylactic Acid (PLA) and Thermoplastic Starch (TPS) Blends; Synthesis and Evaluation of Extrusion Blown Films Thereof. Ind. Crop. Prod. 2019, 141, 111748. [Google Scholar] [CrossRef]
- Przybytek, A.; Sienkiewicz, M.; Kucińska-Lipka, J.; Janik, H. Preparation and Characterization of Biodegradable and Compostable PLA/TPS/ESO Compositions. Ind. Crop. Prod. 2018, 122, 375–383. [Google Scholar] [CrossRef]
- Pierre Sarazin, M.; Baszil, D.F. Polymer Blends Comprising Phase-Encapsulated Thermoplastic Starch and Process for Making the Same. U.S. Patent Application 13/464,209, 8 November 2012. [Google Scholar]
- Andreae, J.; Eric, M.; Arthur, A.; Zweed, K.; Gordon, S. Capsule and Device for Preparing Beverages and Method for Producing Capsules. U.S. Patent Application 15/101,068, 12 January 2017. [Google Scholar]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of Food Waste Streams for the Production of Biopolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef]
- Jullanun, P.; Yoksan, R. Morphological Characteristics and Properties of TPS/PLA/Cassava Pulp Biocomposites. Polym. Test. 2020, 88, 106522. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Composites of Thermoplastic Starch and Nanoclays Produced by Extrusion and Thermopressing. Carbohydr. Polym. 2012, 89, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.A.; López, O.V.; García, M.A.; Barbosa, S.E.; Villar, M.A. Crystalline Morphology of Thermoplastic Starch/Talc Nanocomposites Induced by Thermal Processing. Heliyon 2019, 5, e01877. [Google Scholar] [CrossRef] [PubMed]
- Ayana, B.; Suin, S.; Khatua, B.B. Highly Exfoliated Eco-Friendly Thermoplastic Starch (TPS)/Poly (Lactic Acid)(PLA)/Clay Nanocomposites Using Unmodified Nanoclay. Carbohydr. Polym. 2014, 110, 430–439. [Google Scholar] [CrossRef]
- Amin, M.R.; Chowdhury, M.A.; Kowser, M.A. Characterization and Performance Analysis of Composite Bioplastics Synthesized Using Titanium Dioxide Nanoparticles with Corn Starch. Heliyon 2019, 5, e02009. [Google Scholar] [CrossRef] [PubMed]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving Properties of Thermoplastic Starch Films by Incorporating Active Extracts and Cellulose Fibres Isolated from Rice or Coffee Husk. Food Packag. Shelf Life 2019, 22, 100383. [Google Scholar] [CrossRef]
- Ji, M.; Li, F.; Li, J.; Li, J.; Zhang, C.; Sun, K.; Guo, Z. Enhanced Mechanical Properties, Water Resistance, Thermal Stability, and Biodegradation of the Starch-Sisal Fibre Composites with Various Fillers. Mater. Des. 2021, 198, 109373. [Google Scholar] [CrossRef]
- Wu, N.N.; Qiao, C.C.; Tian, X.H.; Tan, B.; Fang, Y. Retrogradation Inhibition of Rice Starch with Dietary Fiber from Extruded and Unextruded Rice Bran. Food Hydrocoll. 2020, 113, 106488. [Google Scholar] [CrossRef]
- Kahvand, F.; Fasihi, M. Plasticizing and Anti-Plasticizing Effects of Polyvinyl Alcohol in Blend with Thermoplastic Starch. Int. J. Biol. Macromol. 2019, 140, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.; Zhang, Y.; Smeets, N.; Cunningham, M.; Dub, M.A. On the Use of Starch in Emulsion Polymerizations. Processes 2019, 7, 140. [Google Scholar] [CrossRef]
- Caicedo, C.; Aguirre Loredo, R.Y.; Fonseca García, A.; Ossa, O.H.; Vázquez Arce, A.; Calambás Pulgarin, H.L.; Ávila Torres, Y. Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid. Molecules 2019, 24, 4433. [Google Scholar] [CrossRef] [PubMed]
- Ačkar, D.; Babić, J.; Jozinović, A.; Miličević, B.; Jokić, S.; Miličević, R.; Rajič, M.; Šubarić, D. Starch Modification by Organic Acids and Their Derivatives: A Review. Molecules 2015, 20, 19554–19570. [Google Scholar] [CrossRef] [PubMed]
- Tawakaltu, A.-A.; Egwim, E.C.; Ochigbo, S.S.; Ossai, P.C. Effect of Acetic Acid and Citric Acid Modification on Biodegradability of Cassava starch Nanocomposite Films. J. Mater. Sci. Eng. B 2015, 5, 372–379. [Google Scholar] [CrossRef]
- Boruczkowska, H.; Boruczkowski, T.; Leszczyński, W.; Tomaszewska-Ciosk, E.; Miedzianka, J.; Drozdz, W.; Regiec, P. The Obtaining of Starch—and Oleic Acid—Based Ester and Its Properties. Nauka Technol. 2012, 4, 98–107. [Google Scholar]
- Gálvez, J.; Correa Aguirre, J.P.; Hidalgo Salazar, M.A.; Mondragón, B.V.; Wagner, E.; Caicedo, C. Effect of Extrusion Screw Speed and Plasticizer Proportions on the Rheological, Thermal, Mechanical, Morphological and Superficial Properties of PLA. Polymers 2020, 12, 2111. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Kakoli, M.; Davarpanah, A.; Ahmadi, A.; Jahangiri, M.M. Recommendations for Compatibility of Different Types of Polymers with Potassium/Sodium Formate-Based Fluids for Drilling Operations: An Experimental Comparative Analysis. J. Mater. Sci. Eng. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Yu, L.; Petinakis, E.; Dean, K.; Liu, H.; Yuan, Q. Enhancing Compatibilizer Function by Controlled Distribution in Hydrophobic Polylactic Acid/Hydrophilic Starch Blends. J. Appl. Polym. Sci. 2011, 119, 2189–2195. [Google Scholar] [CrossRef]
- Shirai, M.A.; Grossmann, M.V.E.; Mali, S.; Yamashita, F.; Garcia, P.S.; Müller, C.M.O. Development of Biodegradable Flexible Films of Starch and Poly(Lactic Acid) Plasticized with Adipate or Citrate Esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Boix, A.C.; Malinconico, M.; Rippa, M.; Di Serio, M.; Santagata, G. Poly (Lactic Acid)/Thermoplastic Starch Films: Effect of Cardoon Seed Epoxidized Oil on Their Chemicophysical, Mechanical, and Barrier Properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef]
- Fourati, Y.; Tarrés, Q.; Mutjé, P.; Boufi, S. PBAT/Thermoplastic Starch Blends: Effect of Compatibilizers on the Rheological, Mechanical and Morphological Properties. Carbohydr. Polym. 2018, 199, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Sánchez, M.; Campo-Erazo, S.D.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. Structural Changes of Cassava Starch and Polylactic Acid Films Submitted to Biodegradation Process. Int. J. Biol. Macromol. 2019, 129, 442–447. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Alex, L. Epoxidised Sesame Oil as a Biobased Coupling Agent and Plasticiser in Polylactic Acid/Thermoplastic Yam Starch Blends. Heliyon 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Yi, T.; Qi, M.; Mo, Q.; Huang, L.; Zhao, H.; Liu, D.; Xu, H. Ecofriendly Preparation and Characterization of a Cassava Starch/Polybutylene Adipate. Processes 2020, 8, 329. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, Y.; Tian, J. Study on the Biodegradability of Modified Starch/Polylactic Acid (PLA) Composite Materials. RSC Adv. 2020, 10, 26298–26307. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wahab, M.K.A.; Uylan, D.N.; Ismail, H. Physical and Degradation Properties of Polylactic Acid and Thermoplastic Starch Blends—Effect of Citric Acid Treatment on Starch Structures. BioResources 2017, 12, 3076–3087. [Google Scholar] [CrossRef]





| Sample | Starch (wt.%) | Sorbitol (wt.%) | Acid Agent (wt.%) | PLA (wt.%) | ||
|---|---|---|---|---|---|---|
| Lactic Acid | Acetic Acid | Oleic Acid | ||||
| TPS | 70 | 30 | - | - | - | - |
| TPS-L | 70 | 24 | 6 | - | - | - |
| TPS-A | 70 | 24 | - | 6 | - | - |
| PLA | - | - | - | - | - | 100 |
| TPS-PLA | 49 | 21 | 30 | |||
| TPS-L-PLA | 47.5 | 18.5 | 4 | - | - | 30 |
| TPS-A-PLA | 47.5 | 18.5 | - | 4 | - | 30 |
| TPS-L-PLA-O | 47.5 | 14.5 | 4 | - | 4 | 30 |
| TPS-A-PLA-O | 47.5 | 14.5 | - | 4 | 4 | 30 |
| Sample | Tensile Strength (MPa) * | Young Modulus (MPa) * | Deformation at Break (%) * |
|---|---|---|---|
| TPS | 5.40 ± 0.86 a | 77.00 ± 15.00 a | 51.00 ± 3.00 a |
| TPS-L | 6.10 ± 0.62 a | 509.12 ± 24.09 b | 7.49 ± 0.81 b |
| TPS-A | 5.75 ± 0.59 a | 498.06 ± 30.21 b | 4.49 ± 0.59 c |
| PLA | 64.00 ± 2.70 | 3198.00 ± 132.00 | 2.50 ± 0.10 |
| TPS-PLA | 5.60 ± 0.90 A | 135.71 ± 14.08 A | 2.62 ± 0.59 A |
| TPS-L-PLA | 7.23 ± 1.40 B | 1801.81 ± 22.15 B | 2.70 ± 0.11 A |
| TPS-A-PLA | 6.49 ± 0.60 A,B | 1456.55 ± 41.81 C | 2.81 ± 0.27 A |
| TPS-L-PLA-O | 3.68 ± 0.69 D | 1502.32 ± 40.24 D | 0.59 ± 0.17 B |
| TPS-A-PLA-O | 2.92 ± 0.25 D | 1114.27 ± 52.57 E | 0.55 ± 0.17 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, C.; Pulgarin, H.L.C. Study of the Physical and Mechanical Properties of Thermoplastic Starch/Poly(Lactic Acid) Blends Modified with Acid Agents. Processes 2021, 9, 578. https://doi.org/10.3390/pr9040578
Caicedo C, Pulgarin HLC. Study of the Physical and Mechanical Properties of Thermoplastic Starch/Poly(Lactic Acid) Blends Modified with Acid Agents. Processes. 2021; 9(4):578. https://doi.org/10.3390/pr9040578
Chicago/Turabian StyleCaicedo, Carolina, and Heidy Lorena Calambás Pulgarin. 2021. "Study of the Physical and Mechanical Properties of Thermoplastic Starch/Poly(Lactic Acid) Blends Modified with Acid Agents" Processes 9, no. 4: 578. https://doi.org/10.3390/pr9040578
APA StyleCaicedo, C., & Pulgarin, H. L. C. (2021). Study of the Physical and Mechanical Properties of Thermoplastic Starch/Poly(Lactic Acid) Blends Modified with Acid Agents. Processes, 9(4), 578. https://doi.org/10.3390/pr9040578






