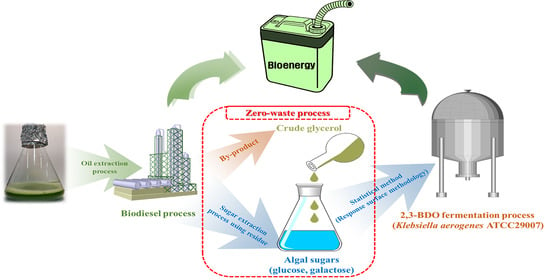
Development of 2,3-Butanediol Production Process from Klebsiella aerogenes ATCC 29007 Using Extracted Sugars of Chlorella pyrenoidosa and Biodiesel-Derived Crude Glycerol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
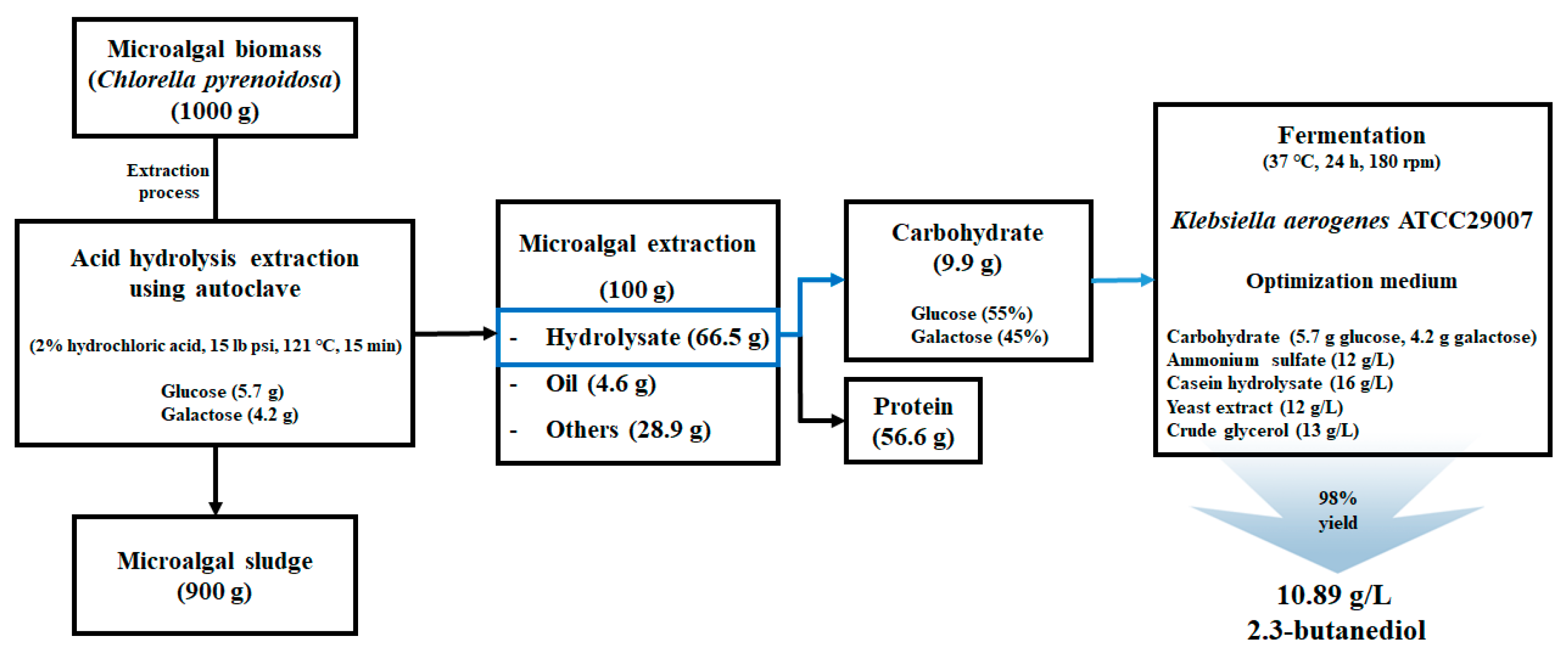
2.2. Sugar Extraction of Chlorella pyrenoidosa Using Hydrochloric Acid
2.3. Microorganism and Culture Conditions
2.4. Experimental Design and Statistical Analysis
2.5. Analytical Methods
3. Results and Discussion
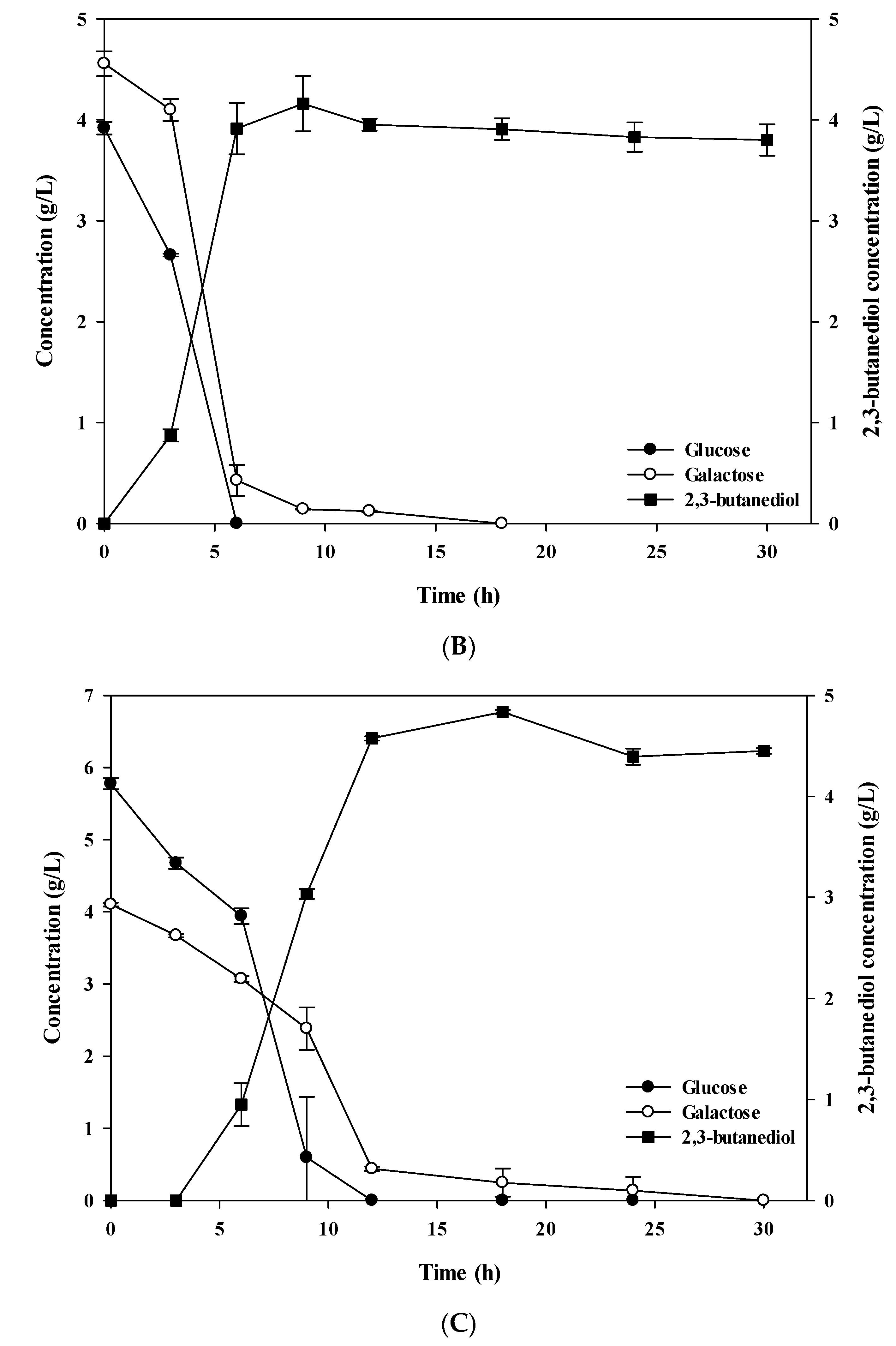
3.1. Comparison of Chemical Sugars and Extracted Algal Sugars for 2,3-BDO Production
3.2. Improvement of 2,3-BDO Production Using Extracted Algal Sugars and Biodiesel-Derived Crude Glycerol
3.3. Statistical Optimization of Medium Components to Improve 2,3-BDO Production from K. aerogenes ATCC 29007
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nwachukwu, R.E.S.; Shahbazi, A.; Wang, L.; Ibrahim, S.; Worku, M.; Schimmel, K. Bioconversion of glycerol to ethanol by a mutant Enterobacter aerogenes. AMB Express 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, X.; Liu, D. A novel route for the flexible preparation of hydrocarbon jet fuels from biomass-based platform chemicals: A case of using furfural and 2,3-butanediol as feedstocks. Green Chem. 2018, 20, 2018–2026. [Google Scholar] [CrossRef]
- Haider, J.; Harvianto, G.R.; Qyyum, M.A.; Lee, M. Cost-and energy-efficient butanol-based extraction-assisted distillation designs for purification of 2,3-butanediol for use as a drop-in fuel. ACS Sustain. Chem. Eng. 2018, 6, 14901–14910. [Google Scholar] [CrossRef]
- Mercado, I.; Alvarez, X.; Verduga, M.E.; Cruz, A. Enhancement of biomass and lipid productivities of scenedesmus sp. cultivated in the wastewater of the dairy industry. Processes 2020, 8, 1458. [Google Scholar] [CrossRef]
- Lee, K.H.; Chun, Y.; Jang, Y.W.; Lee, S.W.; Kim, H.R.; Lee, J.H.; Kim, S.W.; Park, C.; Yoo, H.Y. Fabrication of functional bioelastomer for food packaging from aronia (Aronia melanocarpa) juice processing by-products. Foods 2020, 28, 1565. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Choi, H.S.; Lee, S.J.; Lee, J.H.; Lee, J.H.; Yoo, H.Y.; Han, S.O.; Kim, S.W. Production of xylanase from a novel engineered Pichia pastoris and application to enzymatic hydrolysis process for biorefinery. Process. Biochem. 2018, 65, 130–135. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, J.H.; Lee, S.K.; Chun, Y.; Park, C.; Jin, J.H.; Lee, H.U.; Kim, S.W. Fabricating a modified biochar-based all-solid-state flexible microsupercapacitor using pen lithography. J. Clean Prod. 2021, 284, 125449–125457. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.H.; Yang, X.; Kim, S.B.; Lee, J.H.; Yoo, H.Y.; Park, C.; Kim, S.W. Phenolic compounds: Strong inhibitors derived from lignocellulosic hydrolysate for 2,3-butanediol production by Enterobacter aerogenes. Biotechnol. J. 2015, 10, 1920–1928. [Google Scholar] [CrossRef]
- Regmi, S.; Yoo, H.Y.; Choi, Y.H.; Choi, Y.S.; Yoo, J.C.; Kim, S.W. Prospects for bio-industrial application of an extremely alkaline mannanase from Bacillus subtilis subsp. inaquosorum CSB31. Biotechnol. J. 2017, 12, 1700113–1700120. [Google Scholar] [CrossRef] [PubMed]
- Hazeena, S.H.; Salini, C.N.; Sindhu, R.; Pandey, A.; Binod, P. Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour. Technol. 2019, 278, 145–149. [Google Scholar] [CrossRef]
- Joo, J.; Lee, S.J.; Yoo, H.Y.; Kim, Y.; Jang, M.; Lee, J.; Han, S.O. Improved fermentation of lignocellulosic hydrolysates to 2,3-butanediol through investigation of effects of inhibitory compounds by Enterobacter aerogenes. Chem. Eng. J. 2016, 306, 916–924. [Google Scholar] [CrossRef]
- Correa, P.S.; Morais Junior, W.G.; Martin, A.A.; Caetano, N.S.; Mata, T.M. Microalgae biomolecules: Extraction, separation and purification methods. Processes 2021, 9, 10. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.U.; Lee, J.H.; Lee, S.K.; Yoo, H.Y.; Park, C.; Kim, S.W. Continuous production of bioethanol using microalgal sugars extracted from Nannochloropsis gaditana. Korean J. Chem. Eng. 2019, 36, 71–76. [Google Scholar] [CrossRef]
- Park, C.; Lee, J.H.; Yang, X.; Yoo, H.Y.; Lee, J.H.; Lee, S.K.; Kim, S.W. Enhancement of hydrolysis of Chlorella vulgaris by hydrochloric acid. Bioprocess. Biosyst. Eng. 2016, 39, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.S.; Yang, J.H.; Yoo, H.Y.; Han, S.O.; Lee, J.; Park, C.; Kim, S.W. The potential of waste microalgal hydrolysate for power generation in enzymatic fuel cell. J. Clean Prod. 2018, 187, 903–909. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.S.; Yang, J.H.; Chun, Y.; Yoo, H.Y.; Han, S.O.; Lee, J.; Park, C.; Kim, S.W. Enhanced electron transfer mediator based on biochar from microalgal sludge for application to bioelectrochemical systems. Bioresour. Technol. 2018, 264, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Kim, D.S.; Yoo, H.Y.; Park, C.; Kim, S.W. Biodiesel production by lipases co-immobilized on the functionalized activated carbon. Bioresour. Technol. Rep. 2019, 7, 100248–100255. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.H.; Yang, X.; Yoo, H.Y.; Han, S.O.; Park, C.; Kim, S.W. Re-utilization of waste glycerol for continuous production of bioethanol by immobilized Enterobacter aerogenes. J. Clean Prod. 2017, 161, 757–764. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Wu, Y.X.; Wang, X.B.; Zheng, A.Q.; Zhao, Z.L.; Li, H.B.; Feng, X.J. Crude glycerol pretreatment for selective saccharifiacation of lignocellulose via fast pyrolysis and enzyme hydrolysis. Energy Conv. Manag. 2019, 199, 111894–111900. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, H.S.; Kim, C.K.; Thapa, L.P.; Park, C.; Kim, S.W. Process strategy for 2,3-butanediol production in fed-batch culture by acetate addition. J. Ind. Eng. Chem. 2017, 56, 157–162. [Google Scholar] [CrossRef]
- Chanthoom, K.; Tanikkul, P.; Sirisukpoka, U.; Pisutpaisal, N. Ethanol production form biodiesel-derived crude glycerol by enterobacter aerogenes. Chem. Eng. Trans. 2016, 50, 211–216. [Google Scholar]
- Ciliverti, C.; Biundo, A.; Albergo, R.; Agrimi, G.; Braccio, G.; Bari, I.; Pisano, I. Syngas derived from lignocellulosic biomass gasification as an alternative resource for innovative bioprocesses. Processes 2020, 8, 1567. [Google Scholar] [CrossRef]
- Harvey, B.G.; Merriman, W.W.; Quintana, R.L. Renewable gasoline, solvents, and fuel additives from 2,3-butanediol. ChemSusChem 2016, 9, 1814–1819. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.R.; Lee, J.H.; Lee, S.K.; Chun, Y.; Han, S.O.; Yoo, H.Y.; Park, C.; Kim, S.W. Enhanced in-vitro hemozoin polymerization by optimized process using histidine-rich protein II (HRPII). Polymers 2019, 11, 1162. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, H.Y.; Lee, S.K.; Chun, Y.; Kim, H.R.; Bankeeree, W.; Lotrakul, P.; Punnapayak, H.; Prasongsuk, S.; Kim, S.W. Significant impact of casein hydrolysate to overcome the low consumption of glycerol by Klebsiella aerogenes ATCC 29007 and its application to bioethanol production. Energy Conv. Manag. 2020, 221, 113181–113190. [Google Scholar] [CrossRef]
- Priya, A.; Lal, B. Efficient valorization of waste glycerol to 2,3-buatanediol using Enterobacter cloacae TERI BD 18 as a biocatalyst. Fuel 2019, 250, 292–305. [Google Scholar] [CrossRef]
- Petrova, P.; Petlichka, S.; Petrov, K. New Bacillus spp. with potential for 2,3-butanediol production from biomass. J. Biosci. Bioeng. 2020, 130, 20–28. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.H.; Kim, H.R.; Chun, Y.; Lee, J.H.; Yoo, H.Y.; Park, C.; Kim, S.W. Improved cordycepin production by Cordyceps militaris KYL05 using casein hydrolysate in submerged conditions. Biomolecules 2019, 9, 461. [Google Scholar] [CrossRef]
- Lee, S.J.; Thapa, L.P.; Lee, J.H.; Choi, H.S.; Kim, S.B.; Park, C.; Kim, S.W. Stimulation of 2,3-butanediol production by upregulation of alsR gene transcription level with acetate addition in Enterobacter aerogenes ATCC 29007. Process. Biochem. 2016, 51, 1904–1910. [Google Scholar] [CrossRef]
- Maina, S.; Stylianou, E.; Vogiatzi, E.; Vlysidis, A.; Mallouchos, A.; Nychas, G.J.E.; Castro, A.M.; Dheskali, E.; Kookos, I.K.; Koutinas, A. Improvement on bioprocess economics for 2,3-butanediol production from very high polarity cane sugar via oprimisation of bioreactor operation. Bioresour. Technol. 2019, 274, 343–352. [Google Scholar] [CrossRef]
- Stafford, W.; Lange, W.D.; Nahman, A.; Chunilall, V.; Lekha, P.; Andrew, J.; Johakimu, J.; Sithole, B.; Trotter, D. Forestry biorefineries. Renew. Energy 2020, 154, 461–475. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Sindhu, R.; Pandey, A.; Binod, P. Lignocellulosic bio-refinery approach for microbial 2,3-butanediol production. Bioresour. Technol. 2020, 302, 122873–122880. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Dureja, P.; Rathi, R.; Lal, B. Comparative assessment of separation techniques for downstream processiong of 2,3-butanediol. Fuel 2021, 292, 120351–120358. [Google Scholar] [CrossRef]



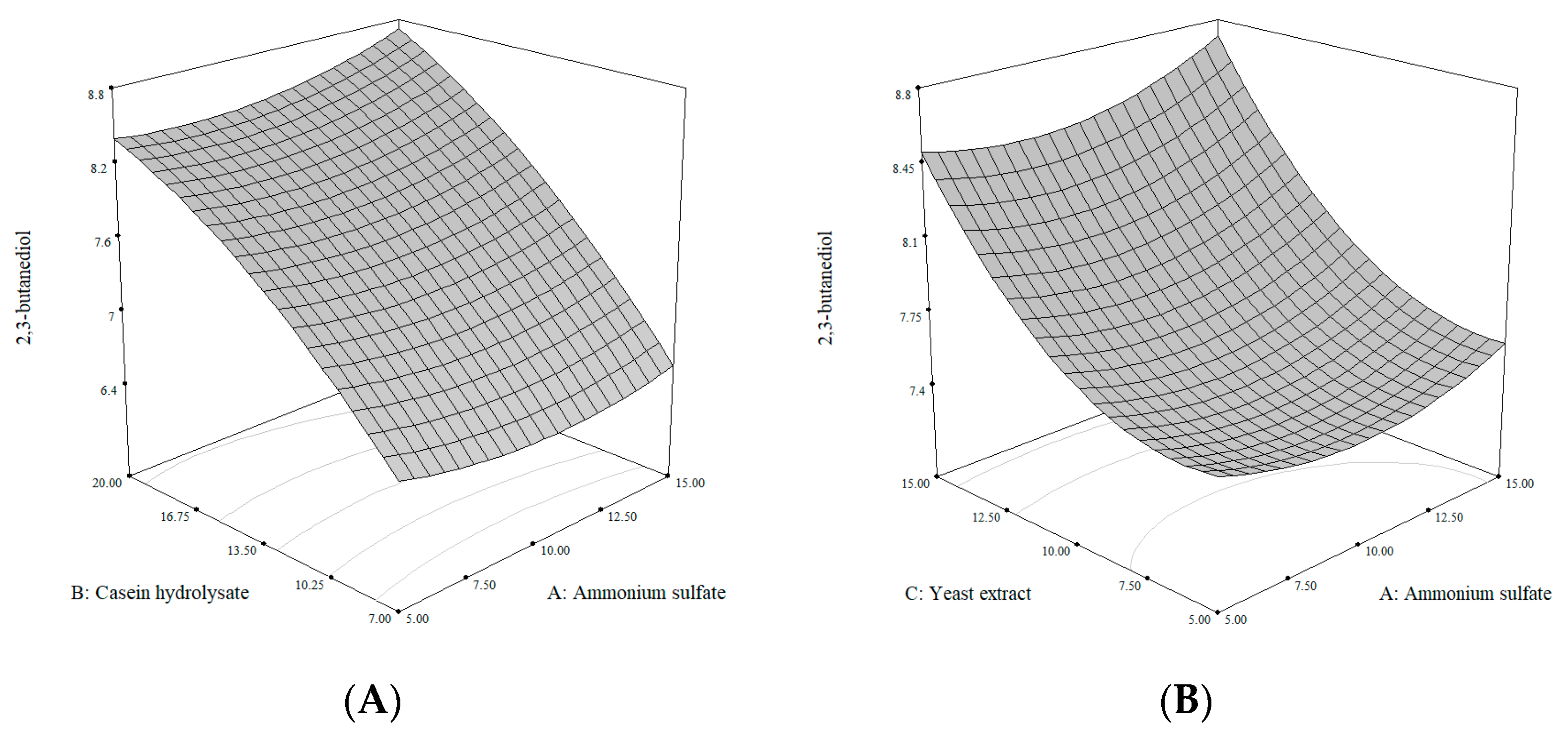

| Variable (g/L) | Symbol | Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Ammonium sulfate | X1 | 5 | 7.5 | 10 | 12.5 | 15 |
| Casein hydrolysate | X2 | 7 | 10.25 | 13.5 | 16.75 | 20 |
| Yeast extract | X3 | 5 | 7.5 | 10 | 12.5 | 15 |
| Crude glycerol | X4 | 5 | 7.5 | 10 | 12.5 | 15 |
| Std.# | Coded Values | 2,3-Butanediol Conc. (g/L) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Predicted | Actual | |
| 1 | −1 | −1 | −1 | −1 | 6.82 | 6.73 |
| 2 | +1 | −1 | −1 | −1 | 6.64 | 6.40 |
| 3 | −1 | +1 | −1 | −1 | 7.27 | 7.21 |
| 4 | +1 | +1 | −1 | −1 | 7.46 | 7.56 |
| 5 | −1 | −1 | +1 | −1 | 7.15 | 7.13 |
| 6 | +1 | −1 | +1 | −1 | 7.12 | 7.36 |
| 7 | −1 | +1 | +1 | −1 | 9.21 | 8.99 |
| 8 | +1 | +1 | +1 | −1 | 9.56 | 9.53 |
| 9 | −1 | −1 | −1 | +1 | 7.11 | 7.15 |
| 10 | +1 | −1 | −1 | +1 | 7.03 | 7.40 |
| 11 | −1 | +1 | −1 | +1 | 8.64 | 8.55 |
| 12 | 1 | +1 | −1 | +1 | 8.93 | 8.95 |
| 13 | −1 | −1 | +1 | +1 | 7.09 | 7.14 |
| 14 | +1 | −1 | +1 | +1 | 7.16 | 7.24 |
| 15 | −1 | +1 | +1 | +1 | 10.23 | 10.48 |
| 16 | 1 | +1 | +1 | +1 | 10.68 | 10.91 |
| 17 | −2 | 0 | 0 | 0 | 8.12 | 8.27 |
| 18 | 2 | 0 | 0 | 0 | 8.39 | 8.08 |
| 19 | 0 | −2 | 0 | 0 | 4.78 | 4.64 |
| 20 | 0 | 2 | 0 | 0 | 8.74 | 8.72 |
| 21 | 0 | 0 | −2 | 0 | 7.81 | 7.86 |
| 22 | 0 | 0 | 2 | 0 | 9.89 | 9.69 |
| 23 | 0 | 0 | 0 | −2 | 7.45 | 7.69 |
| 24 | 0 | 0 | 0 | 2 | 8.86 | 8.46 |
| 25 | 0 | 0 | 0 | 0 | 7.63 | 7.55 |
| 26 | 0 | 0 | 0 | 0 | 7.63 | 7.41 |
| 27 | 0 | 0 | 0 | 0 | 7.63 | 7.79 |
| 28 | 0 | 0 | 0 | 0 | 7.63 | 7.62 |
| 29 | 0 | 0 | 0 | 0 | 7.63 | 7.87 |
| 30 | 0 | 0 | 0 | 0 | 7.63 | 7.57 |
| (A) ANOVA for the Selected Model | |||||
| Source | Sum of squares | DF | Mean squares | f-Value a | Pr > Fb |
| Model | 42.57 | 14 | 3.04 | 45.57 | <0.0001 |
| Lack of fit | 0.85 | 10 | 0.085 | 2.93 | 0.1236 |
| Error | 0.15 | 5 | 0.029 | - | - |
| Corrected total | 43.57 | 29 | - | - | - |
| (B) Statistical Analysis of Factors | |||||
| Factor | Mean square | f-value a | p-value b | ||
| X1 | 0.10 | 1.56 | 0.2309 | ||
| X2 | 23.57 | 353.30 | <0.0001 | ||
| X3 | 6.49 | 97.33 | <0.0001 | ||
| X4 | 2.98 | 44.69 | <0.0001 | ||
| X11 | 0.66 | 9.86 | 0.0067 | ||
| X22 | 1.31 | 19.61 | 0.0005 | ||
| X33 | 2.55 | 38.18 | <0.0001 | ||
| X44 | 0.47 | 6.99 | 0.0184 | ||
| X12 | 0.14 | 2.07 | 0.1708 | ||
| X13 | 0.024 | 0.36 | 0.5591 | ||
| X14 | 0.009 | 0.14 | 0.7178 | ||
| X23 | 2.60 | 38.94 | <0.0001 | ||
| X24 | 1.15 | 17.30 | 0.0008 | ||
| X34 | 0.12 | 1.80 | 0.1998 | ||
| Components | Goal | Importance | Predicted Value (Coded Value) | Experimental Data (g/L) |
|---|---|---|---|---|
| Ammonium sulfate (X1) | In range | 3 | 12.6 | 12.0 |
| Casein hydrolysate (X2) | In range | 3 | 16.0 | 16.0 |
| Yeast extract (X3) | In range | 3 | 12.0 | 12.0 |
| Crude glycerol (X4) | In range | 3 | 13.0 | 13.0 |
| 2,3-butanediol | Maximize | 5 | 8.72 | 10.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Lee, D.Y.; Lee, S.K.; Kim, H.R.; Chun, Y.; Yoo, H.Y.; Kwak, H.S.; Park, C.; Lee, J.H.; Kim, S.W. Development of 2,3-Butanediol Production Process from Klebsiella aerogenes ATCC 29007 Using Extracted Sugars of Chlorella pyrenoidosa and Biodiesel-Derived Crude Glycerol. Processes 2021, 9, 517. https://doi.org/10.3390/pr9030517
Lee JH, Lee DY, Lee SK, Kim HR, Chun Y, Yoo HY, Kwak HS, Park C, Lee JH, Kim SW. Development of 2,3-Butanediol Production Process from Klebsiella aerogenes ATCC 29007 Using Extracted Sugars of Chlorella pyrenoidosa and Biodiesel-Derived Crude Glycerol. Processes. 2021; 9(3):517. https://doi.org/10.3390/pr9030517
Chicago/Turabian StyleLee, Ju Hun, Do Yoon Lee, Soo Kweon Lee, Hyeong Ryeol Kim, Youngsang Chun, Hah Young Yoo, Ho Seok Kwak, Chulhwan Park, Ja Hyun Lee, and Seung Wook Kim. 2021. "Development of 2,3-Butanediol Production Process from Klebsiella aerogenes ATCC 29007 Using Extracted Sugars of Chlorella pyrenoidosa and Biodiesel-Derived Crude Glycerol" Processes 9, no. 3: 517. https://doi.org/10.3390/pr9030517
APA StyleLee, J. H., Lee, D. Y., Lee, S. K., Kim, H. R., Chun, Y., Yoo, H. Y., Kwak, H. S., Park, C., Lee, J. H., & Kim, S. W. (2021). Development of 2,3-Butanediol Production Process from Klebsiella aerogenes ATCC 29007 Using Extracted Sugars of Chlorella pyrenoidosa and Biodiesel-Derived Crude Glycerol. Processes, 9(3), 517. https://doi.org/10.3390/pr9030517