Abstract
This research was focused on developing means of Tintorera grape (Vitis vinifera L.) waste recovery, devising new value-added uses for that material and optimizing of anthocyanin-rich formulations by spray-drying in order to obtain novel ingredients, all for food industry use. First, the identification of phenolic compounds in Tintorera grape extracts by HPLC-DAD-ESI-MSn enabled characterization of the raw material’s health-promoting characteristics. Maintaining the spray-dried products for 4 weeks’ storage enabled study of the formulation’s loss of anthocyanins and antioxidant properties due to drying process temperatures as well as analysis of the retention and stability of such compounds under different conditions (20 and 40 °C). Tintorera grapes presented a significant amount of Malvidin 3-O-hex (5.66 mg g−1 DW). Anthocyanins in spray-dried formulations were stable for 4 weeks. Optimal conditions in the spray-dryer facilitated the products’ antioxidant capacity; for instance, using 10% maltodextrin (w:v) at 90 °C inlet temperature had a little influence on the reduction in encapsulated malvidin 3-O-hex (15%) and presented 3.35 mg GAE g−1 DW of total polyphenol contents, 98.62 µmol Trolox (FRAP assay), and 39.97 µmol Trolox (DPPH assay). Principal component analyses (PCA) showed a high degree of dependence between anthocyanin content and maintenance of antioxidant capacity during storage. These results offer a promising alternative for the industrial management of wine-making wastes in order to implement a sustainable protocol for development of Tintorera grape extracts rich in bioactive compounds for new beverages and functional foods.
1. Introduction
The phenolic compounds in plant-derived foods have been associated with potential health-promoting characteristics due to their ability to act as antioxidants and free-radical scavengers [1]. Most plant phenolic compounds appear as simple phenols and flavonoids, which are polar hydroxylated or glycosylated compounds of relatively low molecular weights. Other plant polyphenols are mostly high molecular polymers such as condensed tannins and proanthocyanidins, which are categorized as non-extractable polyphenols. The abovementioned compounds can be found in grape wastes [2]. Among grapes, Vitis vinifera is the world’s largest fruit crop and it generates a large number of wastes that must be treated adequately to avoid contaminating the areas of production and elaboration of wine [3]. The nature of the wastes produced depends closely on the raw materials (e.g., the grape cultivar), vinification procedures and conditions [3]. The wastes are consumed as table grapes or processed into raisins, juices, jams, or other products [4]. The Tintorera grape (Vitis vinifera L.) is a grape cultivar with a red-colored berry flesh, is a Teinturier cultivar used as color enhancer in high-quality red wines, and is cultivated worldwide for this purpose [5]. In addition, Tintorera varieties typically contain monoglycosylated anthocyanins, being malvidin 3-glucoside the most common [5]. On the other hand, quercetin 3-O-glucoside and quercetin 3-O-glucuronide are the predominant compounds in the flavonol glycoside profile of most grapes [6,7]. Therefore, Tintorera grape by-products can be used for valorization as sources of functional ingredients rich in bioactive phytochemicals [8]. In fact, using such agrowastes to develop new ingredients is a potentially profitable means of reducing their negative environmental impact [3]. Spray-drying is a technique that provides protection and stability to key bioactive compounds which also masks off-flavors by providing a physical barrier [9]. The main advantage found in spray-drying compared to freeze-drying is that this process is more suitable for industrial scale-up and taking into account the large volumes of grape wastes produced by agri-food industry, the management is more handle facing spray-drying process than freeze-drying. In addition, the production time of formulations is an advantage since spray-drying is a process faster than freeze-drying. However, in spray-drying, atomization process influences the size of the droplets formed and therefore the heat-transferring surface between the dry air and the liquid feed. Though highly protective and thus widely employed in the food industry, the process of spray-drying can provoke significant loss of bioactive compounds. Therefore, feed, inlet air, and outlet air temperatures must be optimized during the process, i.e., the liquid feed temperature affects its viscosity and thus its homogeneous spraying capacity, the inlet air temperature is directly proportional to the drying rate and final water content, and the outlet temperature is effectively the control index of the dryer. However, atomization is dependent on additional parameters; geometric aspects of the nozzle, the air:solution volume ratio, and temperature control all have effects on the physical state of the final product [10]. Several authors were able to determine that the different conditions used for spray-drying would protect the Vitis vinifera polyphenols from degradation, mainly oxidation of the bioactive compounds due to oxygen from the environment when these were formulated under correct storage conditions [11]. Some examples of ingredients used to protect the bioactive compounds in Vitis vinifera products (e.g., alginate) previously had been reported [2]. Moreno et al. [12] using different wall materials but maintaining a constant temperature of 25 °C, developed models to explain the degradation of Vitis vinifera polyphenolic compounds (which also had been studied in Vitis labrusca using spray-drying), but that study focused on antimicrobial protection [13]. Thus, the idea of encapsulating phenolic compounds from berries and grapes and their by-products is not new. However, until now, there had been no such studies of agrowastes of Tintorera grapes, a cultivar with high antioxidant capacity, and very rich in anthocyanins which is affected by both the temperature of the process as well as that during storage. Thus, this research focused on evaluating the waste produced by the wine industry related to overproduction of Tintorera grape and transforming that biomass into a source of valuable functional ingredients with great potential for health-promoting biological activities. Moreover, the goal was to provide new knowledge about the composition and operational protocols necessary to obtain the new formulations in order to deliver novel and innovative products derived from Tintorera grape wastes, once recovered and valorized as a source of functional ingredients for industrial applications.
2. Materials and Methods
2.1. Chemicals
The compounds 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), Folin–Ciocalteu reagent, monobasic sodium phosphate, and dibasic sodium phosphate were obtained from Sigma–Aldrich (MilliporeSigma, St. Louis, MO, USA). Meanwhile, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was purchased from Fluka Chemika (Fluka, Neu-Ulm, Switzerland). Ultrapure water was produced using a Millipore water purification system. Formic acid, FeCl3-6H2O, TPTZ and acetate buffer was purchased from Merck (MilliporeSigma, Darmstadt, Germany). Besides, cyanidin 3-glucoside, delphinidin 3-galactoside, malvidin 3-galactoside, chlorogenic acid, rutin trihydrate, quercetin dihydrate, and maltodextrin DE-10 as carrier agents were obtained from Sigma-Aldrich Chemical Co. (MilliporeSigma, St. Louis, MO, USA). For the enzymatic assays, E. electricus AChE, acetylthiocholine iodide (ATCh), 5,5′-dithio-bis(2-nitrobenzoic) acid (DTNB), α-glucosidase, PNP-G, and lipase were all supplied by Sigma-Aldrich Chemical Co. (MilliporeSigma, St. Louis, MO, USA).
2.2. Material Collection
Tintorera grapes were obtained from San Nicolás, Chile (36.49° S, 72.20° W) during two consecutive seasons (2018 and 2019) to carry out a replication of the design of the experiment. After harvesting, all samples were stored at −80 °C until processing and analysis.
2.3. Sample Preparation and Spray-Drying of Tintorera Grape Extracts
An aqueous extract of Tintorera was mixed with 50 mL of maltodextrin (10% and 30%) and stirred at 300 rpm for 2 h. The powder was obtained by means of a spray-drying using a Mini Spray Dryer—B290 (BÜCHI, Flawil, Switzerland). The mixture was fed into the spray-dryer at room temperature with a flow rate of 4 mL min−1. The inlet temperatures were maintained at 90 and 120 °C, whereas outlet air temperatures were 50 and 80 °C, respectively. The dried powder was collected and stored in an opaque, air-tight container at 4 °C for further analysis. Four treatments were carried out LT10 (10% maltodextrin and inlet temperature at 90 °C), LT30 (30% maltodextrin and inlet temperature at 90 °C), HT10 (10% maltodextrin and inlet temperature at 120 °C), and HT30 (30% maltodextrin and inlet temperature at 120 °C). The microspheres obtained in the four treatments (LT10, LT30, HT10, and HT30) were kept in controlled chambers (with controlled relative humidity at 60%) at 20 and 40 °C, and the anthocyanin concentration changes of the encapsulated along one month were studied.
2.4. Identification of Phenolic Compounds of Tintorera Grape Wastes and the New Formulations by HPLC–DAD–ESI-MSn and Quantification by HPLC–DAD
Raw materials and formulations were mixed with 5 volumes of methanol/formic acid/water (25:1:24) for the extraction of anthocyanins, mainly malvidin 3-hexoside and other phenolic compounds, following the protocol described by [14]. Shortly, a Luna C18 column (250 mm × 4.6 mm, 5 mm particle size; Phenomenex, Macclesfield, UK) and 1% formic acid and acetonitrile were used as the mobile phases A and B, respectively, with a flow rate of 1 mL min−1. The HPLC–DAD–ESI-MSn analyses were carried out using an Agilent HPLC 1200 system (Agilent Technologies, Waldbronn, Germany) and coupled to a mass detector in series. The ionization conditions were 350 °C and 4 kV, for capillary temperature and voltage, respectively. The nebulizer pressure and nitrogen flow rate were 65.0 psi and 11 L min−1, respectively. The full-scan mass covered the range of m/z from 100 to 1200. The mass spectrometry data were acquired in the positive ionization mode for anthocyanins and in the negative ionization mode for other flavonoids and phenolic acids. For the quantification, an Agilent HPLC-DAD 1100 system (Agilent Technologies, Waldbronn, Germany) was employed, consisting of a multisolvent delivery system (G1312A), an in-line degasser (G1322A), an autosampler (G1313A) and a photodiode array detector (G1315B). Chromatograms were recorded at 280, 320, 360, and 520 nm, and required standards were used.
2.5. Biological Activity In Vitro of Tintorera Grapes: Acetylcholinesterase Activity Assay, Pancreatic Lipase Activity Assay, and α-Glucosidase Activity Assay
Enzymatic assays were conducted in order to study the biological potential of Tintorera grape extracts. Enzymatic assays of acetylcholinesterase (AChE) and α-Glucosidase activity assay were performed using the methodology described by Noriega et al. [15], whereas lipase activity assay was modified from the previous report by Gilham et al. [16]. For the assays of acetylcholinesterase (AChE), we modified the Ellman method [17] where different concentrations of acetylthiocholine (ATCh) were prepared from a freshly distilled water (1, 0.5, 0.2, and 0.1 mM). The inhibition reaction was done with 100 µL of Vitis vinifera L. extracts and dilutions. The spectrophotometric study was incubated at 37 °C for 15 min and the absorbance was measured by a Thermo Scientific UV-Vis Orion AquaMate 8000 spectrophotometer (Madrid, Spain) at 412 nm. The measurement of enzymatic activity for pancreatic lipase was carried out using 4-nitrophenyl dodecanoate as substrate. The inhibition reaction was done with 100 µL of Vitis vinifera extract and dilutions. The spectrophotometric measures were incubated at 37 °C for 10 min and the absorbance was measured by a Thermo Scientific UV-Vis Orion AquaMate 8000 spectrophotometer (Madrid, Spain) at 400 nm. Finally, inhibitory activity of α-glucosidase was determined by modification of a previously reported method [18]. Moreover, 4-nitrofenil α-D-glucopiranosido (PNP-G) was used as substrate and chromogenic reagent at concentration of 1, 0.5, and 0.2 mM prepared from a freshly distilled water. The inhibition reaction was done with Vitis vinifera L. extracts and dilutions. The spectrophotometric analyses were incubated at 30 °C for 15 min and the absorbance was measured by a Thermo Scientific UV-Vis Orion AquaMate 8000 spectrophotometer (Madrid, Spain) at 400 nm. In all enzymatic assays, a phosphate buffer solution was used as blank and was tested with a mixture of methanol, formic acid, and distilled water (25:1:24). Six replications were performed for each enzymatic assay.
2.6. Total Polyphenol Content and Antioxidant Capacity of Tintorera Grapes and Formulations
The aforementioned hydroalcoholic extracts were prepared to analyze total polyphenol content and antioxidant capacity of formulations of Tintorera grapes. In short, raw materials and formulations were mixed with 5 volumes of methanol/formic acid/water (25:1:24) for the extraction of phenolic compounds. Total phenolic content (TP) was determined by the Folin–Ciocalteu reagent method [19]. The samples were prepared by adding 750 µL of Folin–Ciocalteu 1 N reagent, 750 µL of 20% sodium carbonate, and 500 µL of the Tintorera grape extracts and powders. All of them were maintained for 2 h in the dark and absorbance was measured through a Thermo Scientific UV-Vis Orion AquaMate 8000 spectrophotometer (Madrid, Spain). Results were expressed as milligrams of gallic acid equivalents per grams (mg GAE g−1 DW). Free-radical scavenging activities were determined using the DPPH method according to Mena et al. [20]. Briefly, the antioxidant activity was evaluated by measuring the variation in absorbance at 515 nm after 30 min of reaction with the radical (for DPPH) and FRAP assay (ferric reducing antioxidant power assay) using 30 µL of the extract (per sample), 300 µL of distilled water, and the addition of 3000 µL of FRAP reagent (FeCl3-6H2O 20 mM, TPTZ 10 mM, and acetate buffer 0.3 mM) incubated at 37 °C for 30 min. The assays were performed using Thermo Scientific UV-Vis Orion AquaMate 8000 spectrophotometer (Madrid, Spain). The results were expressed as micromole Trolox mg−1 dry weight. Six replications were carried out per sample.
2.7. Scanning Electron Microscopy of Formulations
Powder samples were attached to double-sided adhesive tape mounted on SEM stubs, covered with gold layer (9 nm) under vacuum and observed in scanning electronic microscope (FEI—Inspect S50) working at 5 kV, with 10,003 and 50,003 of magnification.
2.8. Statistical Analysis
Phenolic compounds, antioxidant capacity, and spray-drying assay were studied by using one-way analysis of variance (ANOVA), with a completely random design (n = 4), and Tukey test at a level of significance p < 0.05. The results of biological activity are presented as descriptive statistics expressed as the mean and standard deviation (2 consecutive years), whereas the inhibition enzyme assays were calculated using the GraphPad Prism v. 6.1 software (GraphPad Prism® software, Inc., San Diego, CA, USA). Finally, Biplot associated with principal components analyses (PCA) was obtained by mean centered data based on the eigen values to determine the correlations among variables, the discrimination of anthocyanin contents of Vitis vinifera and the antioxidants properties, and loss of malvidin 3-hexoside at different process and storage temperatures. All analyses were performed using the InfoStat v. 2017 software.
3. Results and Discussion
3.1. Identification and Quantification of Phenolic Compounds in Tintorera Grapes
The analyses of phenolic compounds in Tintorera grape were carried out by HPLC-DAD-ESI/MSn using the whole grape without separating seeds and flesh, in order to avoid oxidation. Acidified hydromethanolic extracts were prepared for enhancing the extraction of anthocyanins. Eighteen different compounds were identified in the samples, where the major components were the anthocyanins (16 out of 18 detected compounds). A detailed tentative assignment of the anthocyanin peaks identities is shown in Table 1.

Table 1.
Identification and quantification (mg g−1 DW) of phenolic compounds in Tintorera grape (Vitis vinifera L.) by HPLC-Diode Array Detector (DAD)-Electro spray ionization (ESI)/MSn.
The major representative of the phenolic compounds in the Tintorera grape was the malvidin 3-hexoside. Peonidin 3,7-O-diglucoside was also identified, as previously found by [5], since 2 clear productions were generated from m/z 625 (m/z 463 and 301), although it was not quantified. Anthocyanins 3,7-diglucosides and 3,5-diglucosides have been described in other plants and their structures have been unequivocally elucidated by 1H NMR data [21]. Peaks 1, 2, 3, 5, and 6 presented derivatives of the 3-glucosides of the anthocyanidins delphinidin, cyanidin, petunidin, peonidin, and malvidin, respectively. Other anthocyanin derivatives were identified as well, delphinidin 3-O-(6-acetyl)-glucoside (peak 7, m/z 507, 303 and 204), cyanidin 3-O-(6-acetyl)-glucoside (peak 9, m/z 491, 287 and 204), petunidin 3-O-(6-acetyl)-glucoside (peak 10, m/z 521, 317 and 204), and peonidin 3-O-(6-acetyl)-glucoside (peak 11, m/z 505, 301 and 204). These compounds are common to the V. vinifera cultivars, and they were easily identified by their absorbance spectra as well as the mass fragments information [5]. Besides, peaks 14, 15, and 16, represented derivatives of the 3,5-O-diglucosides, peonidin 3,5-O-di-glucoside, malvidin 3,5-O-di-hexoside, and petunidin 3,5-O-di-hexoside. Some other derivatives of the peonidin and malvidin were also detected, though they were only identified by their anthocyanidin. Some additional flavonol glycosides were also detected, and normally, the presence of these compounds may cause interference in the separation and identification [6]. The peaks 4 and 8 were confirmed by comparison of the retention times together with their absorbance and mass spectral information. Myricetin 3-O-glucoside (m/z 316), quercetin 3-O-glucoside (m/z 301), isorhamnetin 3-O-glucoside (m/z 315), and syringetin 3-O-glucoside (m/z 345) were identified (although not all were quantified), also in accordance to previous findings about the flavonol glycosides composition of V. Vinifera red cultivars [6,22]. Other authors found values lower for quercetin 3-O-glucoside ranged from 0.0014 to 0.0335 mg g−1, whereas for isorhamnetin 3-glucoside and syringetin 3-O-glucoside values ranged from 0.0016 to 0.0035 mg g−1 [22] though they were not detected in this experiment. Table 1 also shows the total phenolics content (mg g−1 DW) in Tintorera grape as well, separated by anthocyanins, flavonols, and hydroxycinnamic acid derivatives. In Tintorera grape, total anthocyanin content was high (17.04 mg g−1). Other researchers found values of malvidin 3-O-glucoside around 0.797 mg g−1, malvidin 3-O-6-coumaryl-glucoside around 0.119 mg g−1, and peonidin 3-O-glucoside around 0.614 mg g−1 during raisining process at initial time [23]. These contents of anthocyanins were lower than indicated in our study. In addition, comparison with specifically Tintorera grapes in other manuscript turned out to be difficult because in other works, authors only reported the wine contents [23,24]. In the studied samples, 16 anthocyanins were quantified, being malvidin 3-O-hexoside the major compound detected (5.66 mg g−1), followed by peonidin 3-O-hexoside (3.40 mg g−1). The total flavonols quantified in Tintorera grapes was 0.89 mg g−1, considering only 2 major flavonols detected, the quercetin 3-glucoside (0.67 mg g−1) and the myricetin 3-O-glucoside (0.22 mg g−1). This could be due to the difficulty to analyze minor components stored in external areas of cellular vacuoles and therefore being more sensitive to oxidation and degradation [25]. To observe the effect of temperature, process, and maltodextrin (LT10, LT30, HT10, and HT30) on polyphenols compounds from Tintorera grapes, a Supplementary Materials data have been included, where it can be observed that spray-drying process carried out with 10% maltodextrin and temperature of 90 °C produced lower loss of the content of the main polyphenolic compounds.
3.2. Biological Activity of Tintorera Grapes In Vitro
Table 2 shows the enzymatic inhibition of acetylcholinesterase, lipase, and α-glucosidase enzyme activities by the Tintorera grape extracts.

Table 2.
Inhibitory activity of Tintorera grape (mg mL−1) extract on acetylcholinesterase, lipase, and α-glucosidase enzymes a.
The inhibition of the three enzymes by Tintorera extracts was high, highlighting its activity against acetylcholinesterase and α-glucosidase. Regarding to lipase inhibition, the IC50 of the Tintorera grape extracts was slightly higher than some phenolic standards used in this assay (0.14 mg mL−1 for chlorogenic acid, 0.13 mg mL−1 for delphinidin 3-galactoside, and 0.13 mg mL−1 for kuromanin chloride), with the exception of malvidin 3-galactoside, which presented a similar dose (0.16 mg mL−1). In fact, it is necessary to emphasize that the IC50 values for common inhibitors such as orlistat was 0.123 mg mL−1 [26], a value close to what we have obtained with 0.16 mg/mL, demonstrating a good inhibitory capacity of the Tintorera grape extract.
In relation to AChE enzyme, in previous works, the IC50 values AChE inhibitory drugs were established as follows: physostigmine 1.8 × 10−4 µg mL−1, rivastigmine 1.1 × 10−3 µg mL−1, donepezil 2.5 × 10−3 µg mL−1, and tacrine 1.5 × 10−3 µg mL−1 [27]. The activity (low IC50) of pure compounds and drugs is usually much higher than the activity of the natural extract, however, although the extracts showed less inhibitory activity than that found in these drugs, it must be remembered that they have other advantages. These extracts do not usually have side effects. Therefore, at the concentrations obtained by us, they are still interesting as a dietary alternative.
On the other hand, the IC50 for anti-diabetic drugs, such as Amaryl (glimepiride) (0.88 mg mL−1), Betanorm (gliclazide) (1.40 mg mL−1), and Glucobay (acarbose) (0.75 mg mL−1) [28], were superior than results obtained from Tintorera grape extract, being, therefore, a good candidate for such purpose.
The inhibition kinetics (Figure 1) of the three enzymes was calculated to elucidate inhibition mode. As shown in Figure 1a–c, all inhibition kinetics curves crossed X axis, therefore, the inhibition type of Tintorera grapes for the three enzymes (acetylcholinesterase, lipase, and α-glucosidase) was non-competitive. This situation indicates that phenolic compounds from Tintorera grapes have combined actions between inhibitor and enzyme as well as between inhibitor and enzyme-substrate complex. In addition, it is observed how blanks with buffer phosphate and control with a mixture of methanol, formic acid, and water (25:1:24) presented a lesser inhibition.
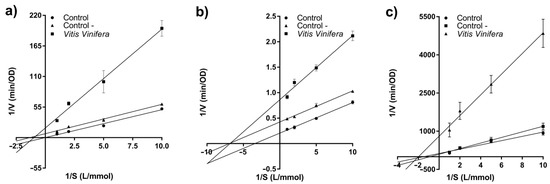
Figure 1.
Lineweaver–Burk plot of extracts from Vitis vinifera on three enzymes. ((a) acetylcholinesterase, (b) lipase, and (c) α-glucosidase).
Other authors have showed interest on biological activity of Vitis vinifera from its leaves, which are widely used in foods in countries such as Greece, Turkey, and Middle East area [29]. They evaluated the neuroprotective activity of leaf extracts by inhibition of acetylcholinesterase (AChE) using Ellman’s method. Results revealed that Frankovka and Italian Riesling varieties presented the best IC50 values among all those studied, with values of 54.5 ± 1.12 and 62.8 ± 1.54 µg mL−1, respectively, compared with galantamine, a drug widely used in the treatment of Alzheimer that expressed a value of 0.09 ± 0.001 µg mL−1. These results reaffirm the importance of natural and inexpensive alternatives in the consumption of food rich in bioactive compounds as well as their use in pharmaceutical industry. Consequently, these results proved that Tintorera grape extracts are potent in vitro inhibitors of acetylcholinesterase, pancreatic lipase, and α-glucosidase, so they may be developed—individually or in synergistic formulations—as natural alternatives for therapies and nutritional interventions.
3.3. Anthocyanins and Antioxidant Capacity in Spray-Drying Formulations of Tintorera Grape
After analyzing the potential of the raw material and focusing on the manage waste produced by the overproduction of Tintorera grapes and their transformation into functional ingredients or beverage, strategies of drying were used.
Both, control treatment at 90 °C combined with 10% of maltodextrin (LT10) and treatment at 120 °C combined with 10% of maltodextrin (HT10) showed higher concentrations for all anthocyanins than the other treatments (Table 3).

Table 3.
Main anthocyanin content (mg g−1 DW) in encapsulated Tintorera grape extracts.
This greater retention of anthocyanins seems to be determined by the optimal percentage of the wall material. In the case of maltodextrin, 10% would be adequate since it is able to immobilize Tintorera extracts.
In Figure 2, formulations from spray drying (inlet air temperature of 90 and 120 °C) of Tintorera grapes with maltodextrin are presented. Treatments HT10 and HT30 (Figure 2c,d) showed spherical shape more piled up due to a strong Tintorera grape extract attraction with maltodextrin to each other. Even though, the shape of formulated powders and attraction between encapsulation agent (maltodextrin) and Tintorera extract were efficient due to temperature, anthocyanins content was affected by temperature conditions.
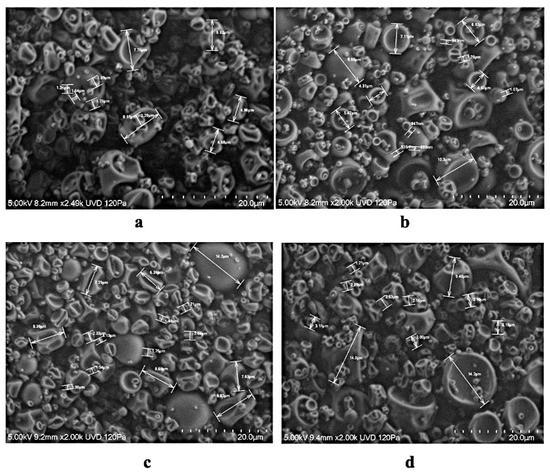
Figure 2.
SEM images of spray-dried Tintorera grapes extracts at two percentage of maltodextrin and two inlet temperatures. ((a) maltodextrin 10% and inlet temperature 90 °C, (b). maltodextrin 30% and inlet temperature 90 °C, (c). maltodextrin 10% and inlet temperature 120 °C, and (d). maltodextrin 30% and inlet temperature 120 °C).
Antioxidant capacity, FRAP and DPPH assay, were carried out for Tintorera grape extracts and formulations by spray-drying (Table 4).

Table 4.
Total Polyphenol contents and antioxidant capacity (ferric reducing antioxidant power (FRAP) and DPPH assays) in Tintorera grapes and their formulations (LT10, LT30, HT10, and HT30).
The values obtained for the grape extracts in the FRAP assay reached to 203.40 μmol Trolox g−1 DW. If we compare these values of FRAP with those found by [14] in other fruits like maqui or açai, we observe that Tintorera grapes presented values as high as those, consequently, they seem to be a promising source of antioxidant compound. Respect to the DPPH* assay, Tintorera grapes (108.27 μmol Trolox g−1 DW) exhibited twice the activity of calafate berry (51.55 μmol Trolox g−1 DW) [21]. These high values for Tintorera grapes are attributed to their remarkable anthocyanin content as tested in other fruits by several authors [30]. Another interesting aspect is that values of DPPH assay were highly correlated with the total polyphenol contents. This did not occur with the FRAP assay. Hence, this would indicate that the polyphenols obtained in Tintorera extracts could be better free radical scavengers than reducers. This is easily observable in biplot associated with principal component analyses (see below in Figure 3). Total polyphenol contents and antioxidant capacity in formulations were lesser than Tintorera grapes due to the losses of anthocyanins and other phenolic compounds. Even though, the treatments LT10 and HT10 presented optimal results. Anthocyanins application in human health is limited to liquid. Nevertheless, liquid formulation is affected by external factors such as light exposure, humidity, the presence of oxygen, and temperature [31]. For that reason, spray drying is a suitable method to formulate and conserve bioactive compounds over time. Above all, in order to take advantage of an overproduction of Tintorera grapes with high antioxidant properties and biological activity.
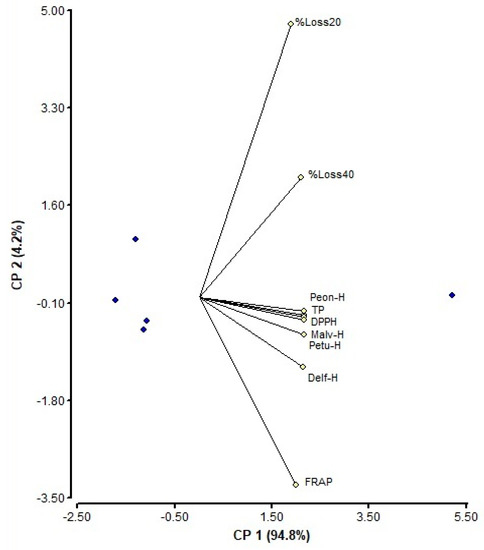
Figure 3.
Biplot associated with principal component analysis (PCA) of variables of Tintorera grape formulations. Percentage of losses of malvidin 3-hexoside in formulations maintained at 20 °C (%Loss20), percentage of losses of malvidin 3-hexoside in formulations maintained at 40 °C (%Loss40), malvidin 3-hexoside content (Malv-H), peonidin 3-hexoside content (Peon-H), petunidin 3-hexoside content (Petu-H), delphinidin 3-hexoside content (Delf-H), total polyphenol contents (TP), DPPH assay (DPPH), and ferric reducing antioxidant power (FRAP) assay (FRAP).
3.4. Effect of Storage Temperature on Anthocyanin-Rich Formulations of Tintorera Grape
Changes in the operational conditions were performed, since anthocyanins are sensitive to high temperature. In addition, two storage temperature (20 and 40 °C for 4 weeks) were used to study degradation of anthocyanins. A study on malvidin 3-hexosides concentration losses in formulations was carried out for 4 weeks, keeping the microspheres at 20 and 40 °C (Figure 4).
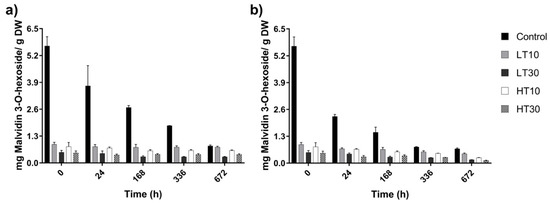
Figure 4.
Malvidin 3-hexosides concentration losses in formulations (LT10, LT30, HT10, and HT30) stored at (a) 20 °C and (b) 40 °C, for 672 h.
Concentrations of malvidin 3-hexoside were higher than in control (without encapsulating), during the 4 weeks of the study at both temperatures, 20 and 40 °C. The losses presented in Tintorera grapes without encapsulating (control) were 86% at 20 °C and 88% at 40 °C. Nonetheless, extracts protected with maltodextrin presented malvidin 3-hexoside losses lesser than 50% in all treatments at 20 °C for 4 weeks. However, it did not occur when the formulations were maintained at 40 °C. In this case, the losses were higher, reaching values between 50% and 75%, but lower when compared with control without protecting. In this assay, also LT10 treatment presented the promising results. Other authors [11] demonstrated that the storage of spray-dried formulated grape extracts improved the stability of anthocyanins over time, although their studies are mainly based on changes in humidity.
Finally, a Biplot associated with principal component analysis (PCA) of variables of Tintorera grape formulations is presented (Figure 3).
PCA was employed by mean centered data based on the eigen values to determine the correlation between variables and the discrimination of anthocyanin contents of Tintorera formulations and antioxidant characteristics in different conditions of temperature of the process and storage temperature. The variables used were percentage of losses of malvidin 3-hexoside in formulations maintained at 20 °C (%Loss20), percentage of losses of malvidin 3-hexoside in formulations maintained at 40 °C (%Loss40), malvidin 3-hexoside content (Malv-H), peonidin 3-hexoside content (Peon-H), petunidin 3-hexoside content (Petu-H), delphinidin 3-hexoside content (Delf-H), total polyphenol contents (TP), DPPH assay (DPPH), and FRAP assay (FRAP).
The correlation between the variables was high and statistically significant. A positive linear relationship was found between the variables. First, the selection criterion of eigen values greater than 1 was used. However, it was observed that the second component explained more fully the variability observed in relation to the FRAP assay and the percentages of loss of malvidin 3-hexoside at temperatures storage of 20 and 40 °C. From the Biplot, a simultaneously visualization of observations and variables are presented. Hence, it can be seen that the individual anthocyanin content in the different spray-drying treatments as well as the total polyphenol content and the DPPH antioxidant capacity assays showed a projection to the right of CP1, while the FRAP assay and the loss percentages malvidin 3-hexoside at storage temperatures of 20 and 40 °C would be represented by both components. Therefore, the variables mentioned above that are projected to the right would be the ones that would be most affected by the different thermal treatments that were performed through spray-drying.
4. Conclusions
Tintorera grape (Vitis vinifera) was studied due to its promising nutritional value in view of managing the overproduction of this grape that is used mainly for wine coloring. Tintorera grapes exhibited high anthocyanin contents and corresponding high antioxidant capacity, as well as potential as inhibitors of enzymes involved in the pathological conditions of energy metabolism (lipase and α-glucosidase) as well as inflammatory and cognitive decline problems (acetylcholinesterase). The encapsulation offers protection to the anthocyanins of Tintorera grapes, combined with adding maltodextrin (10% w:v) and reductions of the incident light and the inlet temperature. Considering the lack of current uses for the agrowaste of the wine-making industry delivering tons of Tintorera grape waste annually, the valorization into functional ingredients offer an innovative waste recovery strategy.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/9/3/518/s1, Table S1: Identification and quantification (mg g−1 DW) of phenolic compounds in formulations (LT10, LT30, HT10 and HT30) by HPLC-DAD-ESI/MS.
Author Contributions
M.D.L.-B.: conceptualization, writing—original draft, and funding acquisition, E.F.C.: methodology, formal analysis, and writing, G.P.: writing and formal analysis, F.N.: formal analysis, data curing, and editing, P.F.-M.: methodology, M.E.R.-R.: writing—reviewing and editing, P.J.: methodology, M.S.: methodology and writing—reviewing and editing, I.S.: reviewing and editing, D.A.M.: methodology and reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the FONDECYT grant 1160899 (CONICYT, Chile).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Recognition and gratitude to the technicians of Chemical Analysis Laboratory, Department of Plant Production—University of Concepción, Chillán.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salehi, B.; Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Delporte, C.; Barra, G.V.; Cazar Ramirez, M.E.; Lopez, M.D.; Ramirez Alarcon, K.; Martins, N.; et al. Ethnopharmacology, Phytochemistry and Biological Activities of Native Chilean Plants. Curr. Pharm. Des. 2020, 26, 1–23. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Ormazabal, M.; Vallejo, A.; Olivares, M.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Optimization of supercritical fluid consecutive extractions of fatty acids and polyphenols from Vitis vinifera grape wastes. J. Food Sci. 2015, 80, E101–E107. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Castillo-Munoz, N.; Fernandez-Gonzalez, M.; Gomez-Alonso, S.; Garcia-Romero, E.; Hermosin-Gutierrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef]
- Castillo-Munoz, N.; Gomez-Alonso, S.; Garcia-Romero, E.; Hermosin-Gutierrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic contents and compositions in skins of red wine grape cultivars among various genetic backgrounds and originations. Int. J. Mol. Sci 2012, 13, 3492–3510. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and chemical variability of grape pomaces from French vineyard. Ind. Crop. Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Spray Drying Techniques for Food Ingredient Encapsulation; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Tolun, A.; Artik, N.; Altintas, Z. Effect of different microencapsulating materials and relative humidities on storage stability of microencapsulated grape pomace extract. Food Chem. 2020, 302, 125347. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.; Cocero, M.J.; Rodríguez-Rojo, S. Storage stability and simulated gastrointestinal release of spray dried grape marc phenolics. Food Bioprod. Process. 2018, 112, 96–107. [Google Scholar] [CrossRef]
- Souza, V.B.; Fujita, A.; Thomazini, M.; Da Silva, E.R.; Lucon, J.F., Jr.; Genovese, M.I.; Favaro-Trindade, C.S. Functional properties and stability of spray-dried pigments from Bordo grape (Vitis labrusca) winemaking pomace. Food Chem. 2014, 164, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Noriega, F.; Mardones, C.; Fischer, S.; García-Viguera, C.; Moreno, D.A.; López, M.D. Seasonal changes in white strawberry: Effect on aroma, phenolic compounds and its biological activity. J. Berry Res. 2020, 11, 103–118. [Google Scholar] [CrossRef]
- Gilham, D.; Lehner, R. Techniques to measure lipase and esterase activity in vitro. Methods 2005, 36, 139–147. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Chan, H.-H.; Sun, H.-D.; Reddy, M.V.B.; Wu, T.-S. Potent α-glucosidase inhibitors from the roots of Panax japonicus CA Meyer var. major. Phytochemistry 2010, 71, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- López, M.D.; Baenas, N.; Retamal-Salgado, J.; Zapata, N.; Moreno, D.A. Underutilized Native Biobío Berries: Opportunities for Foods and Trade. Nat. Prod. Commun. 2018, 13, 1934578X1801301226. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Figueiredo-Gonzalez, M.; Cancho-Grande, B.; Simal-Gandara, J. Evolution of colour and phenolic compounds during Garnacha Tintorera grape raisining. Food Chem. 2013, 141, 3230–3240. [Google Scholar] [CrossRef]
- Figueiredo-Gonzalez, M.; Regueiro, J.; Cancho-Grande, B.; Simal-Gandara, J. Garnacha Tintorera-based sweet wines: Detailed phenolic composition by HPLC/DAD-ESI/MS analysis. Food Chem. 2014, 143, 282–292. [Google Scholar] [CrossRef] [PubMed]
- De Torres, C.; Diaz-Maroto, M.C.; Hermosin-Gutierrez, I.; Perez-Coello, M.S. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; El-Sers, D.A.; Al-Zokeim, N.I.; Gomaa, M.A. Amelioration of experimental metabolic syndrome induced in rats by orlistat and Corchorus olitorius leaf extract; role of adipo/cytokines. J. Pharm. Pharmacol. 2019, 71, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ogura, H.; Kosasa, T.; Kuriya, Y.; Yamanishi, Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 609–613. [Google Scholar] [CrossRef]
- Önal, S.; Timur, S.; Okutucu, B.; Zihnioğlu, F. Inhibition of α-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep. Biochem. Biotechnol. 2005, 35, 29–36. [Google Scholar] [CrossRef]
- Pintac, D.; Cetojevic-Simin, D.; Berezni, S.; Orcic, D.; Mimica-Dukic, N.; Lesjak, M. Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties. Food Chem. 2019, 286, 686–695. [Google Scholar] [CrossRef]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).