Promotional Effects of Rare-Earth Praseodymium (Pr) Modification over MCM-41 for Methyl Mercaptan Catalytic Decomposition
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Catalyst Synthesis
2.2.1. The Preparation of the MCM-41 Support
2.2.2. The Synthesis of Pr/MCM-41 Catalyst
2.3. Characterization
2.4. Catalytic Activity and Stability Measurement
3. Results and Discussion
3.1. Structure of Support and Modified Pr/MCM-41 Catalyst
3.2. The Surface Acid–Base and Reducibility of Support and Pr/MCM-41 Catalysts
3.3. The Surface Composition of the Support and Pr/MCM-41 Catalysts
3.4. CH3SH Reaction Rate and Stability of Catalyst
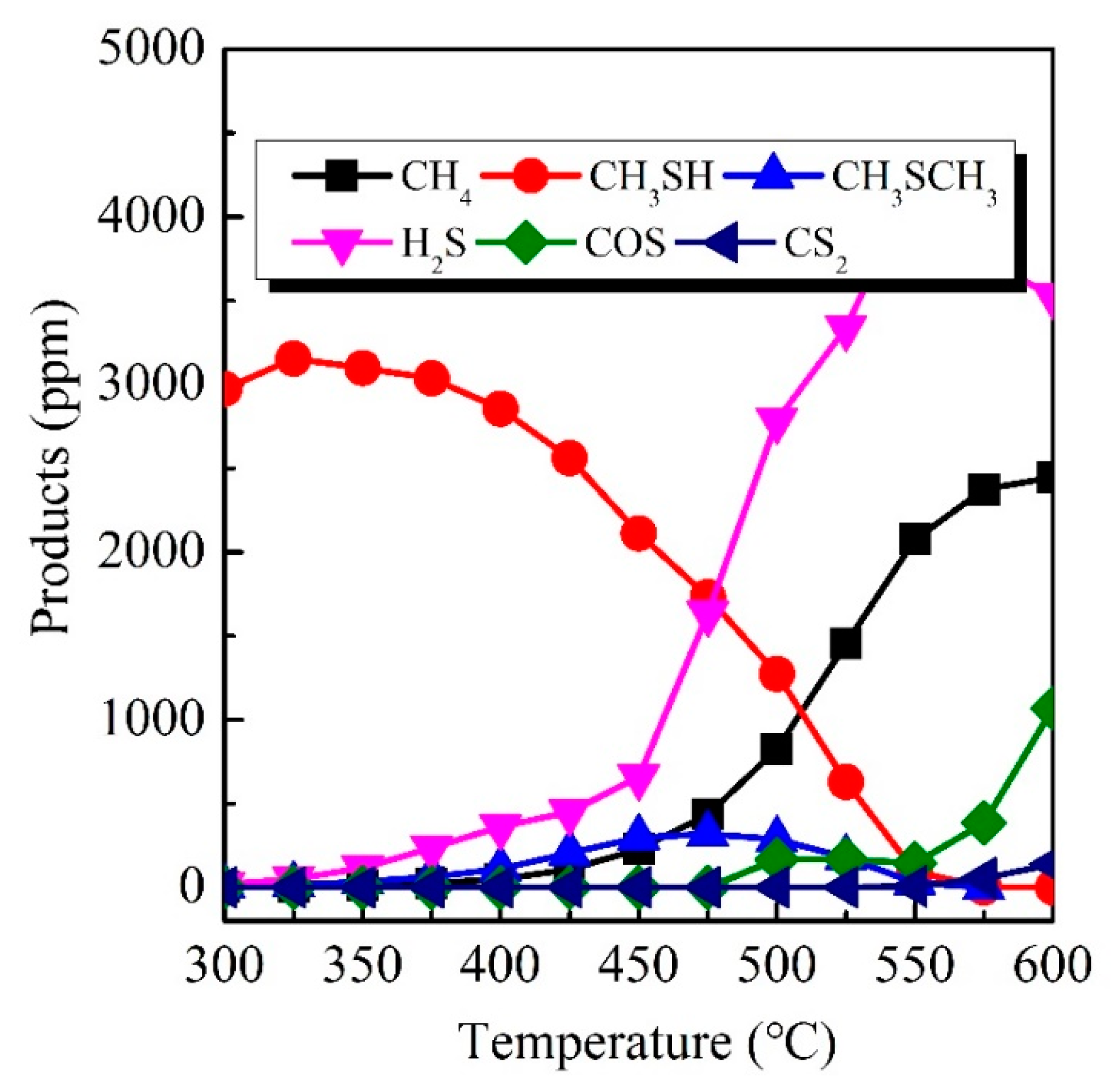
3.5. Product Distribution
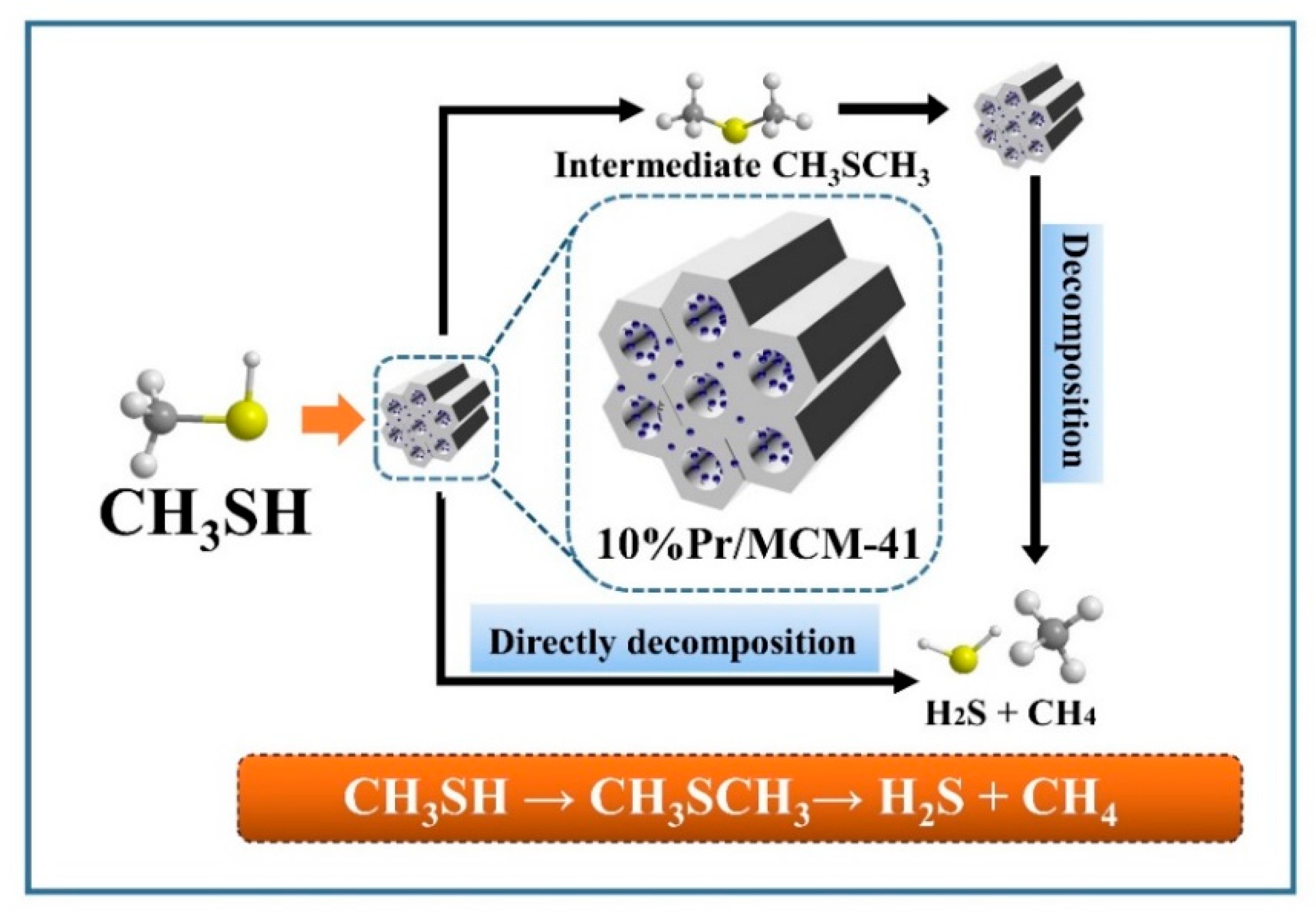
3.6. Possible Reaction Mechanism and Active Sites for the Conversion of CH3SH over the 10% Pr/MCM-41 Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deshmukh, S.; Jana, A.; Bhattacharyya, N.; Bandyopadhyay, R.; Pandey, R.A. Quantitative determination of pulp and paper industry emissions and associated odor intensity in methyl mercaptan equivalent using electronic nose. Atmos. Environ. 2014, 82, 401–409. [Google Scholar] [CrossRef]
- Lee, S.-W.; Daud, W.M.A.W.; Lee, M.-G. Adsorption characteristics of methyl mercaptan, dimethyl disulfide, and trimethylamine on coconut-based activated carbons modified with acid and base. J. Ind. Eng. Chem. 2010, 16, 973–977. [Google Scholar] [CrossRef]
- Darif, B.; Ojala, S.; Pirault-Roy, L.; Bensitel, M.; Brahmi, R.; Keiski, R.L. Study on the catalytic oxidation of DMDS over Pt-Cu catalysts supported on Al2O3, AlSi20 and SiO2. Appl. Catal. B 2016, 181, 24–33. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.; Shang, D.; Zheng, J.; Du, Z.; Wu, Z.; Shao, M.; Zeng, L.; et al. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar] [CrossRef]
- Son, Y.-S.; Kim, J.-C. Decomposition of sulfur compounds by radiolysis: I. Influential factors. Chem. Eng. J. 2015, 262, 217–223. [Google Scholar] [CrossRef]
- Hudson, N.; Ayoko, G.A.; Dunlop, M.; Duperouzel, D.; Burrell, D.; Bell, K.; Gallagher, E.; Nicholas, P.; Heinrich, N. Comparison of odour emission rates measured from various sources using two sampling devices. Bioresour. Technol. 2009, 100, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Sironi, S.; Del Rosso, R.; Centola, P. Predicting odour emissions from wastewater treatment plants by means of odour emission factors. Water Res. 2009, 43, 1977–1985. [Google Scholar] [CrossRef]
- Fang, J.J.; Yang, N.; Cen, D.Y.; Shao, L.M.; He, P.J. Odor compounds from different sources of landfill: Characterization and source identification. Waste Manag. 2012, 32, 1401–1410. [Google Scholar] [CrossRef]
- Lu, N.; Yu, H.T.; Su, Y.; Wu, Y. Water absorption and photocatalytic activity of TiO2 in a scrubber system for odor control at varying pH. Sep. Purif. Technol. 2012, 90, 196–203. [Google Scholar] [CrossRef]
- Montebello, A.M.; Fernández, M.; Almenglo, F.; Ramírez, M.; Cantero, D.; Baeza, M.; Gabriel, D. Simultaneous methylmercaptan and hydrogen sulfide removal in the desulfurization of biogas in aerobic and anoxic biotrickling filters. Chem. Eng. J. 2012, 200–202, 237–246. [Google Scholar] [CrossRef]
- Zhao, S.; Yi, H.; Tang, X.; Gao, F.; Zhang, B.; Wang, Z.; Zuo, Y. Methyl mercaptan removal from gas streams using metal-modified activated carbon. J. Clean. Prod. 2015, 87, 856–861. [Google Scholar] [CrossRef]
- Papurello, D.; Schuhfried, E.; Lanzini, A.; Romano, A.; Cappellin, L.; Märk, T.D.; Silvestri, S.; Biasioli, F. Influence of co-vapors on biogas filtration for fuel cells monitored with PTR-MS (Proton Transfer Reaction-Mass Spectrometry). Fuel Process. Technol. 2014, 118, 133–140. [Google Scholar] [CrossRef]
- Papurello, D.; Schuhfried, E.; Lanzini, A.; Romano, A.; Cappellin, L.; Märk, T.D.; Silvestri, S.; Santarelli, M.; Biasioli, F. Proton transfer reaction-mass spectrometry as a rapid inline tool for filter efficiency of activated charcoal in support of the development of Solid Oxide Fuel Cells fueled with biogas. Fuel Process. Technol. 2015, 130, 78–86. [Google Scholar] [CrossRef]
- Jiang, D.E.; Zhao, B.; Xie, Y.; Pan, G.; Ran, G.; Min, E. Structure and basicity of Al2O3-supported MgO and its application to mercaptan oxidation. Appl. Catal. A 2001, 219, 69–78. [Google Scholar] [CrossRef]
- Kastner, J.R.; Das, K.C.; Buquoi, Q.; Melear, N.D. Low Temperature Catalytic Oxidation of Hydrogen Sulfide and Methanethiol Using Wood and Coal Fly Ash. Environ. Sci. Technol. 2003, 37, 2568–2574. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Chen, D.; Hao, H.; Yu, J.; Liu, J.; Lu, J.; Liu, F.; Wan, G.; He, S.; Luo, Y. Structural/surface characterization and catalytic evaluation of rare-earth (Y, Sm and La) doped ceria composite oxides for CH3SH catalytic decomposition. Appl. Surf. Sci. 2016, 390, 959–967. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Conversion of poisonous methanethiol to hydrogen-rich gas by chemisorption/reforming over nano-scale CeO2: The use of CeO2 as catalyst coating material. Appl. Catal. B 2011, 102, 267–275. [Google Scholar] [CrossRef]
- Hulea, V.; Huguet, E.; Cammarano, C.; Lacarriere, A.; Durand, R.; Leroi, C.; Cadours, R.; Coq, B. Conversion of methyl mercaptan and methanol to hydrocarbons over solid acid catalysts—A comparative study. Appl. Catal. B 2014, 144, 547–553. [Google Scholar] [CrossRef]
- He, D.; Hao, H.; Chen, D.; Lu, J.; Zhong, L.; Chen, R.; Liu, F.; Wan, G.; He, S.; Luo, Y. Rapid synthesis of nano-scale CeO2 by microwave-assisted sol–gel method and its application for CH3SH catalytic decomposition. J. Environ. Chem. Eng. 2016, 4, 311–318. [Google Scholar] [CrossRef]
- Cao, X.; He, D.; Lu, J.; Zhao, Y.; Mei, Y.; Han, C.; Luo, Y. The negative effect of P addition of La/HZSM-5 on the catalytic performance in methyl mercaptan abatement. Appl. Surf. Sci. 2019, 494, 1083–1090. [Google Scholar] [CrossRef]
- Lu, J.; Hao, H.; Zhang, L.; Xu, Z.; Zhong, L.; Zhao, Y.; He, D.; Liu, J.; Chen, D.; Pu, H.; et al. The investigation of the role of basic lanthanum (La) species on the improvement of catalytic activity and stability of HZSM-5 material for eliminating methanethiol (CH3SH). Appl. Catal. B 2018, 237, 185–197. [Google Scholar] [CrossRef]
- He, D.; Wan, G.; Hao, H.; Chen, D.; Lu, J.; Zhang, L.; Liu, F.; Zhong, L.; He, S.; Luo, Y. Microwave-assisted rapid synthesis of CeO2 nanoparticles and its desulfurization processes for CH3SH catalytic decomposition. Chem. Eng. J. 2016, 289, 161–169. [Google Scholar] [CrossRef]
- He, D.; Chen, D.; Hao, H.; Yu, J.; Liu, J.; Lu, J.; Wan, G.; He, S.; Li, K.; Luo, Y. Enhanced activity and stability of Sm-doped HZSM-5 zeolite catalysts for catalytic methyl mercaptan (CH3SH) decomposition. Chem. Eng. J. 2017, 317, 60–69. [Google Scholar] [CrossRef]
- Gallego, G.S.; Marín, J.G.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Influence of Pr and Ce in dry methane reforming catalysts produced from La1−xAxNiO3−δ perovskites. Appl. Catal. A 2009, 369, 97–103. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Zhou, C.; Lim, Z.Y.; Wu, C.; Ye, S.; Wang, W.G. Effect of Pr addition on the properties of Ni/Al2O3 catalysts with an application in the autothermal reforming of methane. Int. J. Hydrog. Energy 2014, 39, 778–787. [Google Scholar] [CrossRef]
- Muñoz, M.; Moreno, S.; Molina, R. Synthesis of Ce and Pr-promoted Ni and Co catalysts from hydrotalcite type precursors by reconstruction method. Int. J. Hydrog. Energy 2012, 37, 18827–18842. [Google Scholar] [CrossRef]
- Lu, J.; Lei, Y.; Wan, G.; Mei, Z.; Yu, J.; Zhao, Y.; He, S.; Luo, Y. Weakening the metal-support strong interaction to enhance catalytic performances of alumina supported Ni-based catalysts for producing hydrogen. Appl. Catal. B 2020, 263, 118177. [Google Scholar] [CrossRef]
- Barroso, M.N.; Galetti, A.E.; Abello, M.C. Ni catalysts supported over MgAl2O4 modified with Pr for hydrogen production from ethanol steam reforming. Appl. Catal. A 2011, 394, 124–131. [Google Scholar] [CrossRef]
- Liu, J.; He, D.; Chen, D.; Hao, H.; Yu, J.; Lu, J.; Liu, F.; Liu, P.; Zhao, Y.; Luo, Y. Promotional effects of rare-earth (La, Ce and Pr) modification over HZSM-5 for methyl mercaptan catalytic decomposition. J. Taiwan Inst. Chem. Eng. 2017, 80, 262–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, D.; Liu, J.; He, D.; Cao, X.; Han, C.; Lu, J.; Luo, Y. Tuning the metal-support interaction on chromium-based catalysts for catalytically eliminate methyl mercaptan: Anchored active chromium species through surface hydroxyl groups. Chem. Eng. J. 2020, 389, 124384. [Google Scholar] [CrossRef]
- Zhao, Y.; He, D.; Chen, D.; Lu, J.; Yu, J.; Liu, J.; Cao, X.; Han, C.; Luo, Y. Investigating the Support Effect for Catalytic Elimination of Methyl Mercaptan: Role of Hydroxyl Groups over Cr-based Catalysts. Catal. Lett. 2020, 150, 2763–2773. [Google Scholar] [CrossRef]
- Lu, J.; Liu, J.; Zhao, Y.; He, D.; Han, C.; He, S.; Luo, Y. The identification of active chromium species to enhance catalytic behaviors of alumina-based catalysts for sulfur-containing VOC abatement. J. Hazard. Mater. 2020, 384, 121289. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, L.; Zhao, Y.; Mei, Y.; Chen, D.; He, S.; Luo, Y. Recycling Spent Cr Adsorbents as Catalyst for Eliminating Methylmercaptan. Environ. Sci. Technol. 2018, 52, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, J.; Chen, D.; Zhang, L.; He, S.; Han, C.; He, D.; Luo, Y. Probing the nature of active chromium species and promotional effects of potassium in Cr/MCM-41 catalysts for methyl mercaptan abatement. New J. Chem. 2019, 43, 12814–12822. [Google Scholar] [CrossRef]
- Bing, J.; Li, L.; Lan, B.; Liao, G.; Zeng, J.; Zhang, Q.; Li, X. Synthesis of cerium-doped MCM-41 for ozonation of p-chlorobenzoic acid in aqueous solution. Appl. Catal. B 2012, 115–116, 16–24. [Google Scholar] [CrossRef]
- Ruiz, J.A.C.; Fraga, M.A.; Pastore, H.O. Methane combustion over Pd supported on MCM-41. Appl. Catal. B 2007, 76, 115–122. [Google Scholar] [CrossRef]
- Asghari, S.; Haghighi, M.; Taghavinezhad, P. Plasma-enhanced dispersion of Cr2O3 over ceria-doped MCM-41 nanostructured catalyst used in CO2 oxidative dehydrogenation of ethane to ethylene. Microporous Mesoporous Mater. 2019, 279, 165–177. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Deaconu, M.; Nicu, I.; Vasile, E.; Mitran, R.-A.; Matei, C.; Berger, D. Heteroatom modified MCM-41-silica carriers for Lomefloxacin delivery systems. Microporous Mesoporous Mater. 2019, 275, 214–222. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Sun, X.; Yang, J.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Reducing Energy Penalty of CO2 Capture Using Fe Promoted SO42−/ZrO2/MCM-41 Catalyst. Environ. Sci. Technol. 2019, 53, 6094–6102. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Nitta, S.; Shimazu, S.; Kurashina, M.; Katoh, M.; Ninomiya, W.; Sugiyama, S. Effect of Introduction of Trace Amount of Chromium Species in Improving Catalytic Performance of MCM-48 in Oxidative Dehydrogenation of Isobutane. J. Chem. Eng. Jpn. 2019, 52, 99–105. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, Y. Enhanced nitrobenzene adsorption in aqueous solution by surface silylated MCM-41. Microporous Mesoporous Mater. 2016, 232, 143–150. [Google Scholar] [CrossRef]
- Ma, T.; Yun, Z.; Xu, W.; Chen, L.; Li, L.; Ding, J.; Shao, R. Pd-H3PW12O40/Zr-MCM-41: An efficient catalyst for the sustainable dehydration of glycerol to acrolein. Chem. Eng. J. 2016, 294, 343–352. [Google Scholar] [CrossRef]
- Li, S.H.; Sun, X.D.; Wang, Y.Z.; Wang, Z.P.; Gu, W.B.; Wang, W.M.; Yao, H.M.; Wang, Y.; Zhu, J.H. Creating an Optimal Microenvironment within Mesoporous Silica MCM-41 for Capture of Tobacco-Specific Nitrosamines in Solution. ACS Appl. Mater. Interfaces 2017, 9, 26805–26817. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Coke- and sintering-resistant monolithic catalysts derived from in situ supported hydrotalcite-like films on Al wires for dry reforming of methane. Nanoscale 2013, 5, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Wang, H.; Sun, Y. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. J. Catal. 2013, 298, 51–60. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.S.; Jung, U.H.; Seo, D.J.; Seo, Y.S.; Yoon, W.L. Combined H2O and CO2 reforming of CH4 over nano-sized Ni/MgO-Al2O3 catalysts for synthesis gas production for gas to liquid (GTL): Effect of Mg/Al mixed ratio on coke formation. Catal. Today 2009, 146, 166–171. [Google Scholar] [CrossRef]
- Rutkowska, M.; Macina, D.; Mirocha-Kubień, N.; Piwowarska, Z.; Chmielarz, L. Hierarchically structured ZSM-5 obtained by desilication as new catalyst for DME synthesis from methanol. Appl. Catal. B 2015, 174–175, 336–343. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.; Moulijn, J.A.; Perez-Ramirez, J. Mechanism of hierarchical porosity development in MFI zeolites by desilication: The role of aluminium as a pore-directing agent. Chemistry 2005, 11, 4983–4994. [Google Scholar] [CrossRef]
- Wolfframm, D.; Ratzke, M.; Kappa, M.; Montenegro, M.J.; Döbeli, M.; Lippert, T.; Reif, J. Pulsed laser deposition of thin PrxOy films on Si(100). Mater. Sci. Eng. B 2004, 109, 24–29. [Google Scholar] [CrossRef]
- Ding, J.-F.; Ma, T.-L.; Yan, C.-X.; Shao, R.; Xu, W.; Guan, R.-F.; Song, X.-Y.; Wang, P.-F. Dehydration of Castor Oil over a NaHSO4/MCM-41 Catalyst Prepared by Supercritical Impregnation. Chem. Eng. Technol. 2018, 41, 2186–2195. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, Q.-Q.; Li, J.; Su, X.-L.; Wu, H.-Y.; Guan, X.-X.; Zheng, X.-C. Tungstophosphoric acid-based mesoporous materials anchored to MCM-41: Characterization and catalytic performance in esterification of levulinic acid with ethanol. J. Porous Mater. 2016, 23, 1329–1338. [Google Scholar] [CrossRef]
- Brasil, H.; Carvalho, A.L.G.d.; Costa, F.F.; Nascimento, L.A.S.d.; Mhadmhan, S.; Pineda, A.; Luque, R.; Valença, G.P. Preparation of novel mesoporous Ca/P MCM-41-based materials for mechanochemical diphenyl sulfide oxidation. Microporous Mesoporous Mater. 2020, 297, 110017. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J.; Zhang, H.; Liu, Y.; Dai, H. Three-dimensionally ordered macroporous Pr6O11 and Tb4O7 with mesoporous walls: Preparation, characterization, and catalytic activity for CO oxidation. Catal. Today 2015, 245, 28–36. [Google Scholar] [CrossRef]
- Gamba, O.; Moreno, S.; Molina, R. Catalytic performance of Ni–Pr supported on delaminated clay in the dry reforming of methane. Int. J. Hydrog. Energy 2011, 36, 1540–1550. [Google Scholar] [CrossRef]
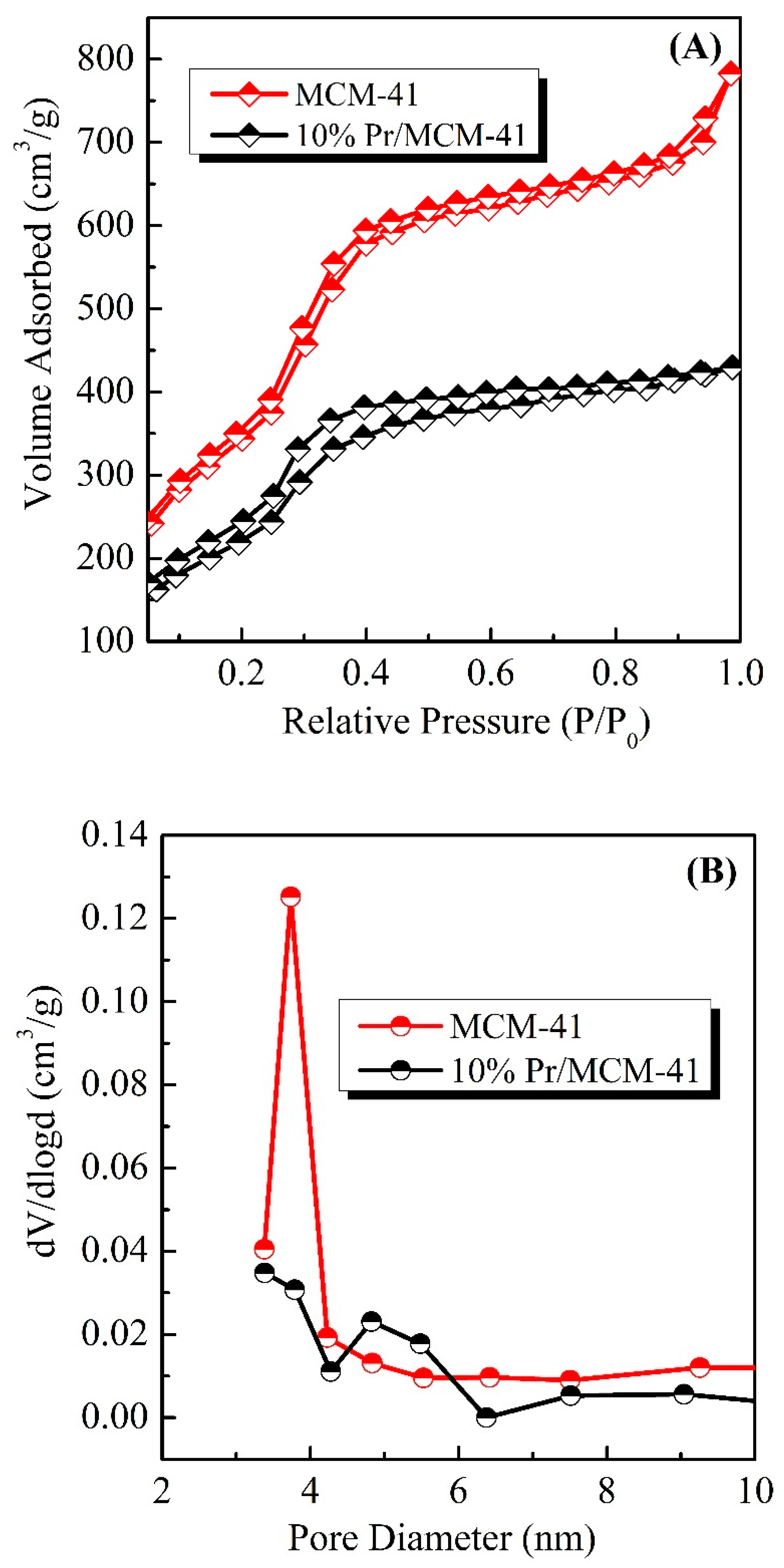


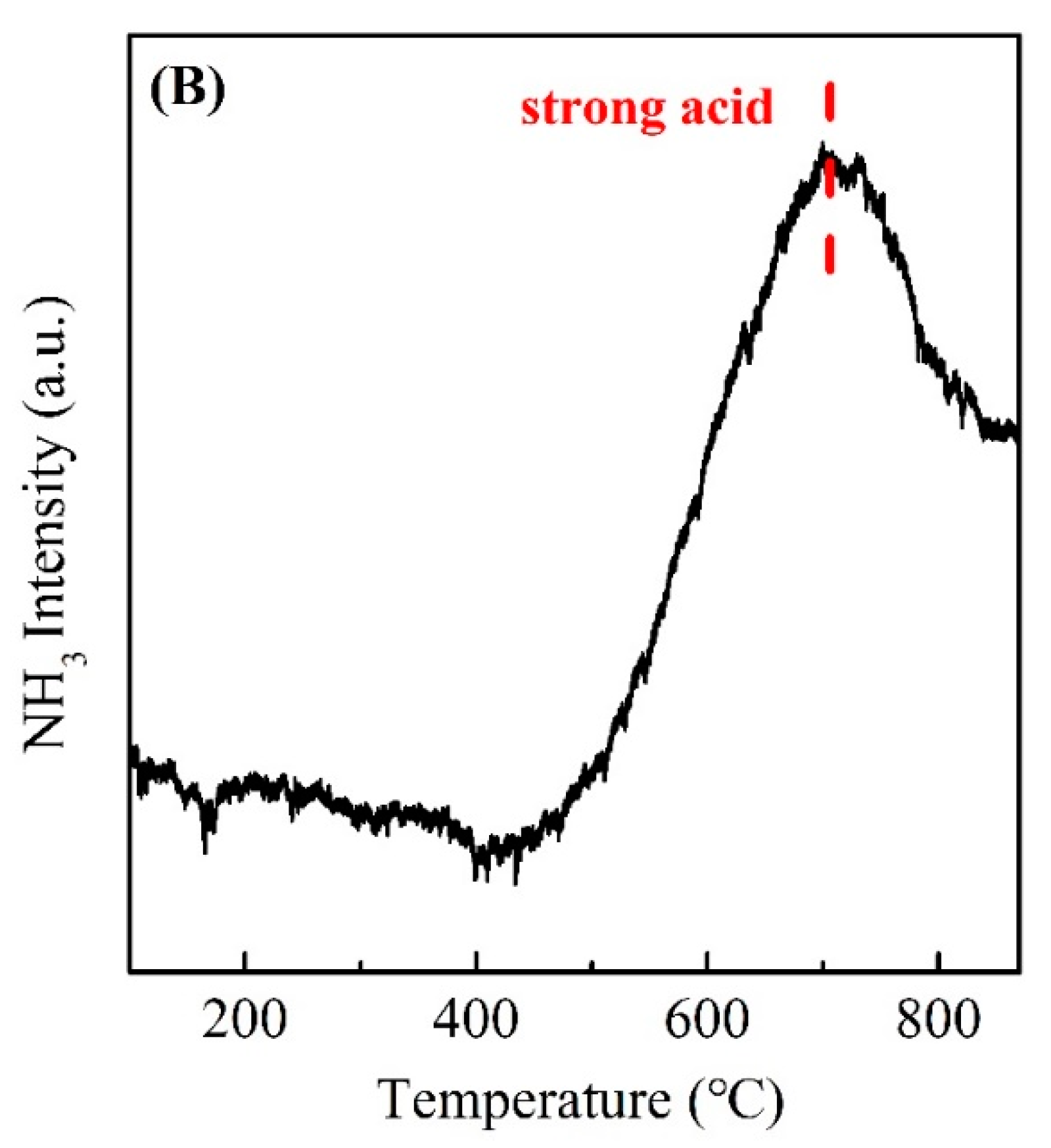
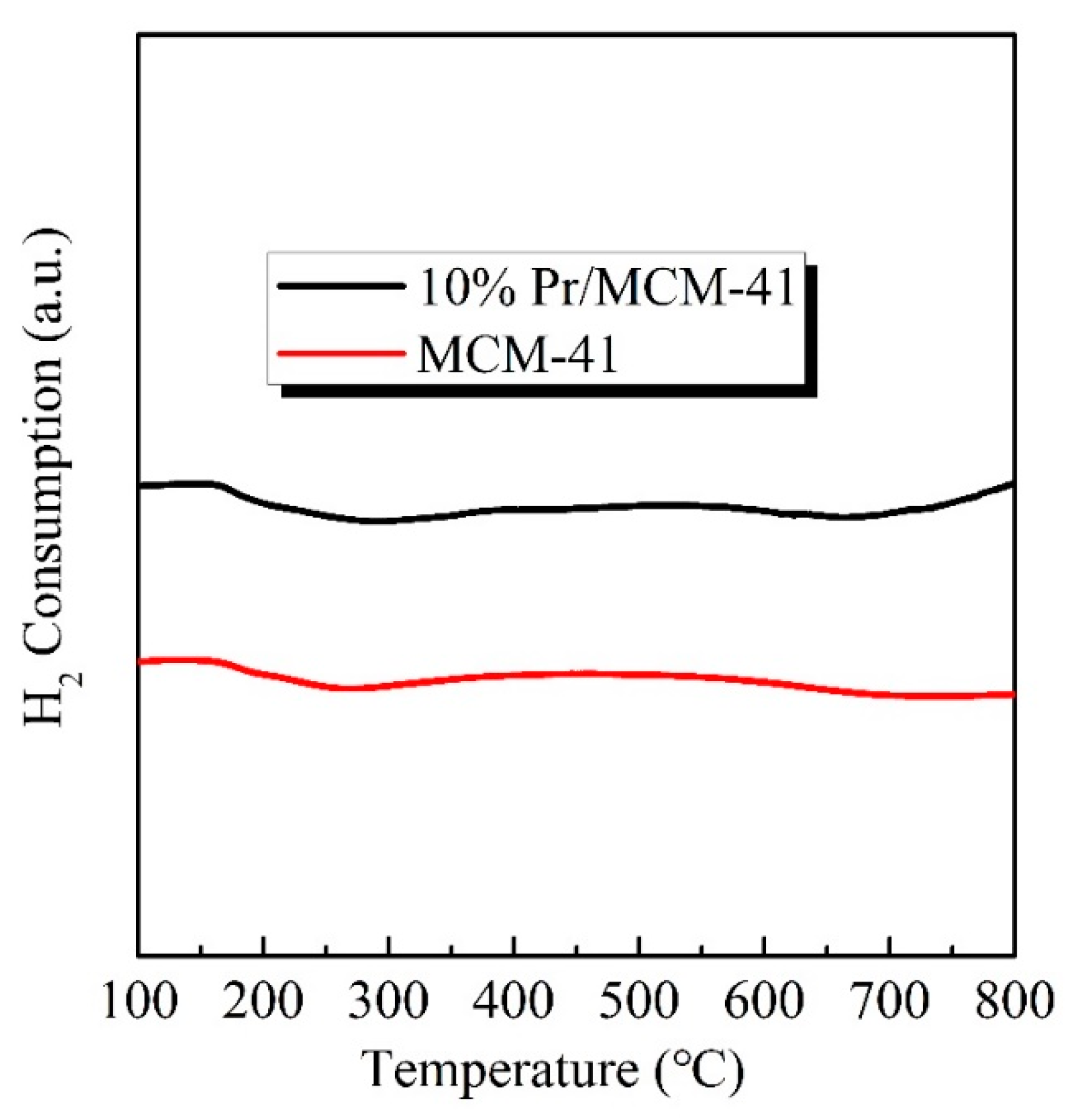

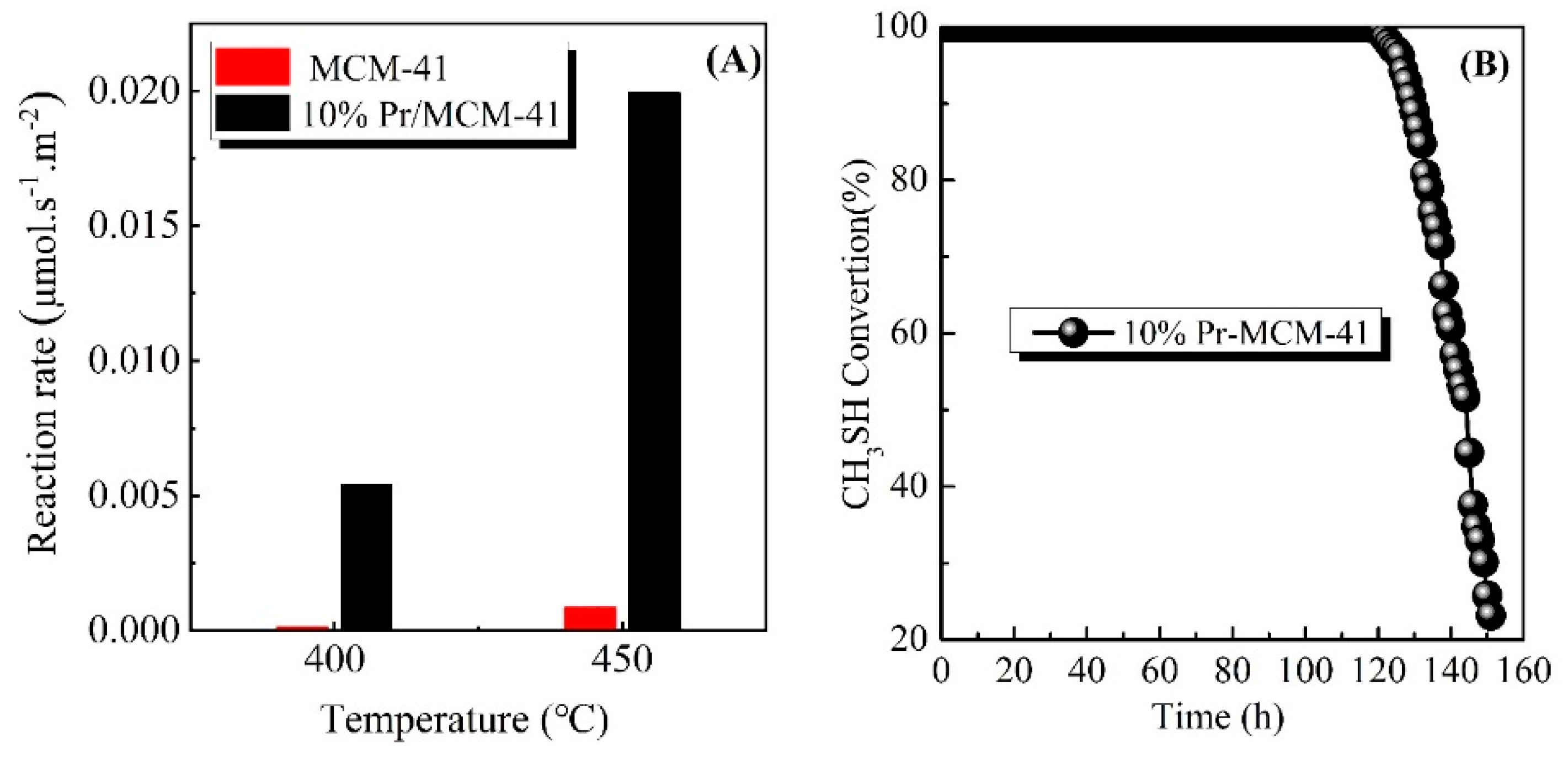
| Sample | SBET (m2/g) | Pore Volume (cc/cm3) | Pore Diameter (nm) |
|---|---|---|---|
| MCM-41 | 1289 | 0.379 | 3.742 |
| 10% Pr/MCM-41 | 920 | 0.111 | 3.382 |
| Sample | Pr4+ | Pr3+ | Pr4+/Pr3+ | Si | OSi-O-Si | ||
|---|---|---|---|---|---|---|---|
| Content (%) | Binding Energy (eV) | Content (%) | Binding Energy (eV) | Binding Energy (eV) | Binding Energy (eV) | ||
| MCM-41 | — | — | — | — | — | 103.85 | 533.03 |
| 10%Pr/MCM-41 | 70.24 | 954.83 | 29.76 | 933.95 | 2.36 | 103.47 | 532.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Lu, J.; Zhao, Y.; Tian, R.; Zhang, W.; He, D.; Luo, Y. Promotional Effects of Rare-Earth Praseodymium (Pr) Modification over MCM-41 for Methyl Mercaptan Catalytic Decomposition. Processes 2021, 9, 400. https://doi.org/10.3390/pr9020400
Cao X, Lu J, Zhao Y, Tian R, Zhang W, He D, Luo Y. Promotional Effects of Rare-Earth Praseodymium (Pr) Modification over MCM-41 for Methyl Mercaptan Catalytic Decomposition. Processes. 2021; 9(2):400. https://doi.org/10.3390/pr9020400
Chicago/Turabian StyleCao, Xiaohua, Jichang Lu, Yutong Zhao, Rui Tian, Wenjun Zhang, Dedong He, and Yongming Luo. 2021. "Promotional Effects of Rare-Earth Praseodymium (Pr) Modification over MCM-41 for Methyl Mercaptan Catalytic Decomposition" Processes 9, no. 2: 400. https://doi.org/10.3390/pr9020400
APA StyleCao, X., Lu, J., Zhao, Y., Tian, R., Zhang, W., He, D., & Luo, Y. (2021). Promotional Effects of Rare-Earth Praseodymium (Pr) Modification over MCM-41 for Methyl Mercaptan Catalytic Decomposition. Processes, 9(2), 400. https://doi.org/10.3390/pr9020400






