Research on Double-Stage and Multi-Stage Capacitive Deionization Absorption Air-Conditioning System
Abstract
1. Introduction
2. Materials and Methods
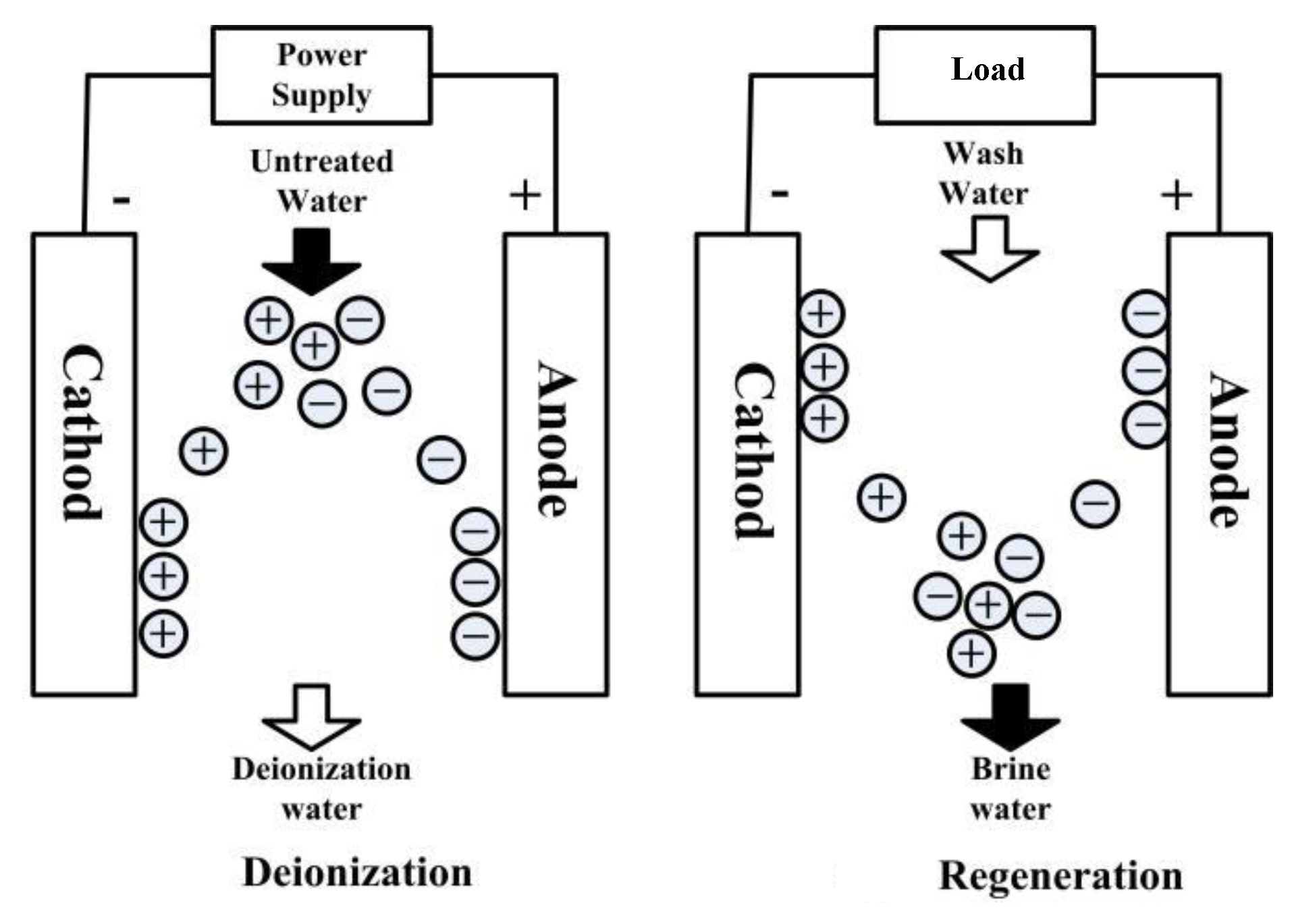
2.1. Principle of the Capacitive Deionization Process
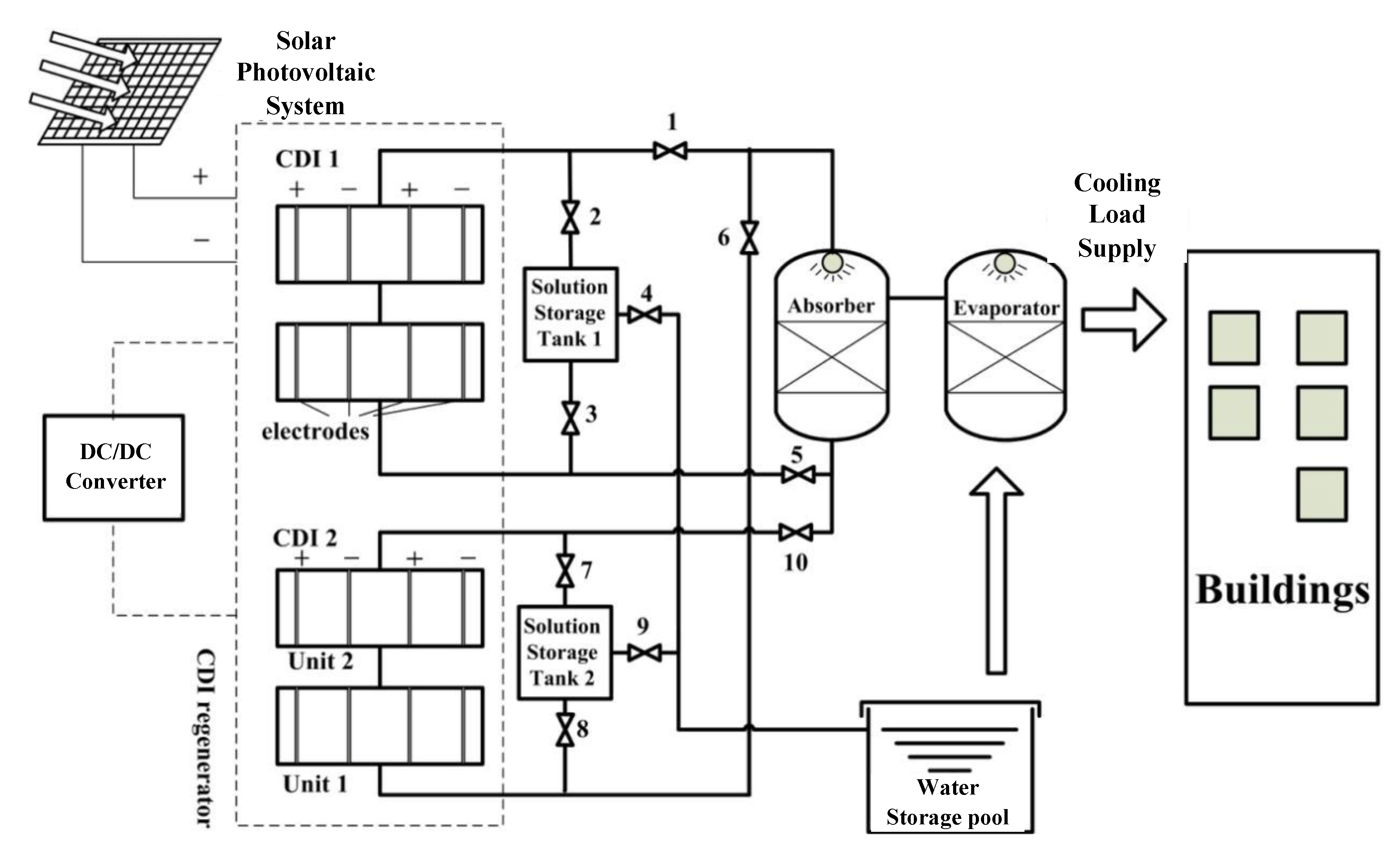
2.2. Single-Stage CDI Absorption Air-Conditioning System
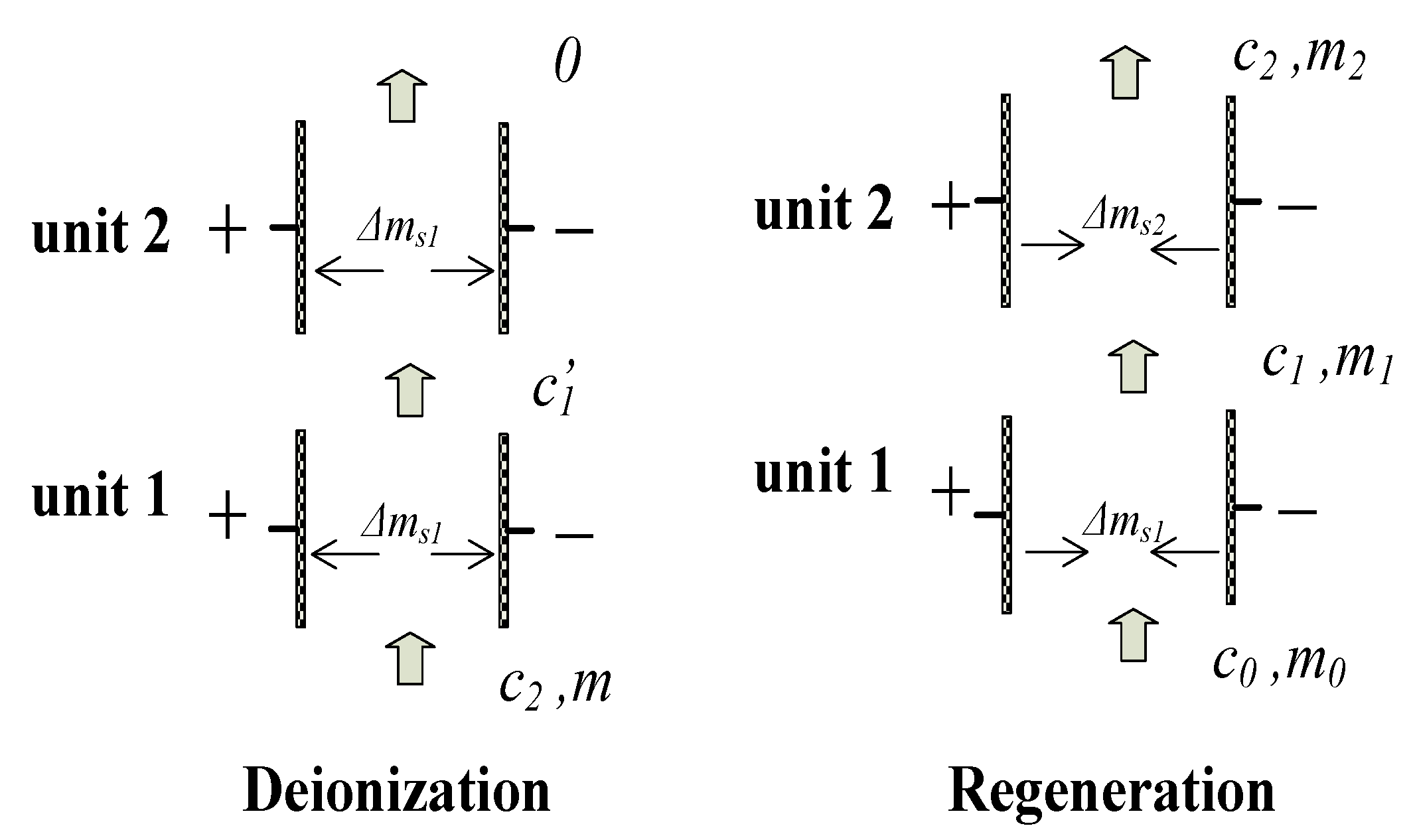
2.3. Double-Stage CDI Absorption Air-Conditioning System
3. Results and Discussion
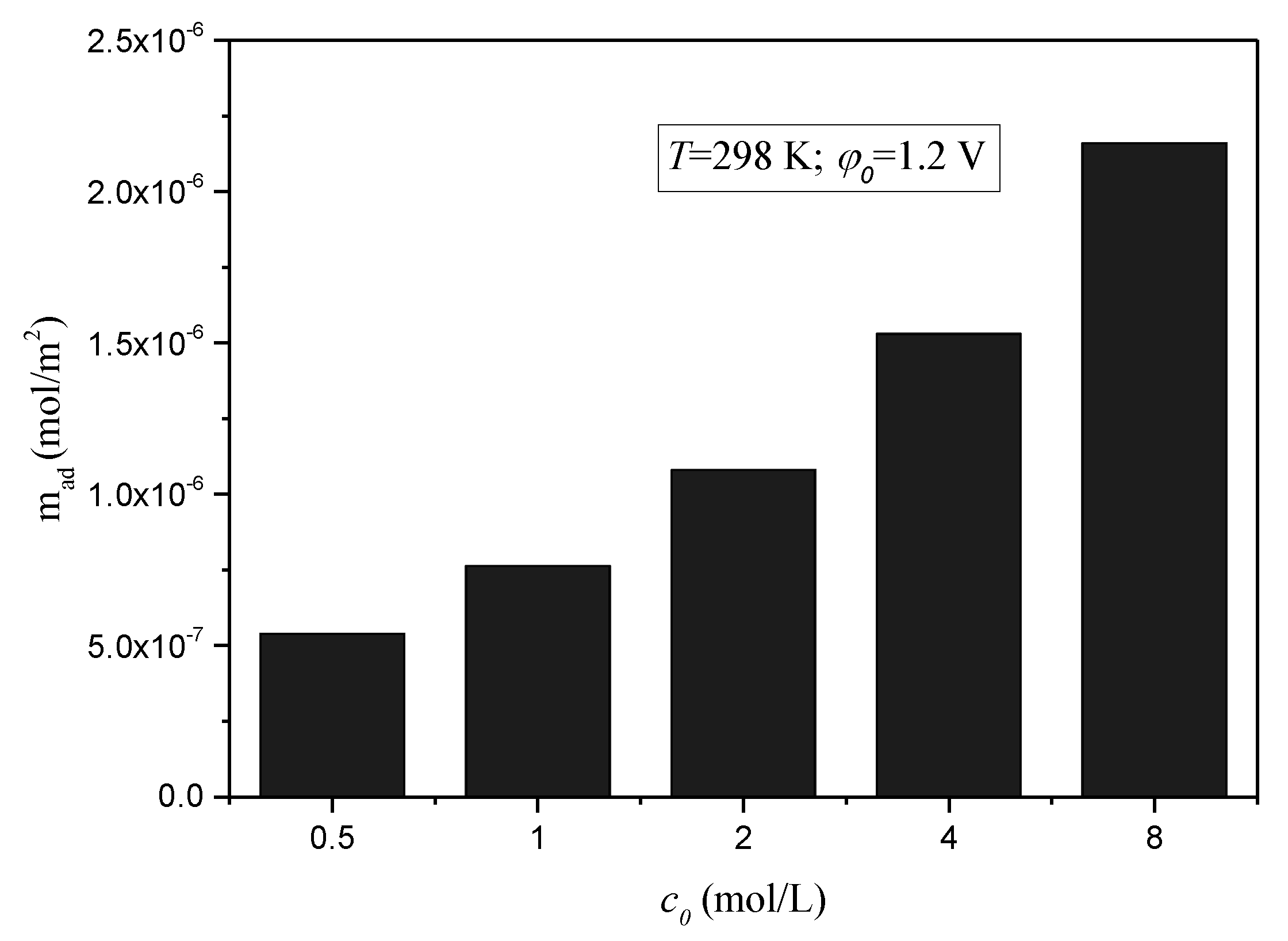
3.1. The Adsorption Properties of CDI Method
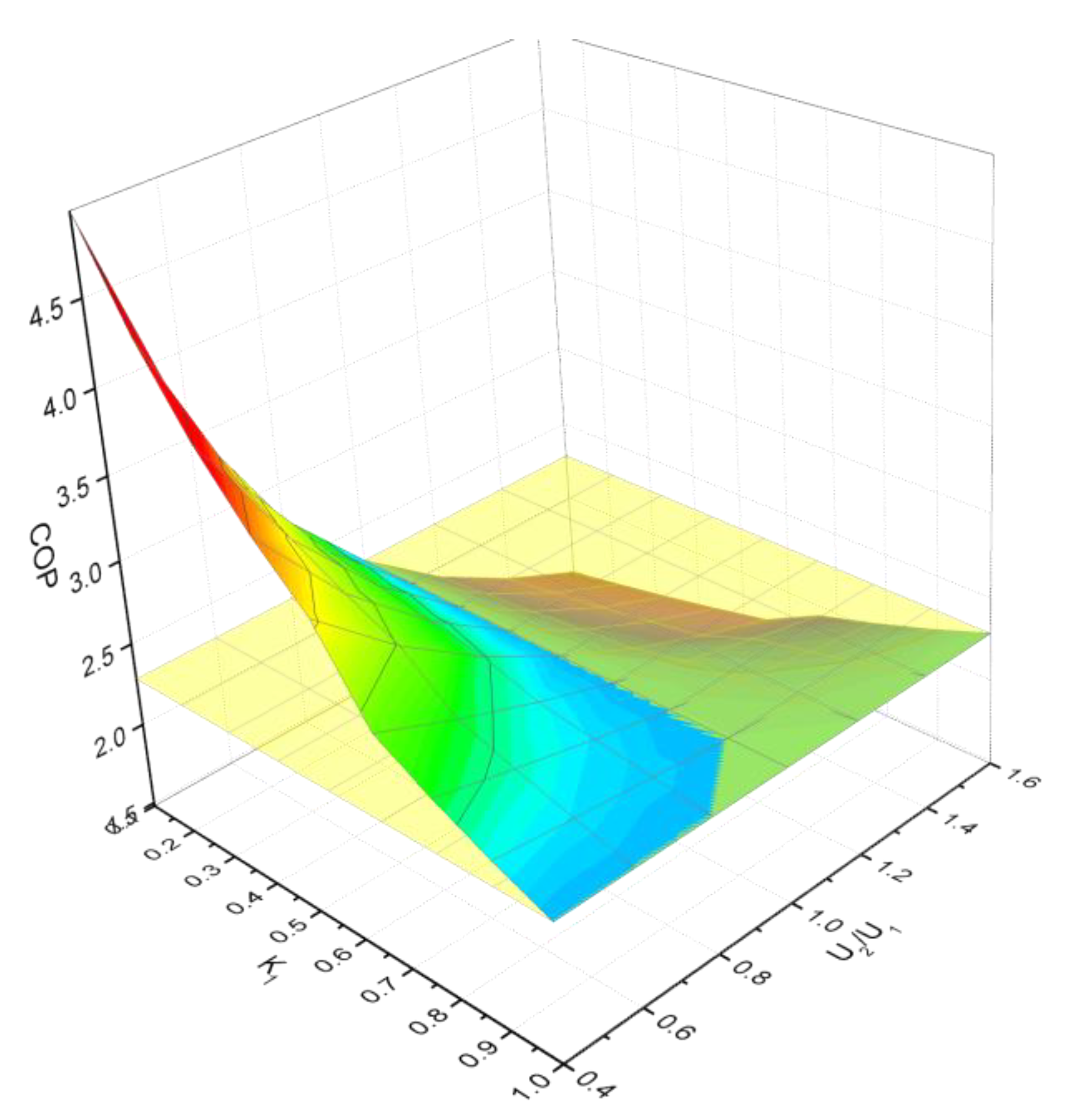
3.2. Performance Analysis of the Double-Stage CDI System
- If k1 < 0.5, it indicates the ions adsorbed by unit 1 are less than that of unit 2; on the other side, c1 < c0, so U2 must be larger than U1, it is U2/U1 > 1.
- It can be found from Figure 9, the COP of the double-stage system is always less than that of the single-stage system when U2/U1 > 1.
3.3. Optimization of the Multi-Stage CDI System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| c | Mass concentration of the solute |
| F | Faraday constant, sA/mol |
| Latent heat of evaporation of water, kJ/(kg × K) | |
| Molecular weight of the solute in the absorbent solution, kg/mol | |
| U | Voltage, V |
| z | Electrochemical valence |
| P | Energy consumption |
| Q | Acquired cooling capacity, kJ |
| m | Mass flow rate, kg/s |
| T | Temperature, K |
| Greek letters | |
| φ | Potential, V |
| ρ | Charge density, C/m2 |
| λ | Charge efficiency |
| η | Energy recovery efficiency |
| Subscripts | |
| 0 | Initial solution |
| 1 | Solution at exit of CDI unit 1 |
| 2 | Solution at exit of CDI unit 2 |
| ia | Entrance of absorber |
| oa | Exit of absorber |
| n | Number of stages |
References
- Solangi, Y.A.; Cheng, L.; Shah, S.A.A.; Alsanad, A.; Ahmad, M.; Akbar, M.A.; Gumaei, A. Analyzing renewable energy sources of a developing country for sustainable development: An integrated fuzzy based-decision methodology. Processes 2020, 8, 825. [Google Scholar] [CrossRef]
- Zhang, X.; Reible, D. Exploring the function of ion-exchange membrane in membrane capacitive deionization via a fully coupled two-dimensional process model. Processes 2020, 8, 1312. [Google Scholar] [CrossRef]
- Ma, Z.; Song, J.; Zhang, J. Energy consumption prediction of air-conditioning systems in buildings by selecting similar days based on combined weights. Energy Build. 2017, 151, 157–166. [Google Scholar] [CrossRef]
- Al-Ugla, A.A.; El-Shaarawi, M.A.I.; Said, S.A.M. Alternative designs for a 24-h operating solar-powered LiBr e water absorption air-conditioning technology. Int. J. Refrig. 2015, 53, 90–100. [Google Scholar] [CrossRef]
- Siddiqui, M.U.; Said, S.A.M. A review of solar powered absorption systems. Renew. Sustain. Energy Rev. 2015, 42, 93–115. [Google Scholar] [CrossRef]
- Han, W.; Sun, L.; Zheng, D.; Jin, H.; Ma, S.; Jing, X. New hybrid absorption-compression refrigeration system based on cascade use of mid-temperature waste heat. Appl. Energy 2013, 106, 383–390. [Google Scholar] [CrossRef]
- Jain, V.; Kachhwaha, S.S.; Sachdeva, G. Thermodynamic performance analysis of a vapor compression-absorption cascaded refrigeration system. Energy Convers. Manag. 2013, 75, 685–700. [Google Scholar] [CrossRef]
- Avanessian, T.; Ameri, M. Energy, exergy, and economic analysis of single and double effect LiBr-H2O absorption chillers. Energy Build. 2014, 73, 26–36. [Google Scholar] [CrossRef]
- Li, Z.; Ye, X.; Liu, J. Performance analysis of solar air cooled double effect LiBr/H2O absorption cooling system in subtropical city. Energy Convers. Manag. 2014, 85, 302–312. [Google Scholar] [CrossRef]
- Bouaziz, N.; Lounissi, D. Energy and exergy investigation of a novel double effect hybrid absorption refrigeration system for solar cooling. Int. J. Hydrog. Energy 2015, 40, 13849–13856. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X. Membrane air-conditioning system driven by renewable energy. Energy Convers. Manag. 2012, 53, 189–195. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Quan, S. Single-stage and double-stage photovoltaic driven regeneration for liquid desiccant cooling system. Appl. Energy 2011, 88, 4908–4917. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Chen, Q. Research on mass transfer and actual performance of the membrane regeneration air-conditioning system. Energy Convers. Manag. 2015, 106, 84–92. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, H.; Zhang, Z. Capacitive deionization regeneration as a possible improvement of membrane regeneration method for absorption air-conditioning system. Appl. Energy 2016, 171, 405–414. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, H.; Zhang, Z. The potentials of capacitive deionization regeneration method for absorption air-conditioning system. Energy Convers. Manag. 2016, 114, 303–311. [Google Scholar] [CrossRef]
- Yao, Q.; Tang, H.L. Occurrence of re-adsorption in desorption cycles of capacitive deionization. J. Ind. Eng. Chem. 2016, 34, 180–185. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Niu, R. Improved capacitive deionization performance by coupling TiO2 nanoparticles with carbon nanotubes. Sep. Purif. Technol. 2016, 171, 93–100. [Google Scholar] [CrossRef]
- Qu, Y.; Campbell, P.G.; Gu, L.; Knipe, J.M.; Dzenitis, E.; Santiago, J.G.; Stadermann, M. Energy consumption analysis of constant voltage and constant current operations in capacitive deionization. Desalination 2016, 400, 18–24. [Google Scholar] [CrossRef]
- Andres, G.L.; Yoshihara, Y. A capacitive deionization system with high energy recovery and effective re-use. Energy 2016, 103, 605–617. [Google Scholar] [CrossRef]
- Kang, J.; Kim, T.; Shin, H.; Lee, J.; Ha, J.I.; Yoon, J. Direct energy recovery system for membrane capacitive deionization. Desalination 2016, 398, 144–150. [Google Scholar] [CrossRef]
- Tang, W.; Kovalsky, P.; He, D.; Waite, T.D. Fluoride and nitrate removal from brackish groundwaters by batch-mode capacitive deionization. Water Res. 2015, 84, 342–349. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P.M. Progress in Materials Science Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Laxman, K.; Tay, M.; Myint, Z.; Al, M.; Sathe, P.; Dobretsov, S.; Dutta, J. Desalination and disinfection of inland brackish ground water in a capacitive deionization cell using nanoporous activated carbon cloth electrodes. Desalination 2015, 362, 126–132. [Google Scholar] [CrossRef]
- Jande, Y.A.C.; Kim, W.S. Desalination using capacitive deionization at constant current. Desalination 2013, 329, 29–34. [Google Scholar] [CrossRef]
- Jande, Y.A.C.; Kim, W.S. Predicting the lowest effluent concentration in capacitive deionization. Sep. Purif. Technol. 2013, 115, 224–230. [Google Scholar] [CrossRef]





| Parameter | Value | Remarks |
|---|---|---|
| c2 | 55% | |
| z | 1 | The absorbent is LiBr solution |
| λ | 50% | This value is from a typical CDI product |
| η | 50% | This value is from a typical CDI product |
| U1 | 0.8 V | The common voltage of a CDI product |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.; Ding, B.; Li, X. Research on Double-Stage and Multi-Stage Capacitive Deionization Absorption Air-Conditioning System. Processes 2021, 9, 395. https://doi.org/10.3390/pr9020395
Cheng F, Ding B, Li X. Research on Double-Stage and Multi-Stage Capacitive Deionization Absorption Air-Conditioning System. Processes. 2021; 9(2):395. https://doi.org/10.3390/pr9020395
Chicago/Turabian StyleCheng, Feng, Boqing Ding, and Xiuwei Li. 2021. "Research on Double-Stage and Multi-Stage Capacitive Deionization Absorption Air-Conditioning System" Processes 9, no. 2: 395. https://doi.org/10.3390/pr9020395
APA StyleCheng, F., Ding, B., & Li, X. (2021). Research on Double-Stage and Multi-Stage Capacitive Deionization Absorption Air-Conditioning System. Processes, 9(2), 395. https://doi.org/10.3390/pr9020395







