3.1. Thermal Reaction Characteristics of High-Sulfur Bauxite
During the oxidation roasting process, various reactions occur, including the dehydration of hydrous mineral (e.g., diaspore and kaolinite) and the desulfurization of pyrite.
Figure 3 shows TG/DTG curves of high-sulfur bauxite at different heating rates.
As shown in
Figure 3, the obvious weight loss of high sulfur bauxite begins at 420 °C and ends at about 790 °C. It can be seen from the DTG curve in
Figure 2 that the decomposition process of high-sulfur bauxite consists of two stages. The first stage starts at about 420 °C and ends at 560–625 °C. The peak value of weight loss rate appears between 495.4 and 526.4 °C. In the second stage, the temperature range of weight loss is 670 –790 °C, and the peak temperature of weight loss is 705–735 °C. The initial temperature, peak temperature, and end temperature of the reaction shift to a high-temperature region with increasing heating rate.
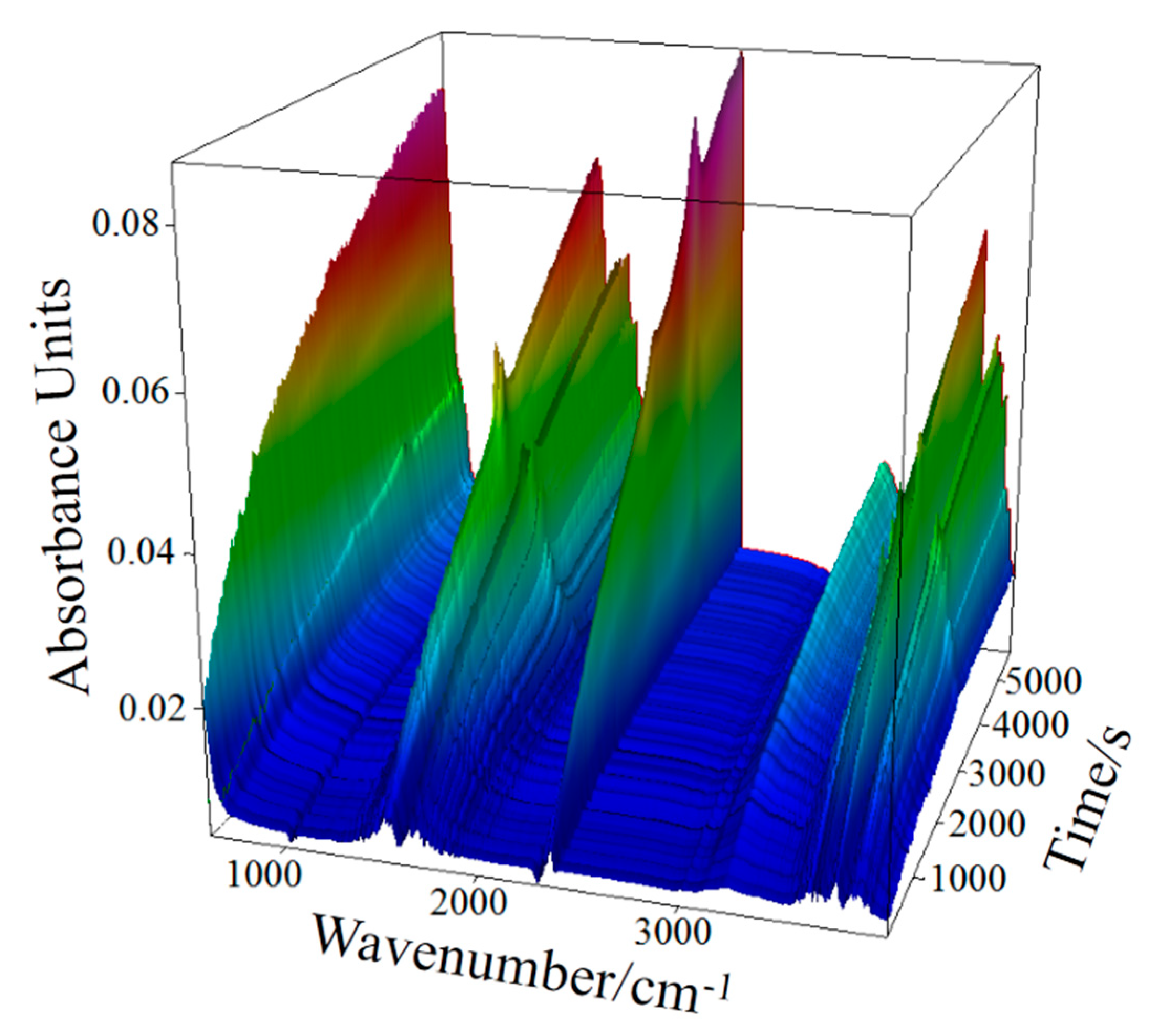
Thermal infrared spectroscopy results show the relationship between the amount of gas produced and the respective temperature regions.
Figure 4 shows a 3D infrared absorption spectrum under conditions of 15 °C/min. The absorption spectra of gases released at different temperatures (times) are presented in
Figure 4. The infrared absorption spectra of gas released from high-sulfur bauxite mainly include O-H, S-O, and C-O groups. In accordance with the results of the mineral composition analysis of high-sulfur bauxite, it is determined that the gases corresponding to the three groups are H
2O (g), SO
2, and CO
2, respectively. The relationship between the temperature and gas flow of SO
2, H
2O, and CO
2 is shown in
Figure 5, where the infrared absorption spectrum of a single component is converted and integrated using the Gram–Schmidt algorithm.
During the heating process at 15 °C/min, high-sulfur bauxite undergoes the oxidation of organic matter (around 352.7 °C), the dehydration of hydrous mineral (447.5–672.8 °C), the oxidation desulfurization of pyrite (367.7–704.3 °C), and the decomposition of carbonate minerals (around 530.5 and 696.8 °C), in which the decomposition temperature of siderite and dolomite is around 530.5 and 696.8 °C, respectively.
Furthermore, the temperature range of pyrite oxidation desulfurization, the dehydration of hydrous minerals such as diaspore and kaolinite, and siderite decomposition occur in an area of considerable overlap.
Table 3 shows the possible reaction equations of bauxite and the standard reaction heat of each reaction at different temperatures, calculated using the Kirchhoff equation. The oxidation desulfurization of pyrite is a strong exothermic reaction, while the dehydration of hydrous minerals, such as diaspore and kaolinite, is an endothermic reaction. The desulfurization rate of pyrite and the dehydration of hydrous minerals determine the heat demand of the roasting process of high-sulfur bauxite, as determined by mineral composition (shown in
Table 2).
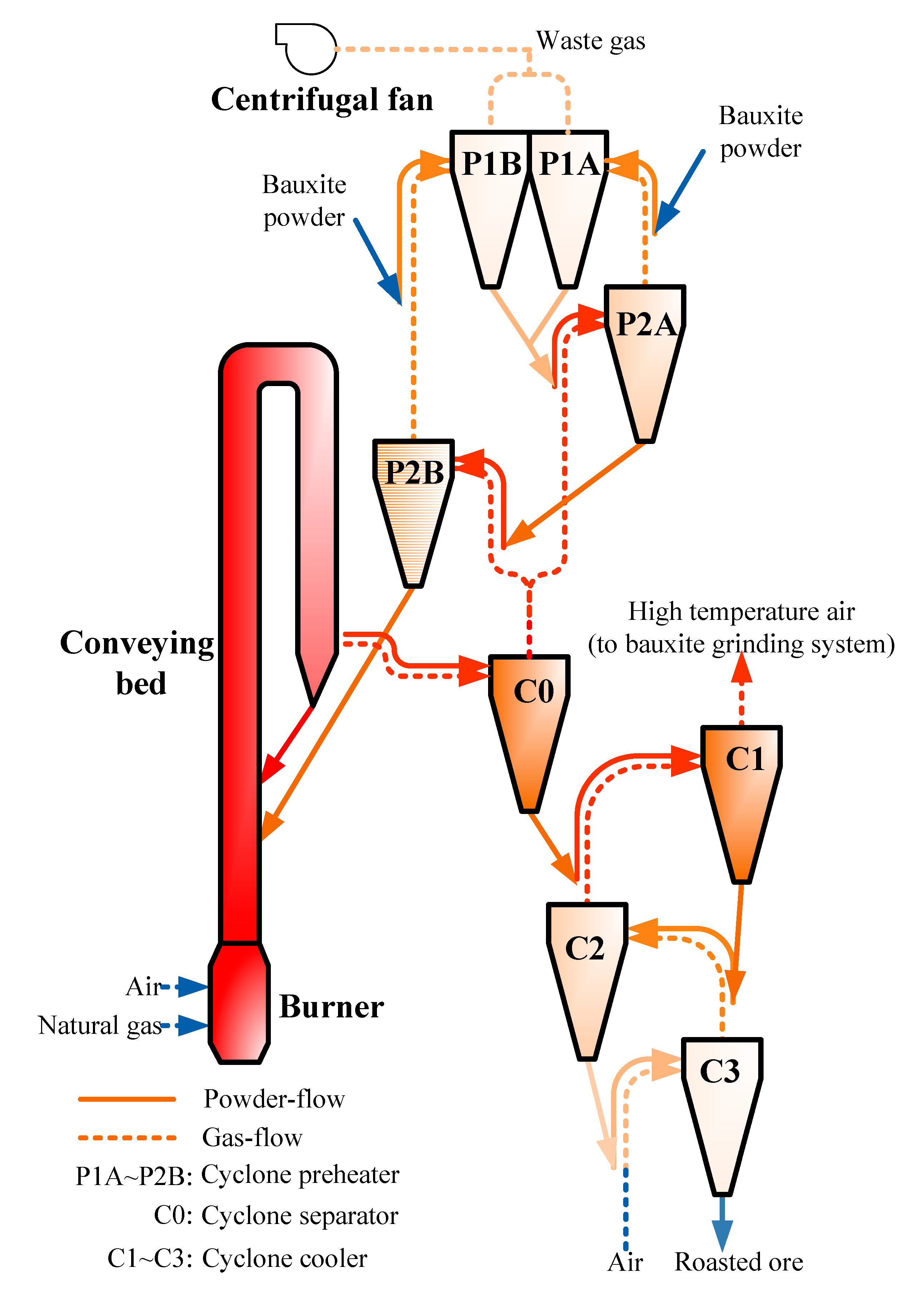
Thermoanalysis results within the heating rate range of 5–20 °C/min show that with increasing heating rate, the temperature range of the dehydration and desulfurization reaction shifts to a high-temperature region; correspondingly, the temperature difference in the inflection point displays a slight increase. Hence, the temperature range of the reactions shifts to a high-temperature region with an increasing heating rate. Compared with the dense-phase bed, such as a rotary kiln or fluidized bed, the dilute-phase conveying bed exhibits a greater contact between gas and powders, and the reaction interface and transmission power in the boundary layer are larger. Furthermore, the heat and mass transfer coefficients are two magnitudes higher than those of the dense-phase bed. Heat transfer is completed quickly after powder flows into the conveying bed. Theoretical calculations show that 99.5% of the heat transfer between particles and gas is completed, and only in the initial region of the acceleration section in the whole powder dispersion process [
23,
24]. Therefore, the temperature corresponding to the maximum rate of the desulfurization and dehydration reactions of high-sulfur bauxite in the conveying bed is higher than that of thermoanalysis experiments, which were conducted in the stacking state.
3.2. Effect of Roasting Temperature on High-Sulfur Bauxite in Conveying Bed
The oxidation reaction of pyrite and the dehydration reaction of hydrous minerals may occur in the roasting process of high-sulfur bauxite, as well as ”adsorption” and ”desorption” between sulfur dioxide and powder particles. Therefore, in order to investigate the effect of the roasting process, the concepts of desulfurization rate (α), sulfate sulfur growth rate (β), and dehydration rate of hydrous minerals (γ) are introduced, where alumina is used as a fixed basis to calculate the above three rates. The three rates were calculated as follows:
α—Desulfurization rate of sulfide sulfur, %;
β—Increasing rate of sulfate sulfur, %;
γ—Dehydration rate of hydrous mineral, %;
ω(Ssulfide)R—The mass fraction of sulfide sulfur in roasted ores, %;
ω(Ssulfide)O—The mass fraction of sulfide sulfur in raw ores, %;
ω(AO)R—The mass fraction of Al2O3 in roasted ores, %;
ω(AO)O—The mass fraction of Al2O3 in raw ores, %;
ω(Ssulfate)R—The mass fraction of sulfate sulfur in roasted ores, %;
ω(Ssulfate)O—The mass fraction of sulfate sulfur in raw ores, %;
ω(H2O)R—The mass fraction of combined water in roasted ores, %;
ω(H2O)O—The mass fraction of combined water in raw ores, %.
Table 4 shows Al
2O
3, ignition loss, and sulfur phase analyses of ore roasted at various roasting temperatures.
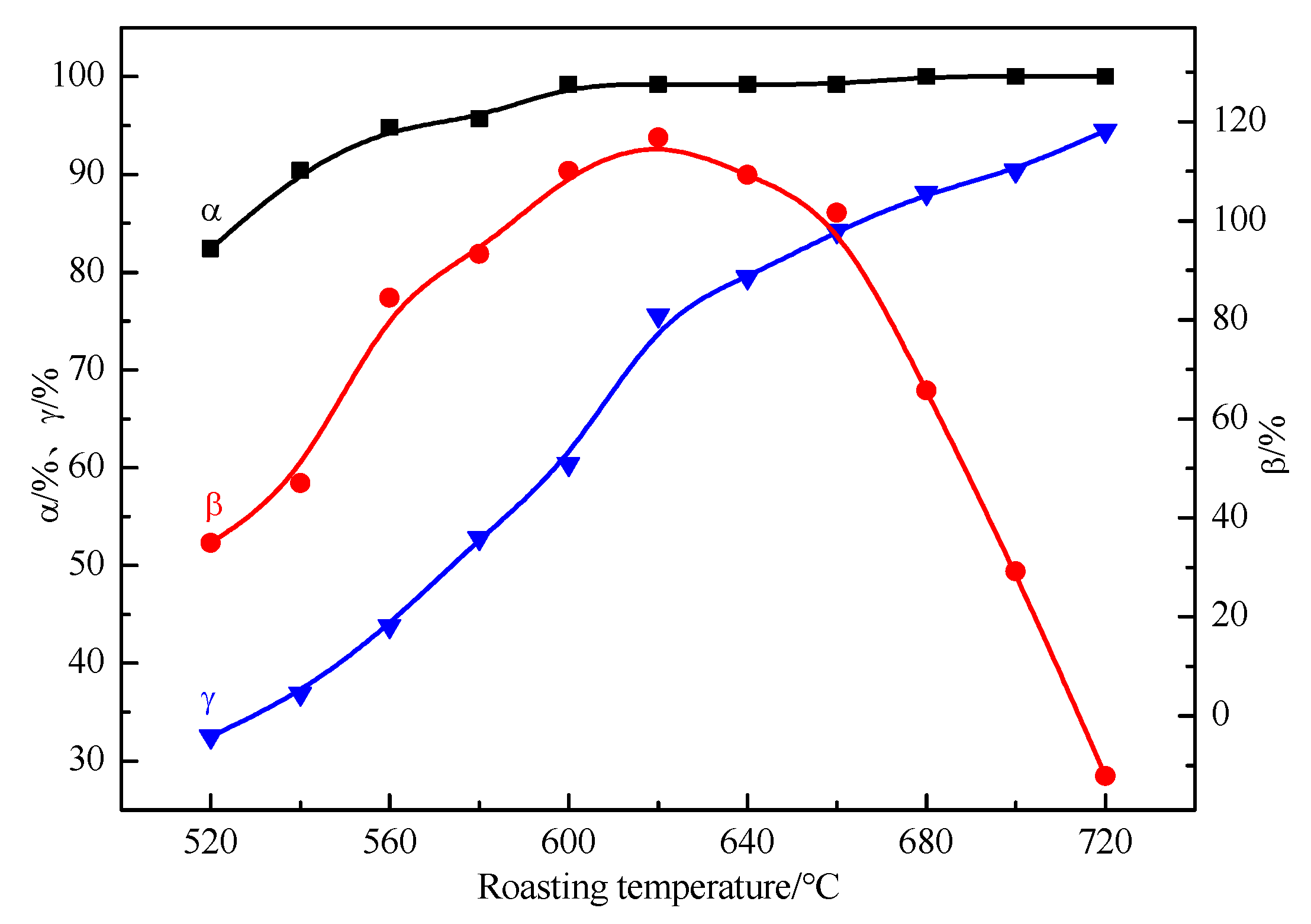
Figure 6 shows the trend of the relationship between the roasting temperature and the α, β, and γ of ore roasted at different roasting temperatures. Under the eleven selected roasting temperatures in the range of 520 to 720 °C, the rapid desulfurization of high-sulfur bauxite can be achieved in the conveying bed after approx. 2 s. Except for ore roasted at 520 °C, the total sulfur content of the other roasted ores is less than 0.37%, and the sulfide sulfur content of the roasted ore is below 0.11%.
As shown in
Figure 6, the roasting temperature is positively correlated with α and γ. In the range of 520–620 °C, α and γ increase rapidly. At 520 °C, α reaches 80.4%, while γ is only 32.5%. At 600 °C, the sulfide sulfur content in the roasted ore decreases to 0.01%, and, compared with α 99.2%, γ is only 60.4%. Hence, the oxidation desulfurization reaction rate of FeS
2 is much higher than the dehydration reaction rate of hydrous minerals in the conveying bed. When the roasting temperature exceeds 620 °C, γ shows a significant decrease. At the temperature of 680 °C, the sulfide sulfur in roasted ore is completely removed, α reaches 100%, correspondingly, while γ is only 88.1%, which indicates that the thermal reaction rate of both reactions in the transport bed is quite different. Therefore, by controlling the roasting temperature in the conveying bed, pyrite can achieve a greater desulfurization rate, while the dehydration rates of hydrous minerals are relatively small, meaning that the energy consumption for the roasting of high-sulfur bauxite can be maintained at a relatively low level, which reduces the overall energy consumption of the entire process.
Compared with dense-phase beds, such as rotary kilns, fluidized beds, and circulating fluidized beds, bauxite particles can be relatively dispersed in the gas phase in the dilute-phase conveying bed, the accumulation and mutual squeezing is greatly weakened, the effective contact area between particles and gas increases sharply, and the interface of heat and mass transfer expands, which is conducive to an increase in the heat and mass transfer rate. The fully developed turbulence of gas and powder exaggerate the Reynolds coefficient. Furthermore, the boundary layers of temperature and mass transfer become thinner, and the temperature gradient and concentration gradient become larger [
25,
26]. This results in the increased power of transmission which, in turn, improves the heat and mass transfer rate. Therefore, heat exchange and reaction between phases are realized within a few seconds.
The sulfate sulfur content initially shows an increasing tread followed by an obvious decrease, indicating that sulfate is formed in the roasting process of high-sulfur bauxite. β reaches the highest value of 116.8% at 620 °C and decreases beyond this temperature, which indicates that the sulfate sulfur is decomposed. Additionally, the total amount of sulfate sulfur in the roasted ore is lower than that of protogenetic sulfate in the raw ore when the value of β becomes negative at 720 °C.
The mechanism of sulfur dioxide absorption on ferric oxide was analyzed [
27,
28]. Fe
2O
3 has a positive influence on the absorption and fixing sulfur in the temperature range of 380–580 °C, where α-Fe
2O
3 displays an optimal effect of fixing sulfur, whose product is Fe
2(SO
4)
3; however, ferric sulfate decomposes into Fe
2O
3, SO
3, and SO
2 above 600 °C. During the roasting process of high-sulfur bauxite, Fe
2O
3 is generated via the desulfurization reaction of pyrite and the decomposition reaction of siderite, which combines with SO
2 to form ferric sulfate, promoting augmentation of the sulfate sulfur content in the roasted ore. Sulfate decomposition is a result of increased roasting temperature; at a roasting temperature of approx. 620 °C, the sulfate decomposition reaction rate increases and the growth rate of sulfate sulfur decreases. Therefore, when high-sulfur bauxite is roasted within a certain temperature range, three sulfur-related reaction processes take place: pyrite desulfurization, SO
2 adsorption to form sulfate, and sulfate decomposition.
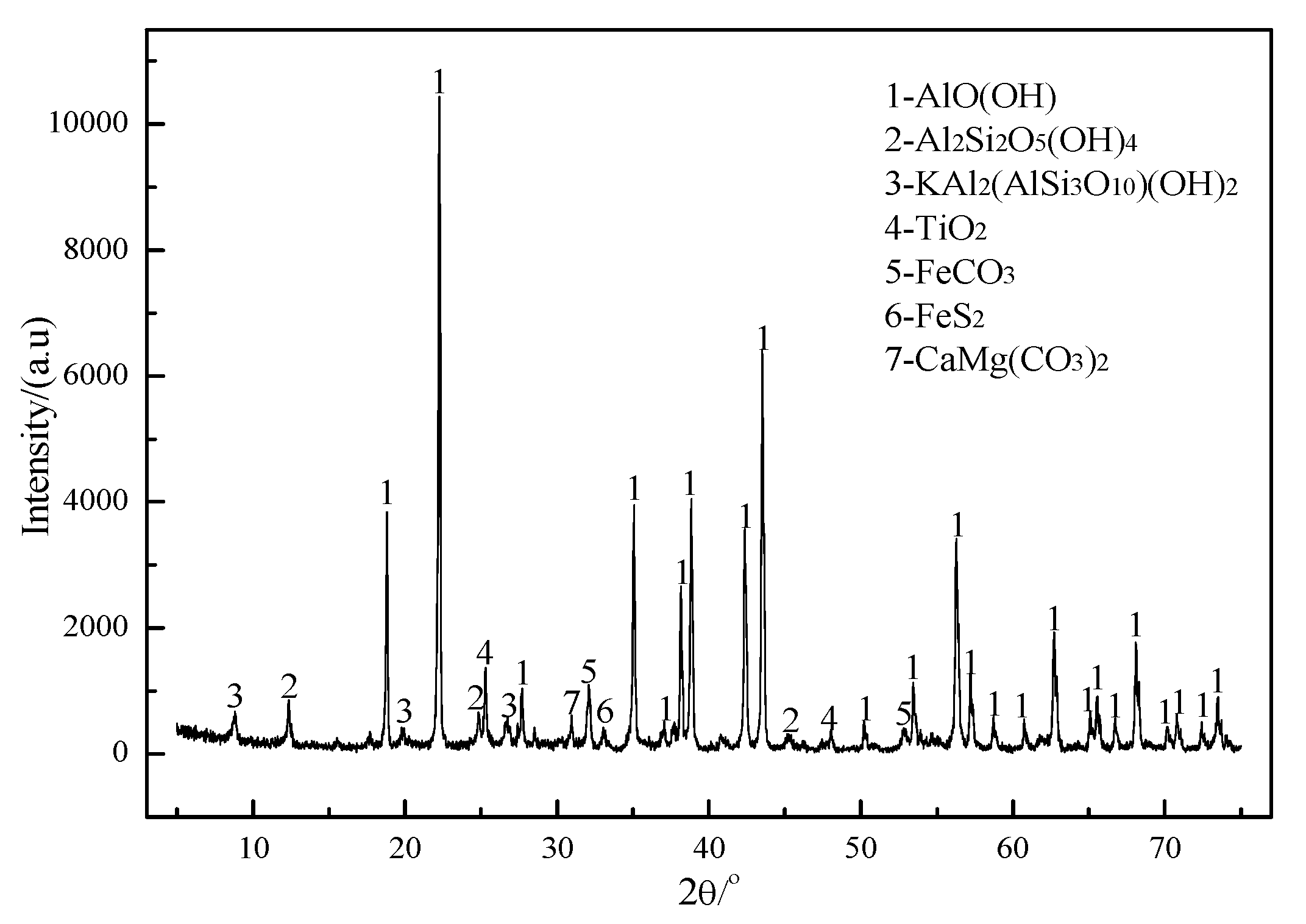
Figure 7 shows the XRD patterns of ores roasted at different roasting temperatures. The intensity of the characteristic diffraction peaks of gibbsite, kaolinite, and siderite reduces rapidly with increasing roasting temperatures. The characteristic diffraction peaks of the three minerals basically disappear at 600 °C, indicating that the dehydration reaction of gibbsite and kaolinite and the decomposition reaction of siderite are basically complete. The characteristic diffraction peaks of pyrite overlap with those of hematite, which cannot be distinguished in an XRD comparison chart. Combined with the sulfide sulfur content in the roasted ore (
Table 4), it can be determined that the oxidation reaction of pyrite finishes at 600 °C. As the roasting temperature increases, the heights of the characteristic diffraction peaks of dolomite and mica show only a slight change, which indicates that both minerals do not or rarely react when the roasting temperature is lower than 720 °C.
The desulfurization and dehydration reactions of high-sulfur bauxite are rapidly completed in the conveying bed, where diaspore and kaolinite are the main aluminous minerals. After dehydration, both minerals are transformed into α-Al
2O
3 and Al
2O
3·2SiO
2, respectively, and the chemical activities of the reaction products directly influence the digestion effect of Al
2O
3. By comparing the XRD patterns of
Figure 7 with the XRD patterns of
Figure 1, the diffraction peaks of α-Al
2O
3 in the roasted ore (2θ = 35.3, 43.4, 57.4, and 68.2°) are wide and diffuse, and an increase in the sawtooth wave is observed, which indicates that diaspore dehydrates to form α-Al
2O
3 with fine grains or crystal defects. No obvious diffraction peak or “amorphous package” (2θ between 15 and 30°) of Al
2O
3∙2SiO
2 is detected in the roasted ore, and in the amorphous phase material, it is difficult to observe lattice vibration peaks; hence, metakaolinite is a disordered junction with a high degree of amorphization in the roasted ore. At the dilute-phase conveying state, the dehydration reaction and roasted ore cooling occur within a few seconds. Flash roasting and flash cooling form α-Al
2O
3 and metakaolinite in the conveying system with a sharp temperature change and a great temperature gradient. Thus, it is difficult for α-Al
2O
3 to form a stable structure with regular crystal; the original aluminum octahedron structure of kaolinite is destroyed; and the observed sharp increase in amorphization leads to the dehydration of kaolinite into highly disordered amorphous metakaolinite.
3.3. Digestion Properties of Roasted Ore
Reports have shown that there are many external factors that affect the digestion performance of bauxite, including digestion temperature, digestion time, lime addition, pulp concentration, caustic alkali concentration of mother liquor, and proportioning molecular ratio. The digestion temperature and time have a considerable influence on the industrial digestion process compared to other external factors. Therefore, only these two factors were taken as variables to investigate the variation in the relative digestion rate of alumina by alternating digestion temperature and time. Chemical reactions between roasted ore and alkali during digestion process mainly involve aluminous minerals and siliceous minerals. The reaction between α-Al
2O
3 in roasted ore and alkali are as follows:
All aluminosilicate minerals are first decomposed into sodium aluminate and sodium silicate into the solution, and then both of them form hydrated sodium aluminosilicate. Taking metakaolinite as an example, the reaction is as follows:
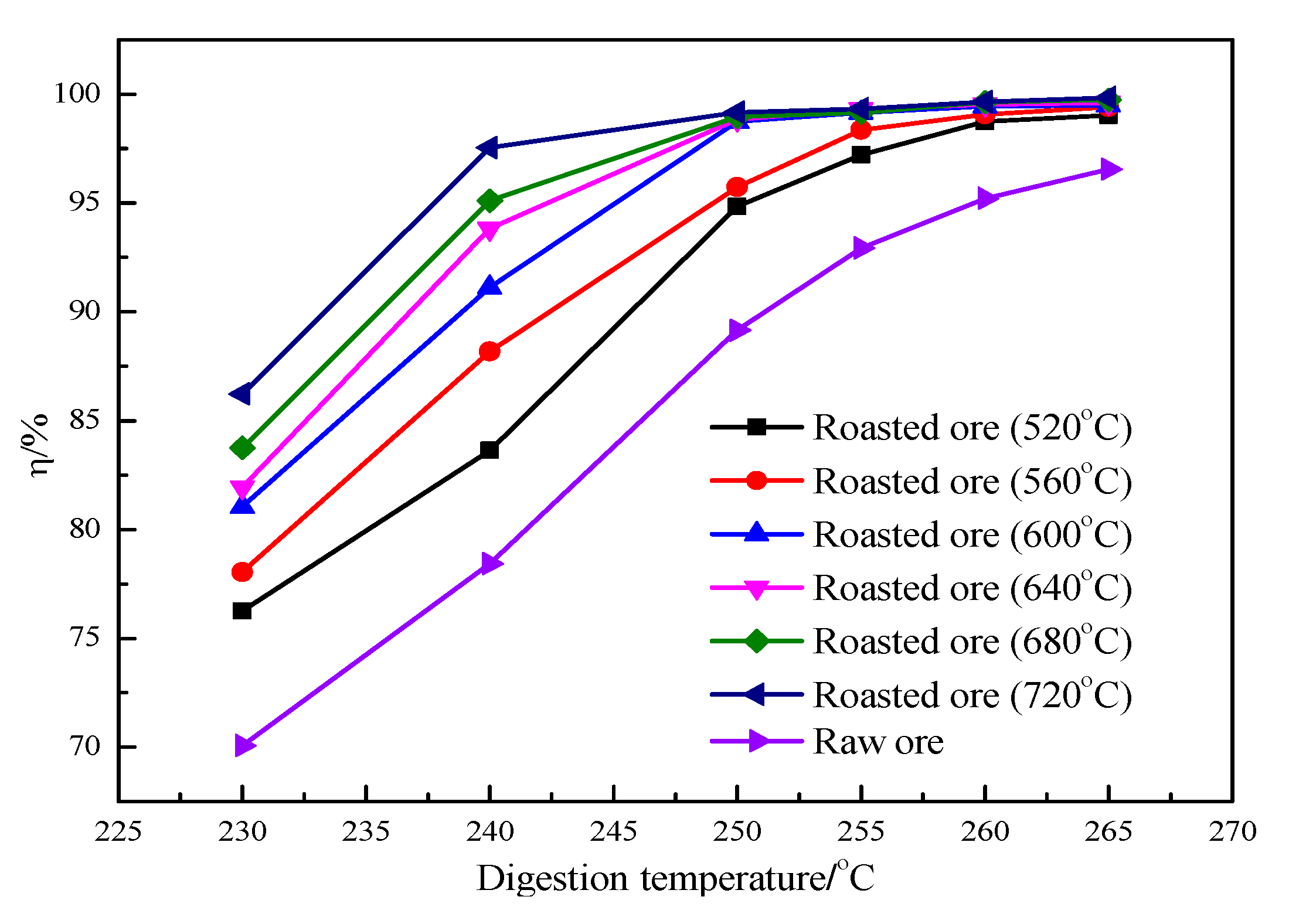
Figure 8 shows the influence of digestion temperature on the relative digestion rate of Al
2O
3, including raw ore and ore roasted at different roasting temperatures, under the conditions of 60 min digestion time, 9% lime addition, 236 g/L caustic concentration, and 1.50 ingredient molecular ratio.
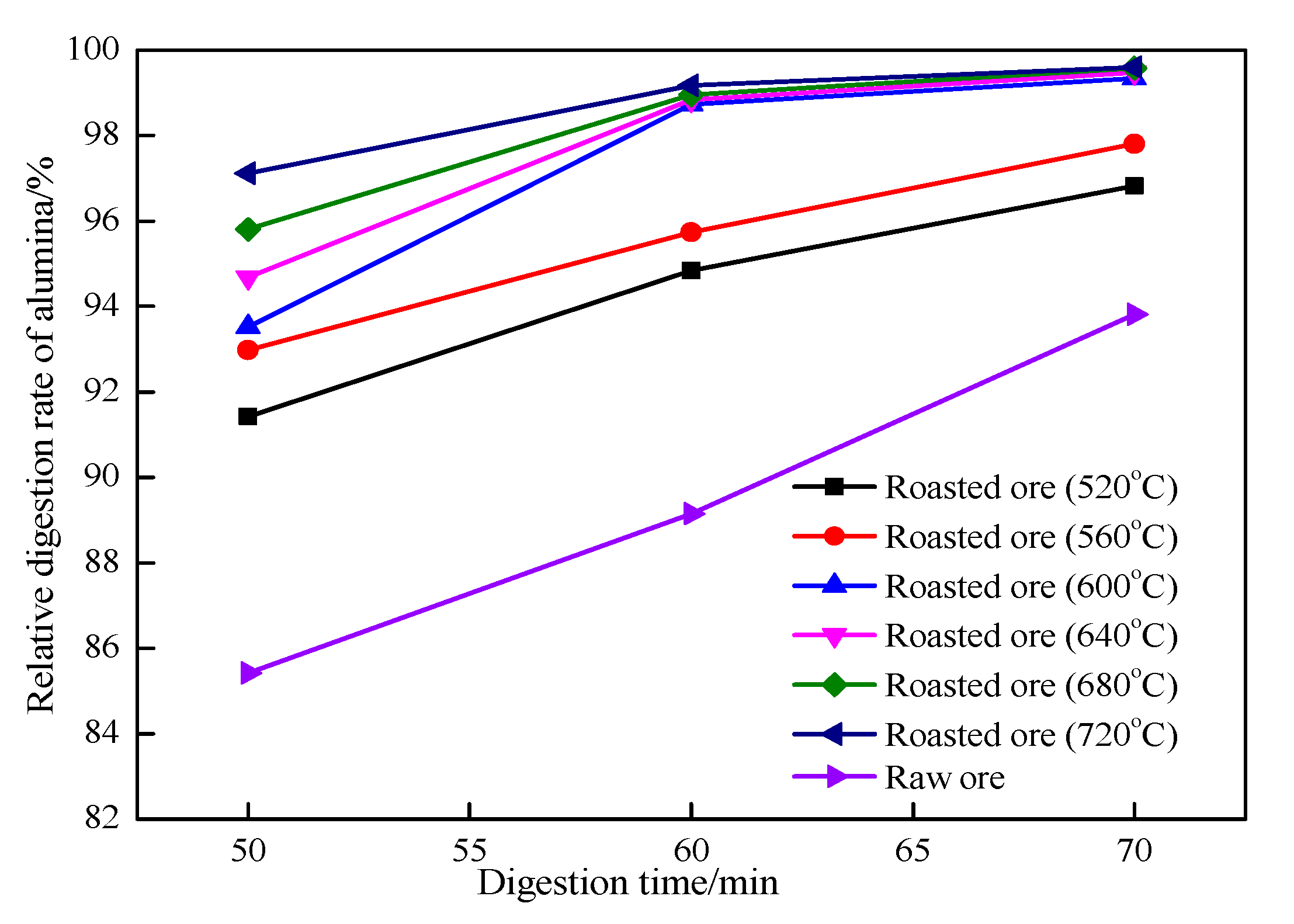
Figure 9 shows the influence of digestion time on the relative digestion rate of Al
2O
3 under the same conditions, except with 260 °C used as the digestion temperature.
As shown in
Figure 8, the digestion temperature significantly influences the relative digestion rate of Al
2O
3, especially that of raw ore, which is positively correlated to the digestion temperature. At the same digestion temperature, the relative digestion rate of roasted ore is higher than that of raw ore. Additionally, the roasting temperature increases with an increase in the relative digestion rate of the roasted ore, indicating that an elevated roasting temperature is conducive to the digestion of alumina in the roasted ore within a roasting temperature range of 520–720 °C and a digestion temperature range of 230–265 °C. Compared with the digestion temperature, the digestion time has no obvious influence on the digestion effect of the roasted ore. However, the relative digestion ratio of the raw ore is significantly affected by the digestion time.
When the digestion temperature is 250 °C and the digestion time is 60 min, the relative digestion rate of alumina in ore roasted at 600–720 °C reaches more than 99.0%, and the alumina in the roasted ore is basically completely dissolved. Using a higher digestion temperature or longer digestion time has no impact on the relative digestion rate of the ore roasted at a higher roasting temperature.
3.4. Activation Mechanism of Bauxite by Roasting in Conveying Bed
The obtained results show that the roasted ore has a better digestion performance than raw ore, as the relative digestion rate of alumina is greatly improved, and an enhanced digestion effect can be obtained under a relatively low digestion temperature and short digestion time.
The desulfurization and dehydration reactions of high-sulfur bauxite in the conveying bed are accomplished rapidly. Diaspore and kaolinite dehydrate are transformed into α-Al
2O
3 and Al
2O
3∙2SiO
2, respectively. The microcrystalline structures of diaspore in raw ore and alumina in roasted ore were analyzed by XRD, and the microcrystalline structure parameter
Dhkl was calculated using the Debye–Scherrer formula in order to understand the changes that occur in the crystallite sizes of Al-O minerals after roasting in the conveying bed. The Debye–Scherrer formula was calculated as follows:
Dhkl—The size of microcrystals in the direction perpendicular to the crystal plane (hkl);
K—The parameter, 0.89;
λ—The wavelength of radiation, calculated according to the wavelength of Kα1, for the copper target, the value is 0.154056 nm;
θ—The half diffraction angle;
β—The broadening of diffraction peak, ;
B—The diffraction peak width (FWHM) of the sample;
b—The physical width of the instrument.
Figure 10 shows the trend of the α-Al
2O
3 crystallite size change with the variation in roasting temperature. The α-Al
2O
3 crystallite size perpendicular to planes 104, 113, 116, and 030 is at the nanometer level, and the maximum crystallite size is smaller than 24.5 nm, while the all-directional crystallite sizes of diaspore in the raw ore are greater than 100 nm. Hence, rapid roasting and dehydration in the conveying bed reduces the size of the crystallite of the Al-O mineral by one magnitude, which increases the microreaction interface. Moreover, the metakaolinite obtained through rapid roasting has a highly disordered amorphous structure. Under the combined action of these two aspects, the reactivity of the roasted ore during digestion greatly improves, which promotes the improvement of the digestion rate of alumina. The average size of α-Al
2O
3 crystallites increases from 14 nm at 520 °C to 20 nm at 720 °C, indicating that the use of a higher roasting temperature coarsens the α-Al
2O
3 microcrystal size, which has a negative impact on the digestion of alumina. However, the order of magnitude of crystallite sizes remains unchanged, making it difficult for them to have a large impact on the digestion of alumina.
Figure 11 shows the results for the specific surface area of raw ore and roasted ore determined by the multi-point BET method. The specific surface area of the raw ore is only 60 m
2/g, and that of the roasted ore increases significantly after rapid roasting in the conveying bed. The specific surface area range of the ore roasted below 560 °C only shows a small increase to 92 m
2/g, whereas above this temperature, it rapidly increases. The specific surface area of ore roasted at 600 °C with a test value of 128 m
2/g is more than double that of raw ore, whereas at 720 °C, it reaches 160 m
2/g. Due to the gas–solid heat transfer reaction being completed in seconds in the conveying bed, the temperature gradient and material concentration gradient between the gas and solid are large, and the heat and mass transfer rate is high, which allows the gas–solid reaction to be completed quickly. The SEM images of raw ore and roasted ore are shown in
Figure 12. The roasting process had a significant influence on the microstructure of bauxite. In the SEM image of raw ore, a few cracks could be found on the particle surface, while many micropores were exposed on the surface of the roasted ore at 600 °C. In the condition of high transfer rate and thermal strength in the conveying bed, more pores and cracks should be produced by the dehydration and desulfurization reactions due to gas escaping, which increases the specific surface area of the roasted ore. In a certain temperature range below 720 °C, the specific surface area of the roasted ore is positively correlated with temperature. Therefore, the liquid–solid reaction interface increases during the digesting process, which is beneficial to the relative digestion rate of alumina and the moderation of digestion conditions.
The rapid roasting of bauxite in the conveying bed reduces the crystallite sizes of Al-O minerals and increases the specific surface area of the roasted ore, which improves the microreaction interface of the subsequent liquid–solid reaction greatly. The roasted ore shows an enhanced digestion effect compared to the one obtained with the raw ore, greatly improving the processing performance of bauxite.