Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications
Abstract
1. Introduction
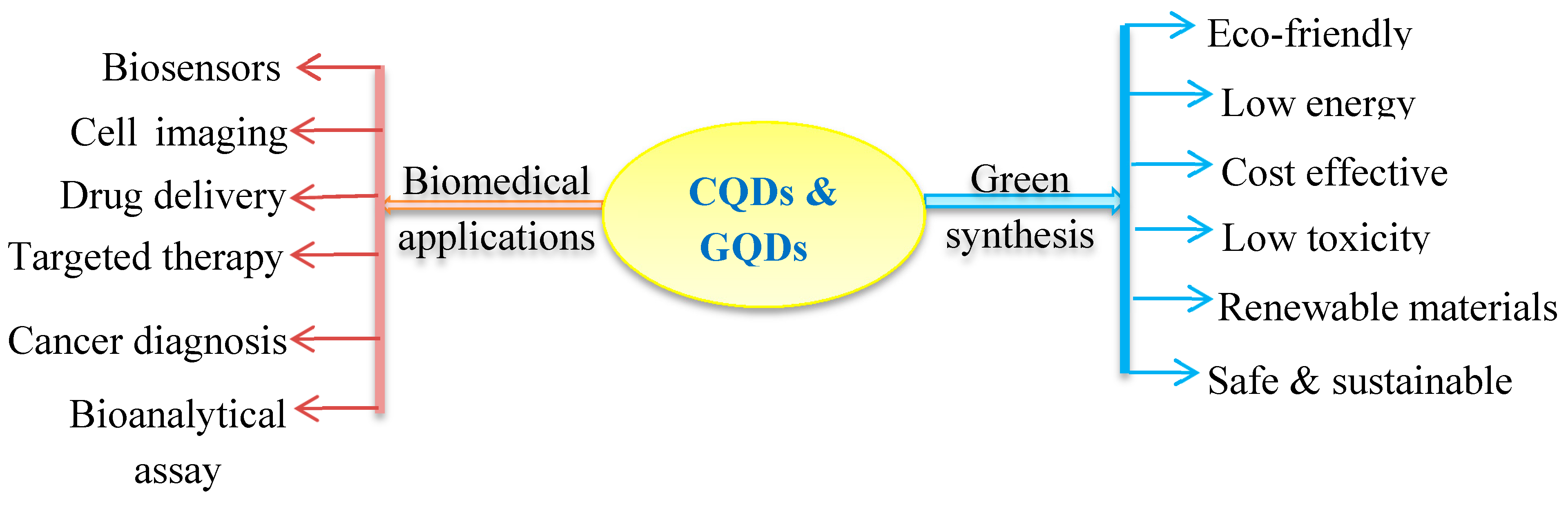
2. Unique Features of Quantum Dots (CQDs and GQDs)
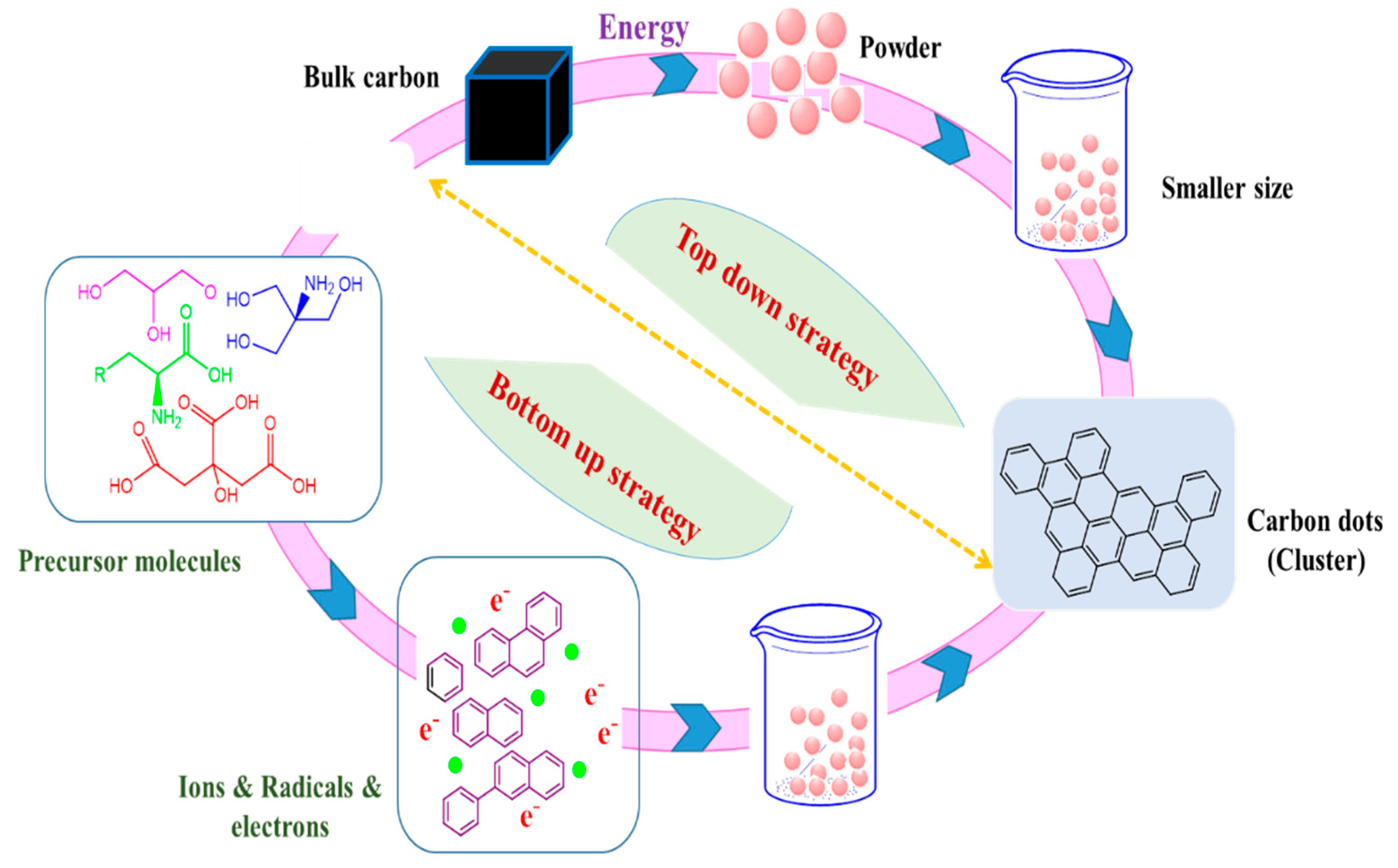
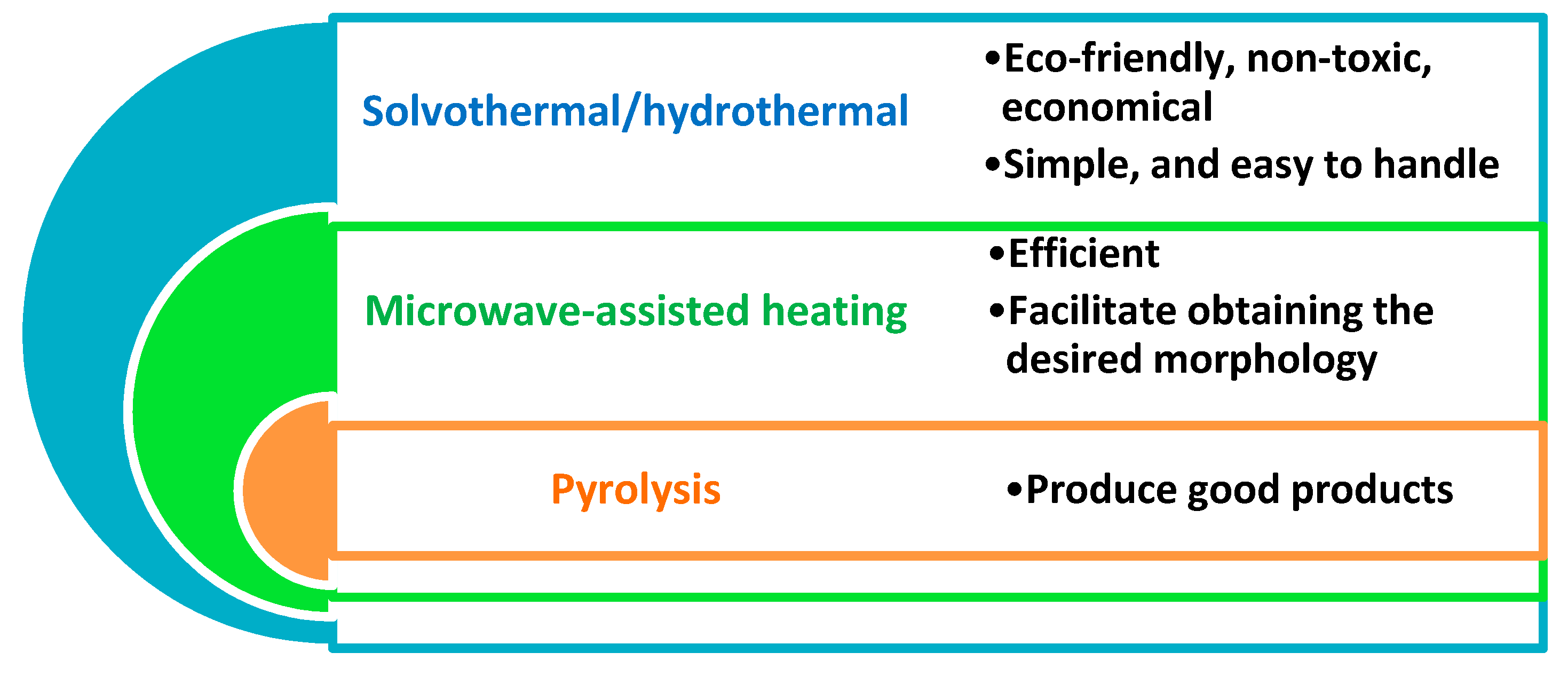
3. Fundamental Approaches of Carbon Dots Fabrication (Green Synthesis)
3.1. Hydrothermal/Solvothermal Process
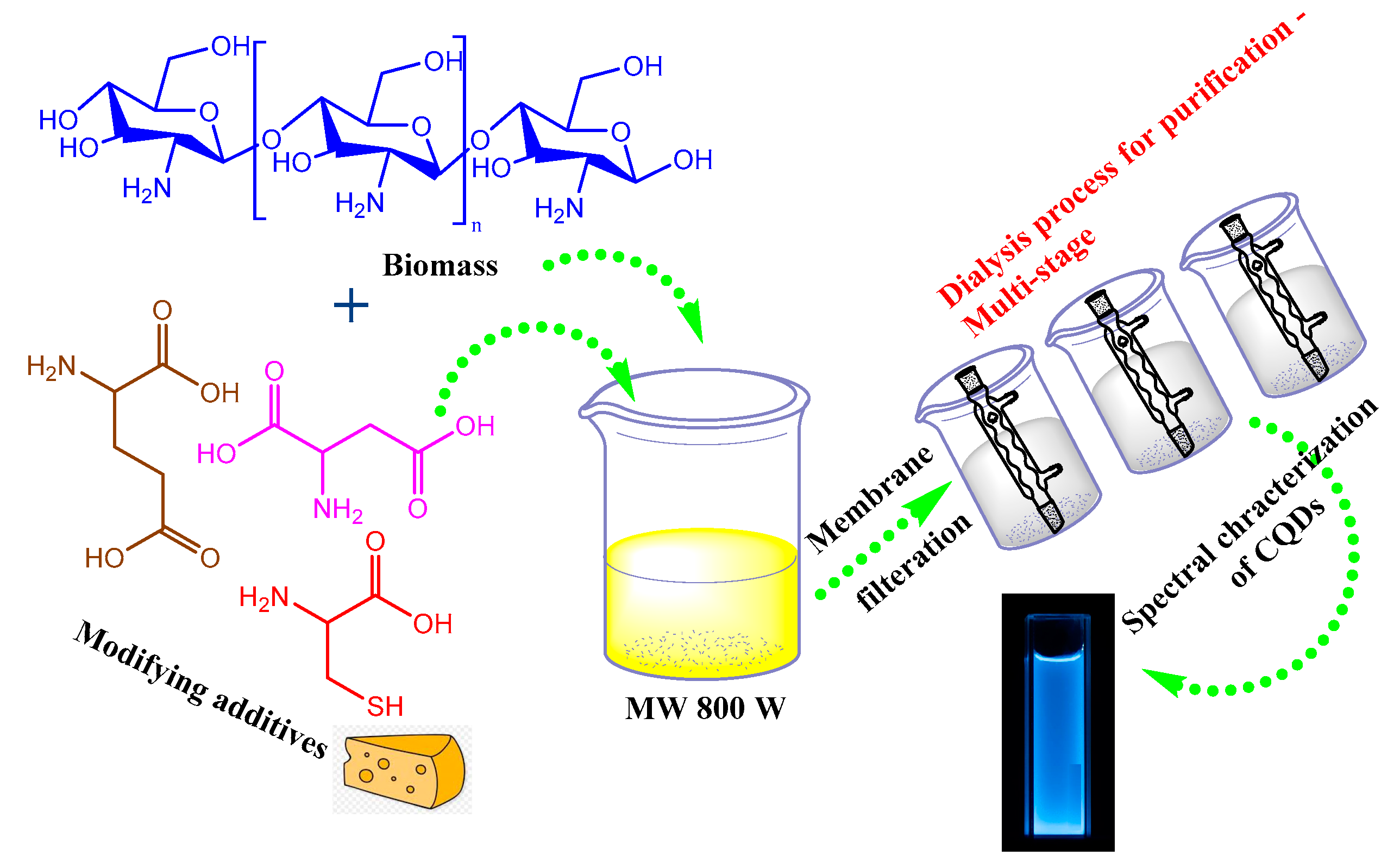
3.2. Microwave-Assisted Heating
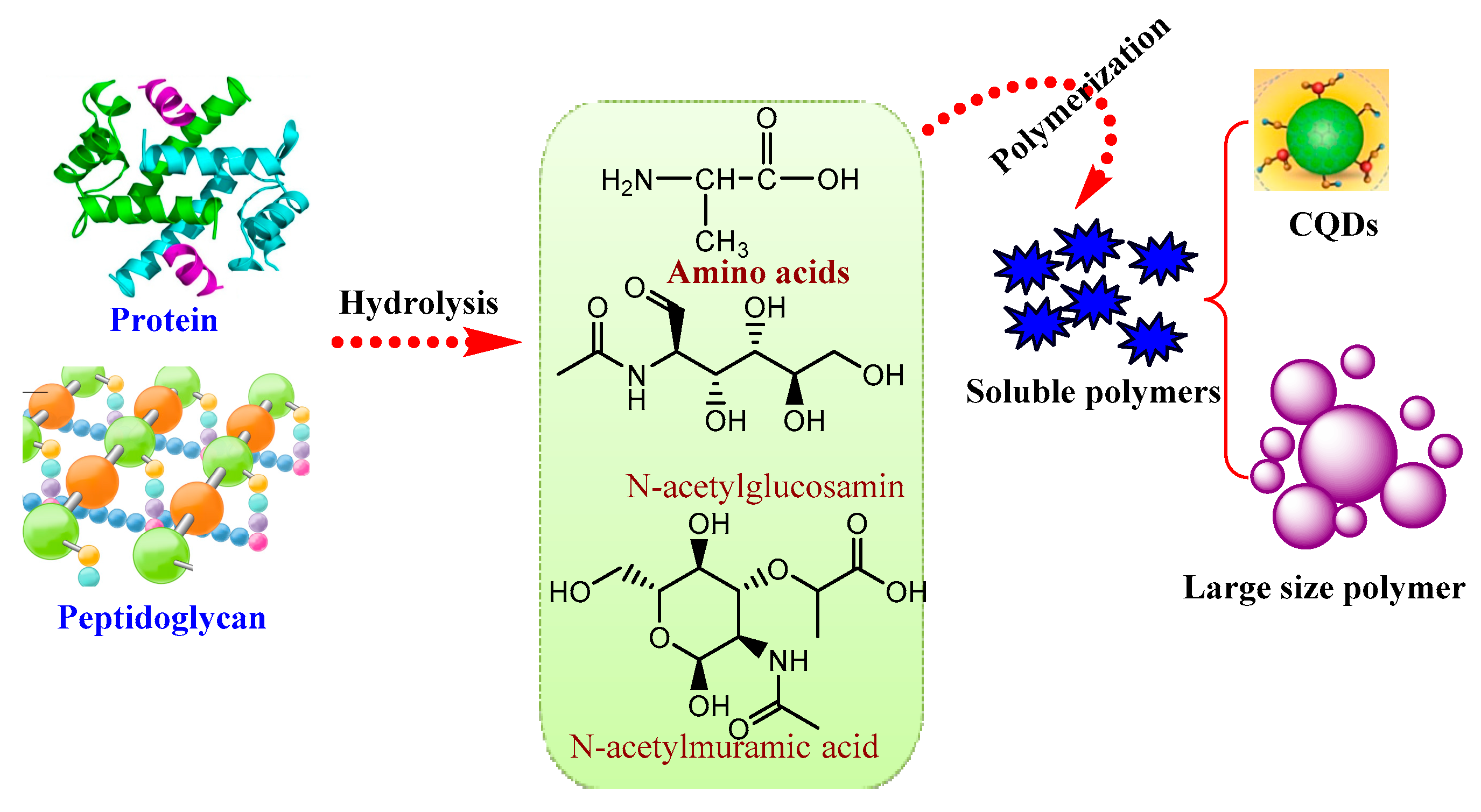
3.3. Pyrolysis
4. Biomedical and Biotechnological Applications
4.1. Cancer Therapy and Drug Delivery
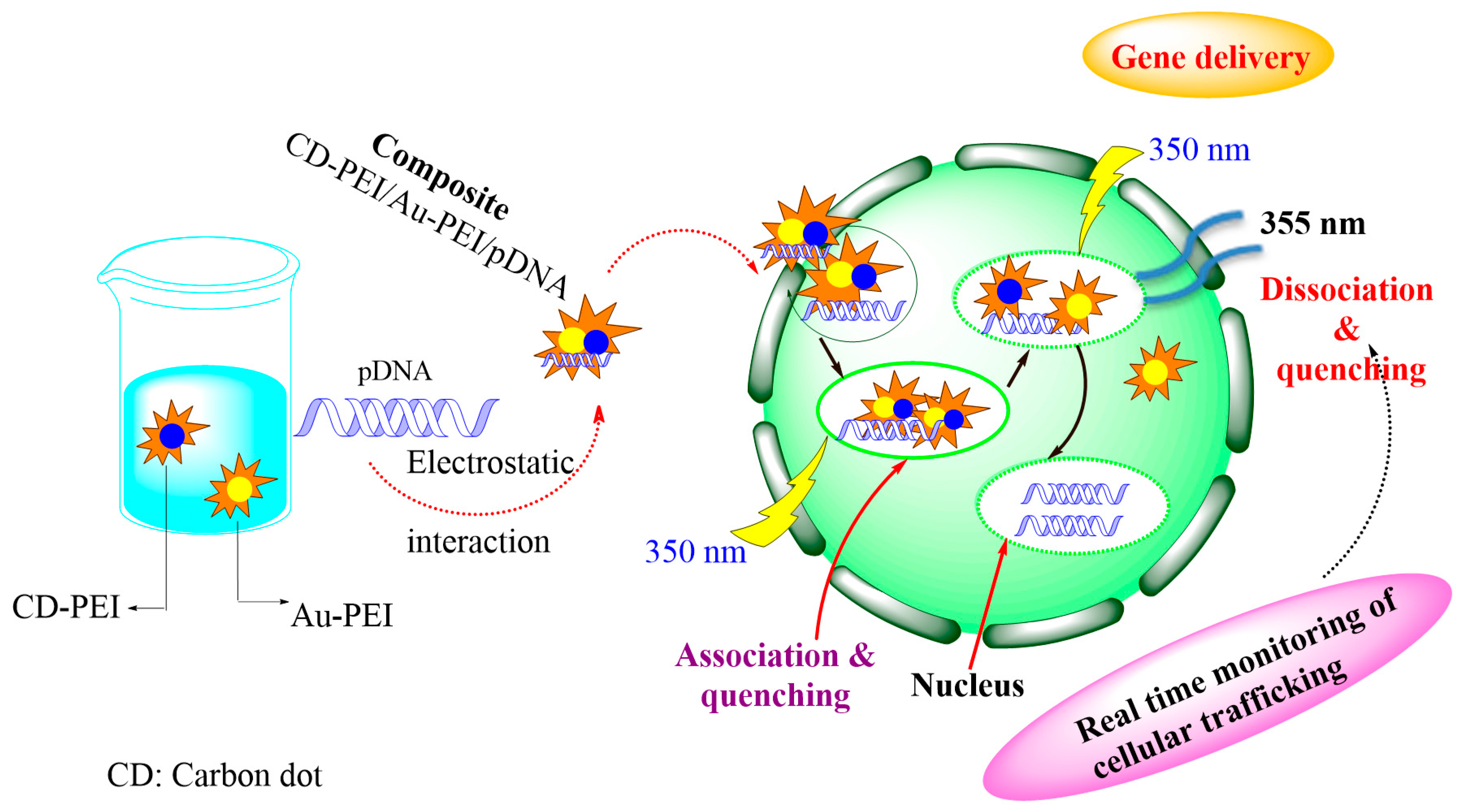
4.2. Imaging and Bioimaging
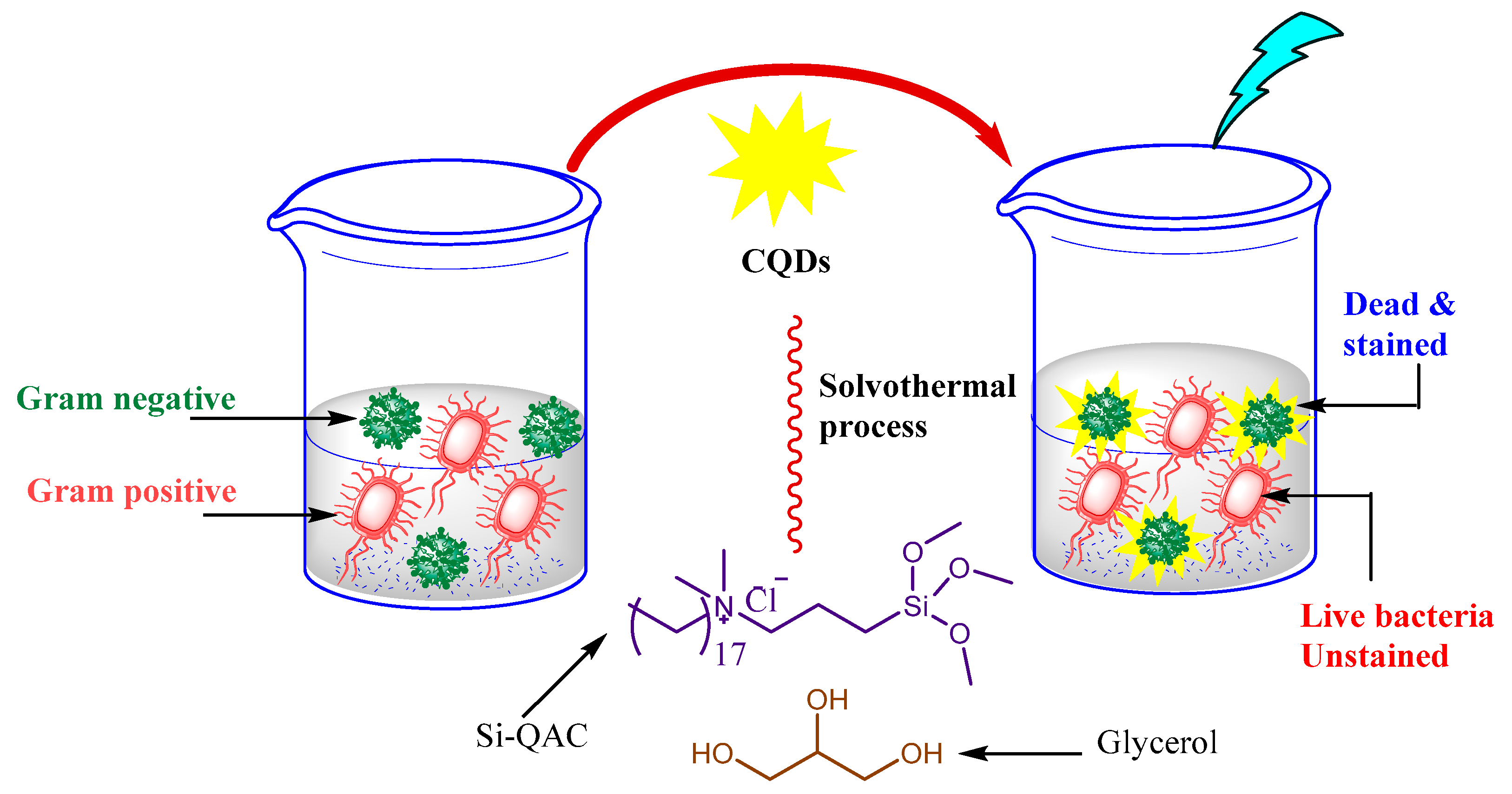
4.3. Anti-Microbial Activity
4.4. Sensors and Biosensors
5. Limitations and Future Prospective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanty, A.; Janowska, I. Tuning the structure of in-situ synthesized few layer graphene/carbon composites into nanoporous vertically aligned graphene electrodes with high volumetric capacitance. Electrochim. Acta. 2019, 308, 206–216. [Google Scholar] [CrossRef]
- Pirzado, A.A.; Le Normand, F.; Romero, T.; Paszkiewicz, S.; Papaefthimiou, V.; Ihiawakrim, D.; Janowska, I. Few-layer graphene from mechanical exfoliation of graphite-based materials: Structure-dependent characteristics. Chem. Eng. 2019, 3, 37. [Google Scholar] [CrossRef]
- Mohanty, W.; Baaziz, M.; Lafjah, M.; Da Costa, V.; Janowska, I. Few layer graphene as a template for Fe-based 2D nanoparticles. Flat Chem. 2018, 9, 15–20. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Mahdy, G.A.; Al-Lohedan, H.A.; Shoueir, K.R. Electrochemical behavior of smart N-isopropyl acrylamide copolymer nanogel on steel for corrosion protection in acidic solution. Int. J. Electrochem. Sci. 2015, 10, 870. [Google Scholar]
- Aljohani, H.; Ahmed, Y.; El-Shafey, O.; El-Shafey, S.; Fouad, R.; Shoueir, K. Decolorization of turbid sugar juice from sugar factory using waste powdered carbon. Appl. Water Sci. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Trache, D.; Thakur, V.K.; Boukherroub, R. Cellulose nanocrystals/graphene hybrids—A promising new class of materials for advanced applications. Nanomaterials 2020, 10, 1523. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lo, P.-Y.; Lee, G.-Y.; Zheng, J.-H.; Cho, E.-C. Carboxylated carbon nanomaterials in cell cycle and apoptotic cell death regulation. J. Biotechnol. 2019, 296, 14–21. [Google Scholar] [CrossRef]
- Thulasi, S.; Kathiravan, A.; Asha Jhonsi, M. Fluorescent carbon dots derived from vehicle exhaust soot and sensing of tartrazine in soft drinks. ACS Omega 2020, 5, 7025–7031. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Suo, W.; Shao, M.; Zhu, Y.; Wang, X.; Feng, J.; Fang, M. Nitrogen doped MoS2 and nitrogen doped carbon dots composite catalyst for electroreduction CO2 to CO with high Faradaic efficiency. Nano Energy 2019, 63, 103834. [Google Scholar] [CrossRef]
- Hu, M.; Li, M.; Qiu, J.; Sun, Y.-P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Ma, J.; Xu, X.; Chu, H.; Zhang, D.; Li, J. Sulfonated glycosaminoglycan bioinspired carbon dots for effective cellular labelling and promotion on the differentiation of mesenchymal stem cells. J. Mater. Chem. B. 2020, 8, 5655–5666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, F.; Zhang, S.; An, Y.; Sun, S. Preparation of N-doped yellow carbon dots and N, P co-doped red carbon dots for bioimaging and photodynamic therapy of tumors. New J. Chem. 2019, 43, 6332–6342. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Su, S.; Qiu, J. Novel fluorescence resonance energy transfer optical sensors for vitamin B 12 detection using thermally reduced carbon dots. New J. Chem. 2015, 39, 501–507. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Wang, M.; Huang, J.; Jiang, X.; Pang, J.; Xu, F.; Zhang, X. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int. J. Biol. Macromol. 2019, 128, 537–545. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Zhai, F.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Hang, D.-R.; Pan, Y.-Q.; Sharma, K.H.; Chou, M.; Islam, S.E.; Wu, H.-F.; Liang, C.-T. 2D CTAB-MoSe2 nanosheets and 0D MoSe2 quantum dots: Facile top-down preparations and their peroxidase-like catalytic activity for colorimetric detection of hydrogen peroxide. Nanomaterials 2020, 10, 2045. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalt. Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Fang, Z.; Niu, Y.; Lou, J.; Wu, Y.; Zou, S.; Xia, S.; Sun, M.; Du, F. Fabrication of HA/PEI-functionalized carbon dots for tumor targeting, intracellular imaging and gene delivery. RSC Adv. 2017, 7, 3369–3375. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano. 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.; Hessel, V.; Lin, L.; Meskers, S.; Gallucci, F. Synthesis of luminescent carbon quantum dots by microplasma process. Chem. Eng. Process. Intensif. 2019, 140, 29–35. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Soltani, P.; Pizzoferrato, R.; Ciotta, E.; Prosposito, P. Graphene quantum dots obtained by unfolding fullerene. Thin. Solid Films 2019, 673, 19–25. [Google Scholar] [CrossRef]
- Khan, Z.M.S.H.; Saifi, S.; Aslam, Z.; Khan, S.A.; Zulfequar, M. A facile one step hydrothermal synthesis of carbon quantum dots for label-free fluorescence sensing approach to detect picric acid in aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112201. [Google Scholar] [CrossRef]
- Vinci, J.C.; Ferrer, I.M.; Seedhouse, S.J.; Bourdon, A.K.; Reynard, J.M.; Foster, B.A.; Bright, F.V.; Coloón, L.A. Hidden properties of carbon dots revealed after HPLC fractionation. J. Phys. Chem. Lett. 2013, 4, 239–243. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chemie 2007, 119, 6593–6595. [Google Scholar] [CrossRef]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef]
- Tan, Q.; Kong, X.; Guan, X.; Wang, C.; Xu, B. Crystallization of zinc oxide quantum dots on graphene sheets as an anode material for lithium ion batteries. Cryst. Eng. Comm. 2020, 22, 320–329. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, L.; Liu, M.; Wan, Q.; Tian, J.; Huang, Q.; Wen, Y.; Liang, S.; Zhang, X.; Wei, Y. Bottom-up preparation of nitrogen doped carbon quantum dots with green emission under microwave-assisted hydrothermal treatment and their biological imaging. Mater. Sci. Eng. C. 2018, 84, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.; Zhao, D.L.; Chung, T.-S. Thin film nanocomposite hollow fiber membranes comprising Na+-functionalized carbon quantum dots for brackish water desalination. Water Res. 2019, 154, 54–61. [Google Scholar] [CrossRef]
- Wang, X.; Hao, J.; Cheng, J.; Li, J.; Miao, J.; Li, R.; Li, Y.; Li, J.; Liu, Y.; Zhu, X. Chiral CdSe nanoplatelets as an ultrasensitive probe for lead ion sensing. Nanoscale 2019, 11, 9327–9334. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, N.; Lin, X.; Lin, J.; Chi, Y.; Chen, G. Extraction of electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chem. Mater. 2010, 22, 5895–5899. [Google Scholar] [CrossRef]
- Hou, J.; Dong, J.; Zhu, H.; Teng, X.; Ai, S.; Mang, M. A simple and sensitive fluorescent sensor for methyl parathion based on l-tyrosine methyl ester functionalized carbon dots. Biosens. Bioelectron. 2015, 68, 20–26. [Google Scholar] [CrossRef]
- Zou, S.; Hou, C.; Fa, L.; Zhang, Y.; Ma, L.; Dong, D.; Li, D.; Huo, D.; Yang, M. An efficient fluorescent probe for fluazinam using N, S co-doped carbon dots from L-cysteine. Sensors Actuators B Chem. 2017, 239, 1033–1041. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chen, J.; Wang, A.-J.; Bao, N.; Feng, J.-J.; Wang, W.; Shao, L. Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury (II) detection and bioimaging. J. Mater. Chem. C. 2015, 3, 73–78. [Google Scholar] [CrossRef]
- Gu, C.; Guo, Z.; Li, Z.; Wang, M.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic ZrHf-based metal-organic framework embedded with carbon dots: Ultra-sensitive platform for early diagnosis of HER2 and HER2-overexpressed living cancer cells. Biosens. Bioelectron. 2019, 134, 8–15. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Z.; Guo, Z.; Ji, Y.; Jin, M.; Wang, X. Cellular distribution and cytotoxicity of graphene quantum dots with different functional groups. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, H.; Wang, F.; Ren, J.; Qu, X. How functional groups influence the ROS generation and cytotoxicity of graphene quantum dots. Chem. Commun. 2017, 53, 10588–10591. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Al-Radadi, N.S.; Eldin, G.M.G.; Almahri, A.; Ahmed, M.K.; Shoueir, K.; Janowska, I. Selenium nanoparticles synthesized using an eco-friendly method: Dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol. 2021, 11, 85–97. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Wassel, A.R.; Shoueir, K. Visible-light driven photocatalytic effectiveness for solid-state synthesis of ZnO/natural clay/TiO2 nanoarchitectures towards complete decolorization of MB from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100425. [Google Scholar]
- El-Desouky, N.; Shoueir, K.R.; El-Mehasseb, I.; El-Kemary, M. Bio-inspired green manufacturing of plasmonic silver nanoparticles/degussa using banana waste peduncles: Photocatalytic, antimicrobial, and cytotoxicity evaluation. J. Mater. Res. Technol. 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Teaima, M.H.; Elasaly, M.K.; Omar, S.A.; El-Nabarawi, M.A.; Shoueir, K.R. Eco-friendly synthesis of functionalized chitosan-based nanoantibiotic system for potential delivery of linezolid as antimicrobial agents. Saudi Pharm. J. 2020, 28, 859–868. [Google Scholar] [CrossRef]
- Shoueir, K.R. Green microwave synthesis of functionalized chitosan with robust adsorption capacities for Cr (VI) and/or RHB in complex aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, L.; Chen, Z.; Zhu, C.; Liu, J.; Zheng, J. Ammonium citrate derived carbon quantum dot as on-off-on fluorescent sensor for detection of chromium (VI) and sulfites. Mater. Lett. 2017, 191, 1–4. [Google Scholar] [CrossRef]
- Wang, B.; Tang, W.; Lu, H.; Huang, Z. Ionic liquid capped carbon dots as a high-performance friction-reducing and antiwear additive for poly (ethylene glycol). J. Mater. Chem. A. 2016, 4, 7257–7265. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasaók, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C. 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Vedamalai, M.; Periasamy, A.P.; Wang, C.-W.; Tseng, Y.-T.; Ho, L.-C.; Shih, C.-C.; Chang, H.-T. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu 2+ ions in cells. Nanoscale 2014, 6, 13119–13125. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Anappara, A.A. White-light-emitting carbon dots prepared by the electrochemical exfoliation of graphite. Chem. Phys. Chem. 2017, 18, 292–298. [Google Scholar] [CrossRef]
- Shinde, D.B.; Pillai, V.K. Electrochemical preparation of luminescent graphene quantum dots from multiwalled carbon nanotubes. Chem. Eur. J. 2012, 18, 12522–12528. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, B.; Li, L. Colloidal graphene quantum dots with well-defined structures. Acc. Chem. Res. 2013, 46, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Noffke, B.W.; Liu, Y.; Li, L.S. Understanding fundamental processes in carbon materials with well-defined colloidal graphene quantum dots. Curr. Opin. Colloid Interface Sci. 2015, 20, 346–353. [Google Scholar] [CrossRef]
- El-Shabasy, R.; Yosri, N.; El-Seedi, H.; Shoueir, K.; El-Kemary, M. A green synthetic approach using chili plant supported Ag/Ag2O@ P25 heterostructure with enhanced photocatalytic properties under solar irradiation. Optik 2019, 192, 162943. [Google Scholar] [CrossRef]
- Sharma, V.; Orejon, D.; Takata, Y.; Krishnan, V.; Harish, S. Gladiolus dalenii based bioinspired structured surface via soft lithography and its application in water vapor condensation and fog harvesting. ACS Sustain. Chem. Eng. 2018, 6, 6981–6993. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators B Chem. 2015, 2013, 434–443. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Sheng, Y.; Shen, J.; Huang, P.; Guo, S.; Pan, J.; Liu, B.; Feng, B. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J. Chem. 2014, 385, 906–909. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green synthesis of luminescent nitrogen-doped carbon dots from milk and its imaging application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low. Dimens. Syst. Nanostruct. 2020, 126, 114417. [Google Scholar] [CrossRef]
- Da Silva Júnior, A.H.; Macuvele, D.L.P.; Riella, H.G.; Soares, C.; Padoin, N. Novel carbon dots for zinc sensing from Campomanesia phaea. Mater. Lett. 2021, 283, 128813. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Facile synthesis of surface-modified carbon quantum dots (CQDs) for biosensing and bioimaging. Materials 2020, 13, 3313. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells. J. Photochem. Photobiol. B Biol. 2019, 191, 150–155. [Google Scholar] [CrossRef]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- Hoan, B.T.; Tam, P.D.; Pham, V.-H. Green synthesis of highly luminescent carbon quantum dots from lemon juice. J. Nanotechnol. 2019, 2019. [Google Scholar] [CrossRef]
- Lai, Z.; Guo, X.; Cheng, Z.; Ruan, G.; Du, F. Green synthesis of fluorescent carbon dots from cherry tomatoes for highly effective detection of trifluralin herbicide in soil samples. Chem. Select. 2020, 5, 1956–1960. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Buszewski, B.; Gadzała-Kopciuch, R. Sulphur and nitrogen doped carbon dots synthesis by microwave assisted method as quantitative analytical nano-tool for mercury ion sensing. Mater. Chem. Phys. 2020, 242, 122484. [Google Scholar] [CrossRef]
- Eskalen, H.; Uruş, S.; Cömertpay, S.; Kurt, A.H.; Özgan, Ş. Microwave-assisted ultra-fast synthesis of carbon quantum dots from linter: Fluorescence cancer imaging and human cell growth inhibition properties. Ind. Crops Prod. 2020, 147, 112209. [Google Scholar] [CrossRef]
- Tadesse, M.; Hagos, D.; RamaDevi, K.; Basavaiah, N.; Belachew, N. Fluorescent-nitrogen-doped carbon quantum dots derived from citrus lemon juice: Green synthesis, mercury (II) ion sensing, and live cell imaging. ACS Omega 2020, 5, 3889–3898. [Google Scholar] [CrossRef]
- Başoğlu, Ü.; Ocak, A.; Gümrükçüoğlu, A. Synthesis of microwave-assisted fluorescence carbon quantum dots using roasted–chickpeas and its applications for sensitive and selective detection of Fe3+ ions. J. Fluoresc. 2020, 30, 1–12. [Google Scholar] [CrossRef]
- Wesoły, M.; Cetó, X.; Del Valle, M.; Ciosek, P.; Wróblewski, W. Quantitative analysis of active pharmaceutical ingredients (APIs) using a potentiometric electronic tongue in a SIA flow system. Electroanalysis 2016, 28, 626–632. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu 2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Thomas, K.V.; Bijlsma, L.; Castiglioni, S.; Covaci, A.; Emke, E.; Grabic, R.; Hernández, F.; Karolak, S.; Kasprzyk-Hordern, B.; Lindberg, R.H. Comparing illicit drug use in 19 European cities through sewage analysis. Sci. Total Environ. 2012, 432, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Al Jahdaly, B.A.; Elsadek, M.F.; Ahmed, B.M.; Farahat, M.F.; Taher, M.M.; Khalil, A.M. Outstanding Graphene Quantum Dots from Carbon Source for Biomedical and Corrosion Inhibition Applications: A Review. Sustainable 2021, 13, 2127. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon N.Y. 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Yang, S.T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Gu, J.; Hu, M.J.; Guo, Q.Q.; Ding, Z.F.; Sun, X.L.; Yang, J. High-yield synthesis of graphene quantum dots with strong green photoluminescence. RSC Adv. 2014, 4, 50141–50144. [Google Scholar] [CrossRef]
- De Yro, P.A.N.; Quaichon, G.M.O.; Cruz, R.A.T.; Emolaga, C.S.; Que, M.C.O.; Magdaluyo, E.J.R.; Basilia, B.A. Hydrothermal synthesis of carbon quantum dots from biowaste for bio-imaging. AIP Conf. Proc. 2019, 2083, 1–6. [Google Scholar] [CrossRef]
- Basavaiah, K.; Tadesse, A.; RamaDevi, D.; Hagos, M.; Battu, G. Facile green synthesis of fluorescent carbon quantum dots from citrus lemon juice for live cell imaging. Asian J. Nanosci. Mater. 2018, 1, 36–46. [Google Scholar]
- Prasannan, P.; Imae, T. One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Y.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of carbon quantum dot nanoparticles derived from byproducts in bio-refinery process for cell imaging and in vivo bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef]
- Wang, X.; Yang, P.; Feng, Q.; Meng, T.; Wei, J.; Xu, C.; Han, J. Green preparation of fluorescent carbon quantum dots from cyanobacteria for biological imaging. Polymers 2019, 11, 616. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 30, 75–82. [Google Scholar] [CrossRef]
- Janus, Ł.; Piatkowski, M.; Radwan-Pragłowska, J.; Bogdał, D.; Matysek, D. Chitosan-based carbon quantum dots for biomedical applications: Synthesis and characterization. Nanomaterials 2019, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, A.K.; Ashraf, P.M. Carbon nanodots synthesized from chitosan and its application as a corrosion inhibitor in boat-building carbon steel BIS2062. Appl. Nanosci. 2020, 10, 1061–1071. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhu, L.; Li, Y.; Yan, Z.; Shen, Z.; Cao, X. Facile microwave assisted synthesis of N-rich carbon quantum dots/dual-phase TiO2 heterostructured nanocomposites with high activity in CO2 photoreduction. Appl. Catal. B Environ. 2018, 231, 269–276. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ehrat, F.; Urban, R.; Teves, R.; Wyrwich, R.; Döblinger, M.; Feldmann, J.; Urban, A.S.; Stolarczyk, J.K. Effect of nitrogen atom positioning on the trade-off between emissive and photocatalytic properties of carbon dots. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Thakur, M.; Mewada, A.; Pandey, A.; Bhori, M.; Singh, K.; Sharon, M.; Sharon, M. Milk-derived multi-fluorescent graphene quantum dot-based cancer theranostic system. Mater. Sci. Eng. C. 2016, 67, 468–477. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.Q.; Huang, H.; Feng, J.J.; Wang, A.J. Eco-friendly and rapid microwave synthesis of green fluorescent graphitic carbon nitride quantum dots for vitro bioimaging. Sens. Actuators B Chem. 2016, 226, 506–511. [Google Scholar] [CrossRef]
- Franco, C.A.; Candela, C.H.; Gallego, J.; Marin, J.; Patino, L.E.; Ospina, N.; Patiño, E.; Molano, M.; Villamil, F.; Bernal, K.M. Easy and rapid synthesis of carbon quantum dots from Mortino (Vaccinium Meridionale Swartz) extract for use as green tracers in the oil and gas industry: Lab-to-field trial development in Colombia. Ind. Eng. Chem. Res. 2020, 59, 11359–11369. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Dager, T.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of mono-disperse carbon quantum dots from fennel seeds: Photoluminescence analysis using machine learning. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, Y.; Wang, J.; Pu, Y.; Xue, W.; Liu, X. Green synthesis of graphene quantum dots and silver nanoparticles compounds with excellent surface enhanced Raman scattering performance. J. Alloys Compd. 2016, 663, 166–171. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chemie. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Gogoi, N.; Chowdhury, D. Novel carbon dot coated alginate beads with superior stability, swelling and pH responsive drug delivery. J. Mater. Chem. B. 2014, 2, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Saha, B.; Ghosh, S.K.; Pradeep Singh, N.D. Photoresponsive quinoline tethered fluorescent carbon dots for regulated anticancer drug delivery. Chem. Commun. 2013, 49, 10471–10473. [Google Scholar] [CrossRef]
- Dong, J.; Wang, K.; Sun, L.; Sun, B.; Yang, M.; Chen, H.; Wang, Y.; Sun, J.; Dong, L. Application of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug delivery. Sens. Actuators B Chem. 2018, 256, 616–623. [Google Scholar] [CrossRef]
- Majumdar, S.; Krishnatreya, G.; Gogoi, N.; Thakur, D.; Chowdhury, D. Carbon-dot-coated alginate beads as a smart stimuli-responsive drug delivery system. ACS Appl. Mater. Interfaces 2016, 8, 34179–34184. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Kim, H.; Singha, K.; Kim, W.J. Transfection and intracellular trafficking properties of carbon dot-gold nanoparticle molecular assembly conjugated with PEI-Pdna. Biomaterials 2013, 34, 7168–7180. [Google Scholar] [CrossRef]
- Yang, K.K.; Chan, G.; Xu, M.; Yin, G.; Lin, X.; Wang, W.J.; Lin, M.D.; Birowosuto, S.; Zeng, T.; Ogi, K.; et al. Biodegradable polymer-coated multifunctional graphene quantum dots for light-triggered synergetic therapy of pancreatic cancer. ACS Appl. Mater. Interfaces. 2019, 11, 2768–2781. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Shen, X.; Su, C.; Yang, J.; Piao, M.; Jia, F.; Gao, G.; Zhang, L.; Lin, Q. One-step synthesis of photoluminescent carbon dots with excitation-independent emission for selective bioimaging and gene delivery. J. Colloid Interface Sci. 2017, 492, 1–7. [Google Scholar] [CrossRef]
- Jhonsi, M.A. Carbon quantum dots for bioimaging, in: State art nano-bioimaging. Intech. Open 2018. [Google Scholar] [CrossRef]
- Wang, M.; Kang, X.; Deng, L.; Xia, Z.; Gao, D. Deep eutectic solvent assisted synthesis of carbon dots using Sophora flavescens Aiton modified with polyethyleneimine: Application in myricetin sensing and cell imaging. Food Chem. 2020, 345, 128817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, G.; Wang, H.; Liang, Z.; Zhou, L.; Yan, B. Protamine assisted rapid synthesis of carbon dots for living nucleolus imaging and gene delivery applications. J. Mater. Sci. 2021, 56, 4396–4406. [Google Scholar] [CrossRef]
- Xie, W.F.; Ma, Y.; Zhang, H.; Fan, M. Louzhen (Department of Chemistry, Beijing Normal University, Beijing 100875); Preparation of fluorescent graphene quantum dots as biological imaging marker for cells. Acta Chim. Sin. 2012, 70, 2169. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Xi, C.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z. Cutting sp 2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.; Cui, Z.; Yang, H.; Guo, C.; Chi, Y.; Li, C.M. One-step and high yield simultaneous preparation of single-and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764–8766. [Google Scholar] [CrossRef]
- Nurunnabi, M.D.; Khatun, Z.; Reeck, G.R.; Lee, D.Y.; Lee, Y. Near infra-red photoluminescent graphene nanoparticles greatly expand their use in noninvasive biomedical imaging. Chem. Commun. 2013, 49, 5079–5081. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent advances in carbon nanodots: Synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: Synthesis, characterization, and bioimaging application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Bin Yang, H.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chemie. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Liu, Y.; Feng, X.Z.; Yin, X.B.; He, X.W.; Zhang, Y.K. Nitrogen-doped carbon dots: A facile and general preparation method, photoluminescence investigation, and imaging applications. Chem. A Eur. J. 2013, 19, 2276–2283. [Google Scholar] [CrossRef]
- Zheng, X.T.; Than, A.; Ananthanaraya, A.; Kim, D.H.; Chen, P. Graphene quantum dots as universal fluorophores and their use in revealing regulated trafficking of insulin receptors in adipocytes. ACS Nano 2013, 7, 6278–6286. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, H.; Song, X.; Ma, X.; Wang, J.; Tan, M. Presence of photoluminescent carbon dots in Nescafe® original instant coffee: Applications to bioimaging. Talanta 2014, 127, 68–74. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, C.; Ding, B.; Li, S.; Zhou, R.; Wang, Y.; Tian, Y. A two-photon “turn-on” fluorescent probe based on carbon nanodots for imaging and selective biosensing of hydrogen sulfide in live cells and tissues. Analyst 2014, 139, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Y.; He, N.; Zhang, Y.; Lu, Z.; Zhang, J.; Zhang, Z. Preparation of graphene quantum dots for bioimaging application. J. Nanosci. Nanotechnol. 2012, 12, 2924–2928. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.F.; Dai, L. Recent advances in graphene quantum dots for fluorescence bioimaging from cells through tissues to animals. Part. Part. Syst. Charact. 2015, 32, 515–523. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, F.; Zheng, T.; Shi, D.; Sun, F.; Yang, N.; Chen, L. Highly luminescent organosilane-functionalized carbon dots as a nanosensor for sensitive and selective detection of quercetin in aqueous solution. Talanta 2015, 135, 145–148. [Google Scholar] [CrossRef]
- Yang, J.; Gao, G.; Zhang, X.; Ma, Y.H.; Chen, X.; Wu, F.G. One-step synthesized carbon dots with bacterial contact-enhanced fluorescence emission property: Fast Gram-type identification and selective Gram-positive bacterial inactivation. Carbon N.Y. 2019, 146, 827–839. [Google Scholar] [CrossRef]
- Huang, S.; Li, W.; Han, P.; Zhou, X.; Cheng, J.; Wen, H.; Xue, W. Carbon quantum dots: Synthesis, properties, and sensing applications as a potential clinical analytical method. Anal. Methods 2019, 11, 2240–2258. [Google Scholar] [CrossRef]
- Fan, Z.; Nie, Y.; Wei, Y.; Zhao, J.; Liao, X.; Zhang, J. Facile and large-scale synthesis of graphene quantum dots for selective targeting and imaging of cell nucleus and mitochondria. Mater. Sci. Eng. C. 2019, 103, 109824. [Google Scholar] [CrossRef] [PubMed]
- Barras, Q.; Pagneux, F.; Sane, Q.; Wang, R.; Boukherroub, D.; Hober, S. Szunerits, high efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces 2016, 8, 9004–9013. [Google Scholar] [CrossRef]
- Nekoueian, K.; Amiri, M.; Sillanpää, M.; Marken, F.; Boukherroub, R.; Szunerits, S. Carbon-based quantum particles: An electroanalytical and biomedical perspective. Chem. Soc. Rev. 2019, 48, 4281–4316. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, E.; Lorentz, K.O.; Stein, G.J.; Mitchell, P.D. Prehistoric schistosomiasis parasite found in the Middle East. Lancet Infect. Dis. 2014, 14, 553–554. [Google Scholar] [CrossRef]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Carbon dots for sensing and killing microorganisms. C. 2019, 5, 33. [Google Scholar] [CrossRef]
- Shoueir, K.R.; Atta, A.M.; Sarhan, A.A.; Akl, M.A. Synthesis of monodisperse core shell PVA@P(AMPS-co-NIPAm) nanogels structured for pre-concentration of Fe (III) ions. Environ. Technol. 2017, 38, 38. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Shouir, K.R.; El-Kemary, M. Activated H2O2 on Ag/SiO2–SrWO4 surface for enhanced dark and visible-light removal of methylene blue and p-nitrophenol. J. Alloys Compd. 2020, 842, 155848. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Al-Radadi, N.S.; Ahmed, M.K.; Shoueir, K.; Elkemary, M. Dye removal, antibacterial properties, and morphological behavior of hydroxyapatite doped with Pd ions. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Ge, S.; Wang, S.; Yan, M.; Zang, D.; Yu, J. Facile and sensitive paper-based chemiluminescence DNA biosensor using carbon dots dotted nanoporous gold signal amplification label. Anal. Methods. 2013, 5, 1328–1336. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Zhao, H.X.; Liu, L.Q.; de Liu, Z.; Wang, Y.; Zhao, X.J.; Huang, C.Z. Highly selective detection of phosphate in very complicated matrixes with an off–on fluorescent probe of europium-adjusted carbon dots. Chem. Commun. 2011, 47, 2604–2606. [Google Scholar] [CrossRef]
- Dai, H.; Yang, C.; Tong, Y.; Xu, G.; Ma, X.; Lin, Y.; Chen, G. Label-free electrochemiluminescent immunosensor for α-fetoprotein: Performance of nafion-carbon nanodots nanocomposite films as antibody carriers. Chem. Commun. 2012, 48, 3055–3057. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, Y.; Zhu, L.; Li, X.; Li, G. An amperometric biosensor for the detection of hydrogen peroxide released from human breast cancer cells. Biosens. Bioelectron. 2013, 41, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xue, W.; Chen, H.; Lin, J. Peroxynitrous-acid-induced chemiluminescence of fluorescent. Anal. Chem. 2011, 83, 8245–8251. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury(II) ions. Sens. Actuators B Chem. 2013, 184, 156–162. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta. 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Appl. Mater. Interfaces 2015, 7, 3058–3067. [Google Scholar] [CrossRef]
- Mohd Yazid, S.N.A.; Chin, S.F.; Pang, S.C.; Ng, S.M. Detection of Sn (II) ions via quenching of the fluorescence of carbon nanodots. Microchim. Acta. 2013, 180, 137–143. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, S.; Liu, M.; Dong, C.; Huang, C. Low-cost synthesis of carbon nanodots from natural products used as a fluorescent probe for the detection of ferrum(iii) ions in lake water. Anal. Methods. 2014, 6, 2086–2090. [Google Scholar] [CrossRef]
- Pramanik, A.; Biswas, S.; Kumbhakar, P. Solvatochromism in highly luminescent environmental friendly carbon quantum dots for sensing applications: Conversion of bio-waste into bio-asset, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 498–512. [Google Scholar] [CrossRef]
- Rawtani, D.; Rao, P.K.; Hussain, C.M. Recent advances in analytical, bioanalytical and miscellaneous applications of green nanomaterial. TrAC Trends Anal. Chem. 2020, 133, 116109. [Google Scholar] [CrossRef]
- Yuan, C.; Qin, X.; Xu, Y.; Li, X.; Chen, Y.; Shi, R.; Wang, Y. Carbon quantum dots originated from chicken blood as peroxidase mimics for colorimetric detection of biothiols. J. Photochem. Photobiol. A Chem. 2020, 396, 112529. [Google Scholar] [CrossRef]
- Bao, R.; Chen, Z.; Zhao, Z.; Sun, X.; Zhang, J.; Hou, L.; Yuan, C. Green and facile synthesis of nitrogen and phosphorus co-doped carbon quantum dots towards fluorescent ink and sensing applications. Nanomaterials 2018, 8, 386. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Liu, M.; Niu, F.; Liu, J.; Wang, E. Enhanced-quantum yield sulfur/nitrogen co-doped fluorescent carbon nanodots produced from biomass Enteromorpha prolifera: Synthesis, posttreatment, applications and mechanism study. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Kumar, V.; Singh, V.K.; Hasan, S.H. Green synthesis of fluorescent carbon quantum dots for the detection of mercury(ii) and glutathione. New, J. Chem. 2018, 42, 5814–5821. [Google Scholar] [CrossRef]
- Shen, P.; Xia, Y. Synthesis-modification integration: One-step fabrication of boronic acid functionalized carbon dots for fluorescent blood sugar sensing. Anal. Chem. 2014, 86, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Di, Y.; Zhu, X.; Ng, T.W.; Xia, J.; Liu, W.; Meng, X.; Wang, P.; Lee, C.S.; Zhang, W. A carbon dot-based fluorescence turn-on sensor for hydrogen peroxide with a photo-induced electron transfer mechanism. Chem. Commun. 2015, 51, 15574–15577. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.C.; Huang, H.; Wang, A.J.; Chen, J.R.; Feng, J.J. Facile synthesis of N, S-codoped fluorescent carbon nanodots for fluorescent resonance energy transfer recognition of methotrexate with high sensitivity and selectivity. Biosens. Bioelectron. 2015, 64, 517–522. [Google Scholar] [CrossRef]
- Shi, J.; Chan, C.; Pang, Y.; Ye, W.; Tian, F.; Lyu, J.; Zhang, Y.; Yang, M. A fluorescence resonance energy transfer (FRET) biosensor based on graphene quantum dots (GQDs) and gold nanoparticles (AuNPs) for the detection of mecA gene sequence of Staphylococcus aureus. Biosens. Bioelectron. 2015, 67, 595–600. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhao, D.; Zeng, D.; Xia, J.; Aldalbahi, A.; Wang, C.; San, L.; Fan, C.; Zuo, X.; et al. Universal fluorescence biosensor platform based on graphene quantum dots and pyrene-functionalized molecular beacons for detection of micrornas. ACS Appl. Mater. Interfaces 2015, 7, 16152–16156. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.S.; Gonçalves, A.S.; De Morais, A.; Benedetti, J.E.; Nogueira, A.F. Graphene-like MoS 2 as a low-cost counter electrode material for dye-sensitized solar cells, This, J. Is © NanoGe. J. Energy Sustain. 2012, 1, 11002–11003. [Google Scholar] [CrossRef]
- Najafiaghdam, H.; Papageorgiou, E.; Torquato, N.A.; Tian, B.; Cohen, B.E.; Anwar, M. A 25 micron-thin microscope for imaging upconverting nanoparticles with NIR-I and NIR-II illumination. Theranostics 2019, 9, 8239. [Google Scholar] [CrossRef]
- Yang, F.; LeCroy, G.E.; Wang, P.; Liang, W.; Chen, J.; Fernando, K.A.S.; Bunker, C.E.H.; Qian, H.; Sun, Y.-P. Functionalization of carbon nanoparticles and defunctionalization toward structural and mechanistic elucidation of carbon “quantum” dots. J. Phys. Chem. C. 2016, 120, 25604–25611. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, Q.; Li, W.; Xie, Y.; Liu, H.; Shang, L.; Liu, Z.; Chen, Z.; Gu, L. A general route to prepare low-ruthenium-content bimetallic electrocatalysts for pH-universal hydrogen evolution reaction by using carbon quantum dots. Angew. Chemie Int. Ed. 2020, 59, 1718–1726. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, L.; Lan, C.; Zhao, J.; Su, Y.; Zhao, S. One-pot green synthesis of oxygen-rich nitrogen-doped graphene quantum dots and their potential application in pH-sensitive photoluminescence and detection of mercury (II) ions. Talanta 2015, 142, 131–139. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Emam, H.E. Environmentally exploitable biocide/fluorescent metal marker carbon quantum dots. RSC Adv. 2020, 10, 42916–42929. [Google Scholar] [CrossRef]
- De Oliveira, M.C.A.; de Elisângela, G.L.; Pires, I.C.B.; Candido, I.C.M.; Rakov, N.; de Oliveira, H.P.; Xing, Y.; Maciel, G.S. Carbon dots-doped electrospun fibers for simultaneous metal ion detection and adsorption of dyes. Adv. Fiber Mater. 2020, 2, 302–313. [Google Scholar] [CrossRef]
- Sharma, N.; Das, G.S.; Yun, K. Green synthesis of multipurpose carbon quantum dots from red cabbage and estimation of their antioxidant potential and bio-labeling activity. Appl. Microbiol. Biotechnol. 2020, 104, 7187–7200. [Google Scholar] [CrossRef] [PubMed]
- Tungare, K.; Bhori, M.; Racherla, K.S.; Sawant, S. Synthesis, characterization and biocompatibility studies of carbon quantum dots from Phoenix dactylifera. 3 Biotech. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]




| Synthetic Approach | Source | Quantum Yield (%) | Size Range (nm) | λem Max | Application | Ref. |
|---|---|---|---|---|---|---|
| Hydrothermal | Banana peel waste | 5 | 4–6 | 355–429 | Bio imaging | [65] |
| Hydrothermal | Cambuci juice (Campomanesia phaea) | 21.3 | 3.7 | 270, 283 | Sensing of Zn2+ | [66] |
| Hydrothermal | Biomass waste | 4.3–8.2 | 1.3 and 4.9 | 445, 435, 43, 435 | Detection of Fe3+ | [67] |
| Hydrothermal | Biomass waste | 14–3.5 | 6 | 205, 260 | Bio imaging | [68] |
| Hydrothermal | Manilkara zapota fruits | 5.7, 7.9, 5.2 | 1.9 ± 0.3, 2.9 ± 0.7, 4.5 ± 1.25 | 405, 488, 561 | Bio imaging | [69] |
| Hydrothermal | Broccoli | - | 2–6 | 330–470 | Ag+ sensing | [70] |
| Hydrothermal | Lemon juice | 79 | 4.5 | 540 | Biosensors | [71] |
| Hydrothermal | Cherry tomatoes | 9.7 | 7 | 430 | Biosensors | [72] |
| Microwave-assisted | ND | 26 | ~10 | ND | sensor of Hg2+ detection | [73] |
| Microwave-assisted | Cotton linter waste | ND | 10.1 | 420 | Bioimaging | [74] |
| Microwave-assisted | Quince fruit | 8.6 | 4.9 | 450 | Bioimaging | [75] |
| Microwave-assisted | Roasted–Chickpeas | 1.8 | 4.5–10.3 | 435 | Detection of Fe3+ | [76] |
| Pyrolysis | Chia seeds | ND | 4 | ND | Sensors | [77] |
| Pyrolysis | Finger millet ragi | ND | 6 | ND | Biosensor | [78] |
| Pyrolysis | Mango | 18.2 | 6 | 525 | Biosensor | [79] |
| Cell Line | Imaging Position | Quantum Dots Conc. | Color | Ref. |
|---|---|---|---|---|
| L929 fibroblasts | Membrane, cytoplasm | 0.30 mg/mL | ND | [68] |
| HeLa | Membrane, cytoplasm | 10 μg/mL | Blue, green, yellow | [69] |
| Nematodes | ND | 100 μg/mL | Blue, green, red | [65] |
| H2452, HUVEC | ND | 50 μL/mL, 100 μL/mL | Blue | [74] |
| HT-129 | ND | ND | Blue | [75] |
| HEK-293 cells | Cell membrane | 40 μg/mL | Multi-color | [113] |
| A549 MCF-7 | Cell cytoplasm | 25 μg/mL | Yellow | [114] |
| HeLa | Cell nucleus | 0.01 mg/mL | Green | [115] |
| MCF-7 | Cell membrane, cytoplasm, nucleus | 100 μg/mL | Green | [116] |
| MDA-MB231 | Cell | 0.1 mg/mL | Red | [117] |
| MC3T3 | Cell cytoplasm | 2.5 mg/mL | Bright green or blue | [118] |
| Analyte | LOD | Range | λem Max | Quantum Yield (%) | Size Range (nm) | Ref. |
|---|---|---|---|---|---|---|
| Zn2+ | 5.4 μM | 0–125 μM, 125–200 μM | 270, 283 | 21.3 | 3.7 | [66] |
| Fe3+ | 0.073 μM | 0.1–0.9 μM | 445, 435, 43, 435 | 4.3–8.2 | 1.3 and 4.9 | [67] |
| Ag+ | 0.5μM | 0–600 μM | 330–470 | - | 2–6 | [70] |
| V5+ | 3.2 ppm | 0–100 ppm | 540 | 79 | 4.5 | [71] |
| Trifluralin | 0.5 nM | 0.050–200 μM | 430 | 9.7 | 7 | [72] |
| Hg2+ | 1.78 μM | 5–50 μM | ND | 26 | ~10 | [73] |
| As3+ | 0.02 μg/mL | 0.1–2 μg/mL | 450 | 8.6 | 4.9 | [75] |
| Fe3+ | 2.8, 8.2 μM | 11.3, 37.5 μM | 435 | 1.8 | 4.5–10.3 | [76] |
| Hydrazine | 39.7 μM | 125–1125 μM | ND | ND | 4 | [77] |
| Cu2+ | 10 nM | 0–100 μM | ND | ND | 6 | [78] |
| Fe2+ | 0.62 ppm | ND | 525 | 18.2 | 6 | [79] |
| Fe3+ | 0.21 μM | 0–300 μM | 450 | 22 | 6 | [153] |
| Hg2+ | 2.3 nM | 5–100 μM | 428 | 6.4 | 2.8 | [154] |
| Hg2+ | 2.6 μM | 10–100 μM | 420 | 9.6 | 8 | [155] |
| Fe3+ | 0.56 μM | 50–350 μM | 493 | 11.2 | 3 | [156] |
| Fe3+ | 0.5 μM | 0–1.7 mM | 450 | 8 | 2.8 | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shabasy, R.M.; Farouk Elsadek, M.; Mohamed Ahmed, B.; Fawzy Farahat, M.; Mosleh, K.N.; Taher, M.M. Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes 2021, 9, 388. https://doi.org/10.3390/pr9020388
El-Shabasy RM, Farouk Elsadek M, Mohamed Ahmed B, Fawzy Farahat M, Mosleh KN, Taher MM. Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes. 2021; 9(2):388. https://doi.org/10.3390/pr9020388
Chicago/Turabian StyleEl-Shabasy, Rehan M., Mohamed Farouk Elsadek, Badreldin Mohamed Ahmed, Mohamed Fawzy Farahat, Khaled N. Mosleh, and Mohamed M. Taher. 2021. "Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications" Processes 9, no. 2: 388. https://doi.org/10.3390/pr9020388
APA StyleEl-Shabasy, R. M., Farouk Elsadek, M., Mohamed Ahmed, B., Fawzy Farahat, M., Mosleh, K. N., & Taher, M. M. (2021). Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes, 9(2), 388. https://doi.org/10.3390/pr9020388






