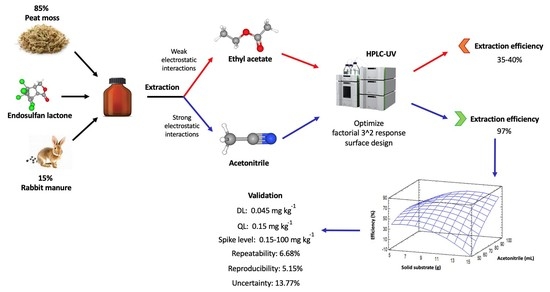
Optimization and Validation of an Extraction Method for Endosulfan Lactone on a Solid Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Analytical Standards
2.2. Raw Material
2.3. Equipment
2.4. Solid Substrate Parameters
2.5. Analytical Method
2.5.1. Extraction Procedure with Acetonitrile
2.5.2. Extraction Procedure with Ethyl Acetate
2.5.3. HPLC UV-VIS Conditions
2.5.4. Experimental Design
2.5.5. Optimization Process
2.6. Validation Method
3. Results
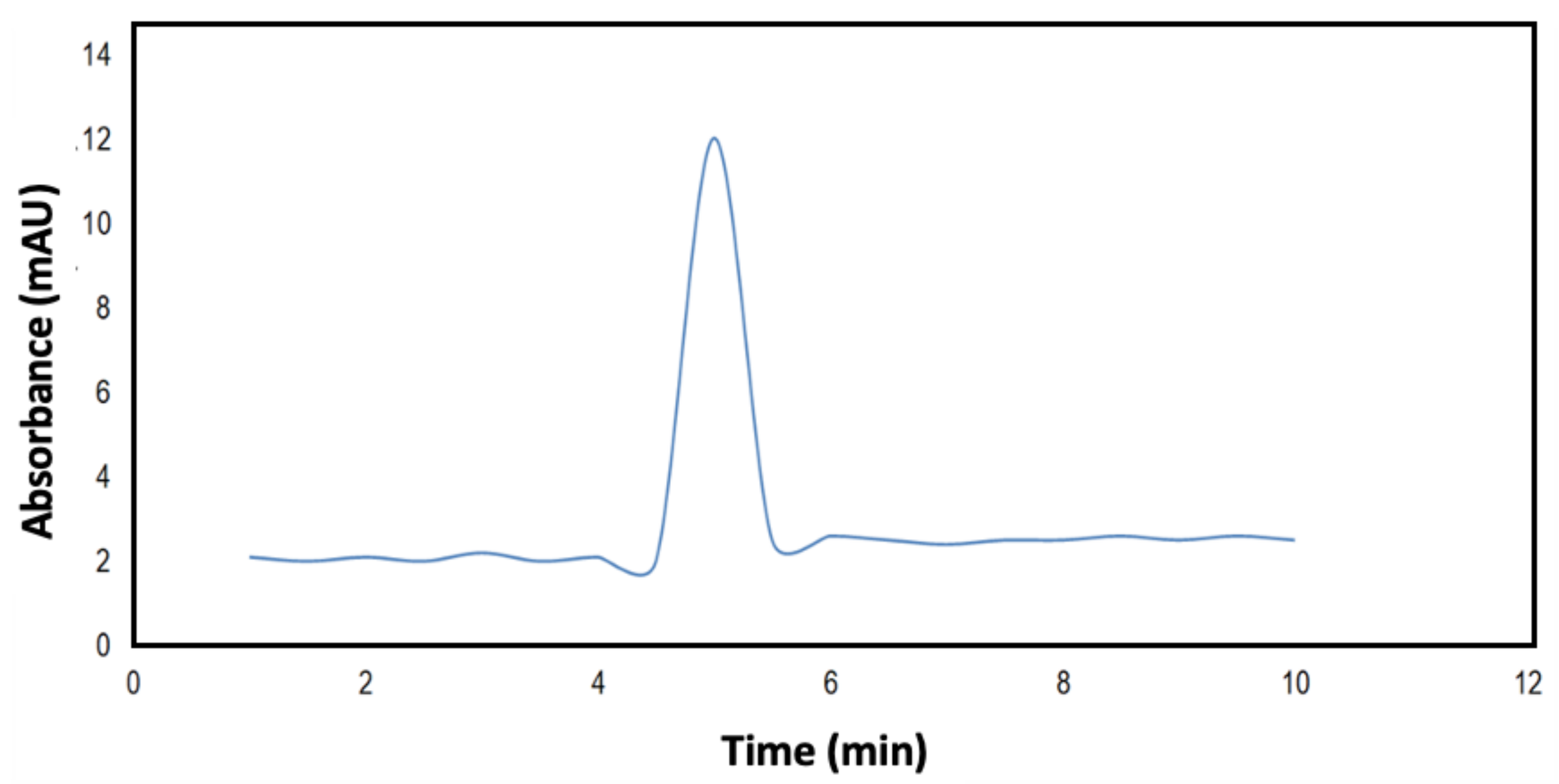
3.1. Solvent Extraction
3.2. Optimization Process
3.3. Validation Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uzcátegui, J.; Mendoza, L. Organochloride pesticides residues and their relation to soil parameters in Pueblo Llano, Mérida state, Venezuela. Bioagro 2011, 23, 115–120. [Google Scholar]
- Bussian, B.N.; Pandelova, M.; Lehnik-Habrink, B.; Henkelmann, B.; Schramm, K.W. Persistent endosulfán sulfate is found with highest abundance among endosulfán I, II and sulfate in German forest soils. Environ. Pollut. 2015, 206, 661–666. [Google Scholar] [CrossRef]
- Betancurt, L.A.; Ocampo, R.; Ríos, L.A. La problemática del endosulfán: Aspectos químicos, analíticos y ambientales. Rev. Luna Azul 2015, 40, 293–313. [Google Scholar]
- Stockholm Convention On Persistent Organic Pollutants; Review Committee UNEP/POPS/POPRC: Geneva, Switzerland, 2011.
- Tiwari, M.K.; Guha, S. Kinetics of the biodegradation pathway of endosulfán in the aerobic and anaerobic environments. Chemosphere 2013, 93, 567–573. [Google Scholar] [CrossRef]
- Annex, C. Risk profile of endosulfán. United Nations Environmental Programme. Geneva 2008, 29, 1–6. [Google Scholar]
- Vázquez-Villegas, P.T.; Meza-Gordillo, R.; Gutiérrez-Miceli, F.A.; Ruíz-Valdiviezo, V.M.; Villalobos-Maldonado, J.J.; Montes-Molina, J.A.; Fernández-Toledo, A.A.J. Determinación de CL50 y CE50 de endosulfán lactona y diazinon en lombriz de tierra (Eisenia foetida). Agroproductividad 2018, 11, 105–112. [Google Scholar]
- Durak, B.Y.; Chormey, D.S.; Firat, M.; Bakirdere, S. Validation of ultrasonic-assisted switchable solvent liquid phase microextraction for trace determination of hormones and organochloride pesticides by GC-MS and combination with QuEChERS. Food Chem. 2020, 305. [Google Scholar] [CrossRef]
- Karim, A.V.; Singh, S.P.; Shrivastav, S. Measurement and removal of endosulfán from contaminated environmental matrices. Environ. Contam. 2018, 145–164. [Google Scholar] [CrossRef]
- Gfrerer, M.; Lankmayr, E. Screening, optimization and validation of microwave-assisted extraction for the determination of persistent organochloride pesticides. Anal. Chim. Acta 2005, 533, 203–211. [Google Scholar] [CrossRef]
- Rashid, A.; Nawaz, S.; Barker, H.; Ahmad, I.; Ashraf, M. Development of a simple extraction and clean-up procedure for determination of organochloride pesticides in soil using gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 2933–2939. [Google Scholar] [CrossRef]
- Parte, S.G.; Mohekar, A.D.; Kharat, A.S. Microbial degradation of pesticide: A review. Afr. J. Biotechnol. 2017, 11, 992–1012. [Google Scholar] [CrossRef]
- Rivera, A.P.T.; Murgas, R.E.C.; Ríos, A.E.M.; Merini, L.J. Effect of glyphosate on microbiota, soil quality and biofortified bean crop in Codazzi, department of Cesar, Colombia. Rev. Argent. Microbiol. 2020, 52, 61–71. [Google Scholar] [CrossRef]
- Masiá, A.; Vázquez, K.; Campo, J.; Picó, Y. Assessment of two extraction methods to determine pesticides in soils, sediments and sludges. Application to the Túria River Basin. J. Chromatogr. A 2015, 1378, 19–31. [Google Scholar] [CrossRef]
- Fernández-Moreno, J.L.; Garrido-Frenich, A.; Plaza-Bolaños, P.; Martínez-Vidal, J.L. Multiresidue method for the analysis of more than 140 pesticides residues in fruits and vegetables by chromatography coupled to triple quadrupole mass spectrometry. J. Mass Spectrom 2008, 43, 1235–1254. [Google Scholar] [CrossRef]
- Li, W.; Dai, Y.; Xue, B.; Li, Y.; Peng, X.; Zhang, J.; Yan, Y. Biodegradation and detoxification of endosulfán in aqueous medium and soil by Achromobacter xylosoxidans strain CS5. J. Hazard. Mater. 2009, 167, 209–2016. [Google Scholar] [CrossRef]
- Tor, A.; Aydin, M.E.; Özcan, S. Ultrasonic solvent extraction of organochlorine pesticides from soil. Anal. Chim. Acta 2006, 559, 173–180. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Y.; Lu, J.; Wilson, C. Copper clean-up procedure for ultrasonic extraction and analysis of pyrethroid and phenylpyrazole pesticides in sediments by gas chromatography-electron capture detection. Sci. Total Environ. 2011, 409, 3482–3491. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Badu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 33. [Google Scholar] [CrossRef]
- Vanaja, K.; Shobha, R.H. Desing of experiments: Concept and applications of Plackett Burman Desing. Clin. Res. Regul. Aff. 2007, 24, 1–23. [Google Scholar] [CrossRef]
- Murphy, R.J. Screening design. Encycl. Biopharm. Stat. 2003, 1, 920. [Google Scholar]
- Jaradat, K.A. Adsorption and Desorption Characteristics of Endosulfán Pesticide in the Three Soils in Palestine; An-Najah National University: Nablus, Palestine, 2009. [Google Scholar]
- Villalobos-Maldonado, J.J.; Meza-Gordillo, R.; Mancilla-Margalli, N.A.; Ayora-Talavera, T.R.; Rodríguez-Mendiola, M.A.; Arias-Castro, C.; Vázquez-Villegas, P.T.; Ruíz-Valdiviezo, V.M. Removal of decachlorobiphenyl in vermicomposting process amended with rabbit manure and peat moss. Water Air Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- NOM-021-RECNAT-2000. Establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreos y análisis. D. Of. Fed. 2000, 14, 17. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Sante, D. Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed SANTE/11813/2017. Bruss. Belg. 2017, 46. [Google Scholar]
- Espín, S.; López, E.M.; Mojica, P.M.; Fernández, A.J.G. Development of an analytical method for the extraction of organochlorine pesticides in feather. An. Vet. Murcia 2010, 26, 77–90. [Google Scholar]
- Jurado, J.M. Aplicación de Microsoft Excel a la Química Analítica: Validación de Métodos Analíticos; Universidad de Sevilla-Departamento de Química Analítica: Seville, Spain, 2017. [Google Scholar]
- Currie, L.A. Nomenclature in an evaluation of analytical method including detection and quantification capabilities: IUPAC recommendations 1995. Anal. Chim. Acta 1999, 391, 105–126. [Google Scholar] [CrossRef]
- Masschelein-Kleiner, L. Solvents. Natl. Cent. Conserv. Restor. 2004, 1, 49–58. [Google Scholar]
- Wetzler, D.E. Solvatación en Mezclas de SOLVENTS, Estudiada por Técnicas Fotoquímicas; University of Buenos Aires: Viamonte, Argentina, 2000. [Google Scholar]
- Vázquez-Villegas, P.T. Toxicological and Physiological Responses of Eisenia fetida Exposed to Endosulfan Lactone. Ph.D. Thesis, Tecnológico Nacional de México/IT Tuxtla Gutiérrez, Chiapas, Mexico, 2019. [Google Scholar]
- Gamarra-Lezcano, C.C.; Díaz-Lezcano, M.I.; Vera-de-Ortíz, M.; Galeano, M.D.P.; Cabrera-Cardús, A.J.N. Relación carbono-nitrógeno en suelos de sistemas silvopastoriles del Chaco paraguayo. Rev. Mex. Cienc. For. 2018, 9, 4–26. [Google Scholar]



| Solid Substrate (g) | Rotary Agitation Time (min) | Acetonitrile (mL) | Particle Size (mm) |
|---|---|---|---|
| 5 | 60 | 50 | 0.2 |
| 10 | 90 | 75 | 0.5 |
| 15 | 120 | 100 | --- |
| Solid Substrate (g) | Sonicated Time (min) | Ethyl Acetate (mL) | Particle Size (mm) |
|---|---|---|---|
| 5 | 10 | 30 | 0.2 |
| 10 | 20 | ||
| 67.5 | 0.5 | ||
| 15 | 30 |
| Parameter | Solid Substrate | AS1 | AS2 |
|---|---|---|---|
| Organic matter (%) | 7.3 ± 0.34 | 7.6 ± 0.15 | 7.2 ± 0.28 |
| C/N | 14.92 | 13.51 | 14.05 |
| Insecticide | Endosulfan Lactone |
|---|---|
| Linearity | y = 16,025x R2 = 0.999 |
| Spike level (mg kg−1) | 0.15–100 |
| Recovery or efficiency (%), (n = 27) | 97 |
| DL (mg kg−1) | 0.045 |
| QL (mg kg−1) | 0.15 |
| Repeatability RSDr (%), (n = 13) | 6.68 |
| Reproducibility RSDr (%), (n = 3) | 5.15 |
| Variation coefficient (%) | 13.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Villegas, P.T.; Meza-Gordillo, R.; Luján-Hidalgo, M.C.; Cruz-Salomón, A.; Ruíz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A.; Villalobos-Maldonado, J.J.; Montes-Molina, J.A. Optimization and Validation of an Extraction Method for Endosulfan Lactone on a Solid Substrate. Processes 2021, 9, 284. https://doi.org/10.3390/pr9020284
Vázquez-Villegas PT, Meza-Gordillo R, Luján-Hidalgo MC, Cruz-Salomón A, Ruíz-Valdiviezo VM, Gutiérrez-Miceli FA, Villalobos-Maldonado JJ, Montes-Molina JA. Optimization and Validation of an Extraction Method for Endosulfan Lactone on a Solid Substrate. Processes. 2021; 9(2):284. https://doi.org/10.3390/pr9020284
Chicago/Turabian StyleVázquez-Villegas, Paola T., Rocío Meza-Gordillo, María C. Luján-Hidalgo, Abumalé Cruz-Salomón, Víctor M. Ruíz-Valdiviezo, Federico A. Gutiérrez-Miceli, Juan J. Villalobos-Maldonado, and Joaquín A. Montes-Molina. 2021. "Optimization and Validation of an Extraction Method for Endosulfan Lactone on a Solid Substrate" Processes 9, no. 2: 284. https://doi.org/10.3390/pr9020284
APA StyleVázquez-Villegas, P. T., Meza-Gordillo, R., Luján-Hidalgo, M. C., Cruz-Salomón, A., Ruíz-Valdiviezo, V. M., Gutiérrez-Miceli, F. A., Villalobos-Maldonado, J. J., & Montes-Molina, J. A. (2021). Optimization and Validation of an Extraction Method for Endosulfan Lactone on a Solid Substrate. Processes, 9(2), 284. https://doi.org/10.3390/pr9020284