1. Introduction
With the depletion of conventional oil reserves, the abundant deposits of heavy oil and natural bitumen are gaining more and more attention [
1]. A distinctive feature of heavy oil and natural bitumen is the high content of polar compounds in their composition—resin-asphaltene associates in the structure of water–oil dispersed system, which create many problems during the extraction, transportation and processing of heavy oil. The microbiological degradation in most deposits of heavy oil and natural bitumen lead to the absence of n-alkanes, which are considered to be the main component of conditioned oils, in their composition. Heavy oil and natural bitumen represent a different type of hydrocarbon resource in their composition, therefore, conventional oil development technologies are of little use. The development of innovative technologies for heavy oil and natural bitumen requires scientific knowledge of their composition and reactive capacity in various natural and technogenic processes [
2,
3].
The hydrothermal-catalytic conversion of heavy oil resources is an efficient and economical method of reducing oil viscosity [
4,
5,
6]. The most suitable catalysts for this process are the systems with strong active centers that can actively destroy C—C, C—S, and C—O bonds of resin and asphaltene molecules with the formation of additional low boiling hydrocarbons with saturated and aromatic hydrocarbons.
The oil-soluble salts of iron (Fe), cobalt (Co), and copper (Cu), used as catalysts, intensified the decrease in the content of high-molecular compounds during thermal catalytic cracking. The main mechanism for transformation of the components includes the destruction of high-molecular compounds by breaking of sulfur-containing bonds, which is confirmed by the data of elemental analysis [
7]. The use of nickel complex Ni (II) 0.5 wt.% during the hydrothermal conversion of heavy oil at 180 °C for 24 h leads to a decrease in viscosity by 75.2% rel., and a pour point decrease by 9.5 °C. Based on the results of the structural group analysis, asphaltenes and resins are partially cracked into small molecules with a regular decrease in the content of heteroatomic compounds [
8]. In [
9,
10], soluble transition metal carboxylates of nickel, iron, cobalt, and copper were used as catalysts for hydrothermal conversion of heavy oil at 300 °C, and propanol and tetralin as modifying additives. The viscosity of the products of catalytic hydrothermal treatment in the presence of carbon dioxide was significantly reduced by using a catalytic composition containing iron, cobalt and copper, along with the addition of propanol. It is associated with an increase in the number of saturated and aromatic hydrocarbons and a decrease in tar content. Mo, W, C, and Ni alloys, Cu and Fe nano oxides, Ni-chelates, supported nano-Fe particles, and Cu
2+ and Fe
3+ toluenesulfonic acid complexes also showed good catalytic activity in hydrothermal treatment of heavy oil. Their catalytic effect is associated with the content of strong active centers accelerating the destruction of C—C, C—S, C—O bonds in the structures of resins and asphaltenes [
11]. The destruction of heteroatomic bonds of high molecular weight components of heavy oil leads to an increase in the concentration of saturated hydrocarbons and lighter aromatics.
The catalytic properties of catalysts can be improved by using various precursors of transition metals, additives, nontraditional metals, new active phases. In an article [
12], NiFe
2O
4 nanocatalyst (OA-NiFe
2O
4) modified with oleic acid is proposed for Liaohe heavy oil secondary refining. Under the specified thermodynamic conditions (240 °C, 3 MPa, 24 h), 9.67% of heavy components of heavy oil are converted to lighter components, resulting in a significant viscosity decrease. Structural, spectral, and morphological characteristics show that oleic acid, deposited in a coordinated manner on the surface of NiFe
2O
4 nanoparticles, prevents aggregation of nanoparticles and leads to the refinement of the composition of heavy oil during hydrothermal catalytic conversion.
Experiments on the simulation in an autoclave of heavy oil enrichment in reservoir conditions using transition metal carboxylates (Ni, Co, Fe) as catalyst precursors were conducted by [
13]. The process was carried out at 250, 300 and 350 °C in the presence of a naphthenoaromatic hydrogen donor. It was found that the significant changes in the oil content occur at 300 and 350 °C, which is accompanied by the increase in the number of light fractions and a decrease in the content of high molecular weight hydrocarbons. The authors of [
14] proved that the morphology of Fe
3O
4 affects the characteristics of the hydrothermal-catalytic effect on heavy oil. L-Fe
3O
4 catalyst with irregular morphology has larger intercrystalline mesopores. The large mesopores break C—S bonds and reduce the ratio of resins to asphaltenes. The Fe
3O
4/heilandite catalyst can effectively reduce the viscosity of crude oil by 85%.
Li G.R. et al. [
15] developed an advanced bifunctional catalyst with a catalytic center in the presence of a hydrogen donor for the process of hydrothermal-catalytic conversion of heavy oil from the Shenli field. PdCl
2/silica gel (LEC) ligand exchange chromatography was used to separate organo-sulfur compounds in order to obtain the critical organo-sulfur compounds, including polycyclic aromatic heterocycles (PAH), polycyclic aromatic sulfur heterocycles (PASH) and polar sulfur compounds (PSC), before and after the hydrothermal catalytic conversions. The characteristics of the isolated organo-sulfur compounds show that nonconjugated C—S bonds of high molecular weight compounds were broken to reduce the viscosity, while the conjugated C—S bonds were stable under the conditions of hydrothermal-catalytic conversion of heavy oil. This work provides valuable information on the conversion of organosulfur compounds of extra-heavy oil to develop more efficient catalysts for hydrothermal processes. The scientists [
16] synthesized an interphase active cobalt complex (cobalt dodecylbenzenesulfonate) for the hydrothermal process. The catalyst was not subjected to the thermal degradation at 400 °C, which indicated its high thermal stability. Catalytic properties of the catalyst were evaluated during hydrothermal-catalytic conversion of heavy oil. The viscosity of heavy oil after hydrothermal catalytic exposure decreased by 38%. The average molecular weight, group composition, and average molecular structure of experimental products were analyzed using the elemental analysis, IR Fourier spectroscopy, ion cyclotron resonance with electrospray Fourier transform, and 1H nuclear magnetic resonance. The results showed that the catalyst had an effect on C—S, C—O bonds in asphaltenes and resins, leading to a decrease in the molecular weight and viscosity of heavy oil.
The catalytic effect of various minerals in the conversion of petroleum hydrocarbons is widely reflected in the literature [
17,
18]. The effect of reservoir rocks exhibiting high catalytic activity is mainly due to the presence of clay minerals in them, which accelerate the processes of isomerization, depolymerization, and dealkylation of hydrocarbons. The integrated effect of hydrothermal factors in the presence of solid porous sorbents: silica and two types of clay minerals—bentonite and kaolin—was studied in [
19] under laboratory conditions. In the presence of clay minerals, there was a greater degree of viscosity reduction and group composition changes in the direction of increasing the saturates (nonresinous oil) content, which indicated the catalytic effect of clays.
Carbonate rock, in contrast to clay rock, has a greater influence on the process of cracking of hydrocarbons by C—C bonds [
20,
21]. The composition changes of crude oil in the presence of carbonate rock were studied on model systems “oil-carbonate rock” [
22]. The impact of rock-forming minerals, quartz, kaolin, montmorillonite, on the composition and characteristics of the products of hydrothermal treatment was studied in [
23,
24]. Based on the results of the experiments, it might be concluded that minerals accelerated hydrothermal transformation of heavy oil from Liaohe field and led to a decrease in the viscosity and average molecular weight of the oil.
The reactions of thermal decomposition of hydrocarbons to a greater extent proceed through a radical-chain mechanism with the regular formation of low molecular weight homologues and condensation products. The presence of oxides of Zn, Cr, Cu, Mg, Ca accessible for oil in the inorganic rock matrix, accelerates the dehydrogenation reactions, while the presence of sulfides and oxides of Mo, Co, Ni contributes to the hydrogenation reactions. The presence of oxygen and TiO
2, V
2O
5, MoO
2, Cu
2O in the reaction medium catalyzes the oxidation reactions [
25]. The presence of aluminum silicate in the rock brings about heterolytic mechanism underlying such reactions as disproportionation, isomerization, and alkylation of hydrocarbons. Thus, metal compounds, present in rocks or introduced from outside, can change the mechanism of the ongoing reactions of hydrothermal transformations of heavy oil, affecting the composition of final products. The crucial factor in hydrothermal transformations of hydrocarbons in rocks is the nature of the chemisorption process occurring at the interface. As a result of this interaction, molecules of hydrocarbons of heavy oil turn into surface radicals or ions with high reaction capacity. Resins and asphaltenes, being the most polar compounds of oil, compensate surface forces of rock-forming minerals by being adsorbed on the surface of mineral particles.
The hydrothermal catalytic conversion of heavy oil is accompanied by the formation of a large amount of free radicals in the reaction mixture, which leads to the polymerization and polycondensation reactions. Water as a hydrogen donor provides additional hydrogen atoms for hydrogenation reactions and hydrodesulphurisation of hydrocarbons. To date, the most studied hydrogen donors are cyclohexane, methylcyclohexane, and tetralin [
26]. They provide active hydrogen ions in the reaction mixture, which contribute to the hydrogenation and desulfurization reactions, as well as the stabilization of intermediate compounds during catalytic hydrothermal exposure [
27].
The fundamental difference between the hydrothermal conversion of hydrocarbons in an enclosed area and in an open continuous process is further involvement of final products in reactions, till an equilibrium state is established. Thermal and hydrothermal transformations of organic matter and petroleum hydrocarbons in a water vapor medium in a closed system are considered in the scientific literature as one of the methods for modeling processes of hydrocarbon conversion during generation, migration, production by thermal steam extraction methods, as well. First experimental studies on high-temperature transformations of petroleum hydrocarbons in the presence of water vapor and mineral compounds were carried out in the early 1970s and 1980s and called aquathermolysis [
28]. Harald Thimm’s work was mainly focused on gas formation. To understand the chemistry of conversion of petroleum hydrocarbons during aquathermolysis, the most popular reaction mechanism was proposed in 1982 by Hyne J.B. [
29]:
The principal aspect of this reaction is associated with breaking of carbon-sulfur bonds, as a result of which a decrease in the oil viscosity is achieved, while the resulting hydrogen participates in hydrogenation reactions, which improves the quality of oil.
The reaction mechanisms of hydrothermal conversion of heavy oil in an enclosed area are poorly known. Therefore, the new knowledge on conversion mechanisms of heavy oil hydrocarbons in an enclosed reactor is of great relevance. Therefore, this work is devoted to identifying change patterns in the composition and rheological properties of heavy Ashalcha oil in laboratory hydrothermal catalytic conversion unit in the presence of metal oxides, their precursors, and organic compounds.
2. Experimental
2.1. Raw Materials
The Ashalcha heavy crude oil, with an aromatic content of more than 13% and resinous asphaltene substances more than 45%, is characterized by a density of 0.9751 g/cm
3. Carbonate (marble) (1, 2, 4, 6 experiments), predominantly consisting of calcite, and kaolin clay (3, 7 experiments), due to the presence of finely dispersed associations of aluminosilicate clay minerals with significant catalytic activity in rocks, were chosen as mineral compounds that make up the oil-bearing rock [
22]. In the reaction mixture, superheated steam is an effective medium for hydrothermal catalytic conversion of heavy oil since it contributes to the uniform distribution of heat throughout the reactor volume and prevents polycondensation and carbonization reactions.
The choice of inorganic compounds was due to the following reasons. Ni (nickel sulphate) and Cu (copper sulfate) salts are highly soluble in water, are strong electrolytes, and are widely used to accelerate chemical reactions (experiment 3). γ-Al2O3 shows catalytic activity in thermal decomposition reactions of hydrocarbons (experiment 1). α-Al2O3 fraction up to 0.25 µm has a developed specific surface area and sorption properties (experiment 4). Nickel (II) oxides are capable of showing an oxidative effect, poly-α-olefins are characterized by a high content of hydrogen (14% by weight), in hydrothermal conditions can serve as its source (experiment 5). The process of hydrothermal catalytic conversion of crude heavy oil with poly-α-olefins can be considered as an effective method of disposal of polymeric wastes to produce synthetic oil.
In order to improve filtration-capacity of carbonate rocks during hydrothermal treatment, the research on the effect of carboxylic acids on the composition and properties of oil and rock-forming minerals was of interest. To this end, carboxylic acids, differing in the number of carboxyl groups, were selected. C2H4O2 acid (experiment 7) was chosen as a representative of monobasic carboxylic acids. As a tribasic weak carboxylic acid, 3-hydroxy, 3-carboxy pentanedioic acid was chosen, which is able to form chelate complexes with Ca+, Mg+, Cu+, Fe+ ions and others (experiment 6). In addition, carboxylic acids can be a source of the hydrogen proton, since their dissociation produces the acetate ion CH3COO− and the proton H+.
2.2. Experimental
The introduction of inorganic compounds into the reaction mixture, pre-exposed to mechanical activation, was carried out through water. The mechanical activation was carried out on the Ultrasonic Processor 400S installation with an ultrasonic wave frequency of 22 kHz and an energy density of 5 W/cm
2. It allowed the increase of the surface area by reducing their sizes for Al
2O
3 particles to ≈5 × 10
−7 m, kaolin to ≈1 × 10
−7 m. In the first experiment, the ratio of oil to water was 2.2:1, carbonate was used as the mineral compound in the amount of 3.6% by weight to oil (
Table 1). In the second experiment, the ratio of oil to water was 2.8 to 1, carbonate was used as the mineral compound in the amount of 7% by weight to oil. In experiment 4, an equal amount of Al
2O
3 was added to the carbonate, the total amount of mineral compounds was 5% wt. to oil. In the fifth experiment, the composition of the initial mixture was similar to the first experiment, the difference being in the addition of NiO with poly-α-olefins in the amount of 16% by weight to oil. In the sixth experiment, the ratio of oil to water was 2.3:1, carbonate to carboxylic acid—1:2. In the seventh experiment kaolin was used as a mineral compound in the amount of 7% by weight to oil, and carboxylic acid C
2H
4O
2. In the third experiment, kaolin was used as a mineral compound in the amount of 3.5% by weight; nickel sulfate NiSO
4 and copper CuSO
4 were used in a 2:1 ratio to accelerate reactions.
In experiments 1, 2, 4, and 6, carbonate is involved in a coarsely dispersed state with a small specific surface, and therefore it does not accelerate the reactions of the hydrothermal transformation of heavy oil in the presence of catalytic compounds. The study of carbonate compounds before and after the hydrothermal catalytic conversion of heavy oil allows the evolution of their changes under the influence of thermodynamic conditions and the composition of final products of experiments.
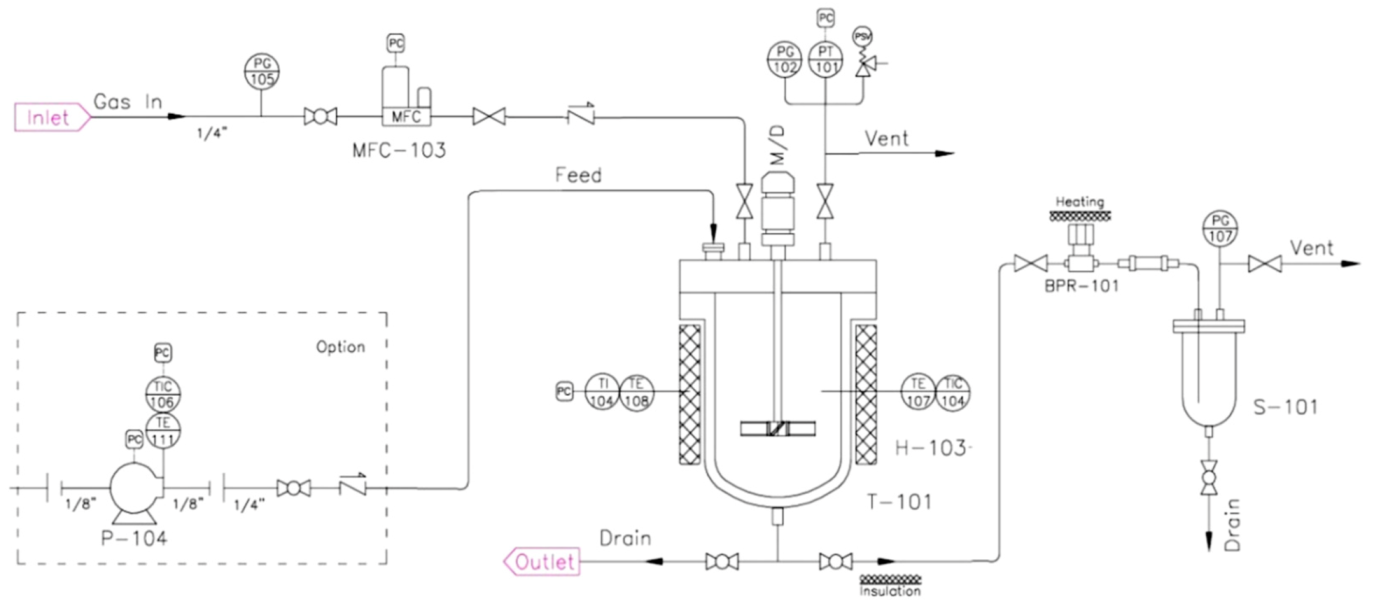
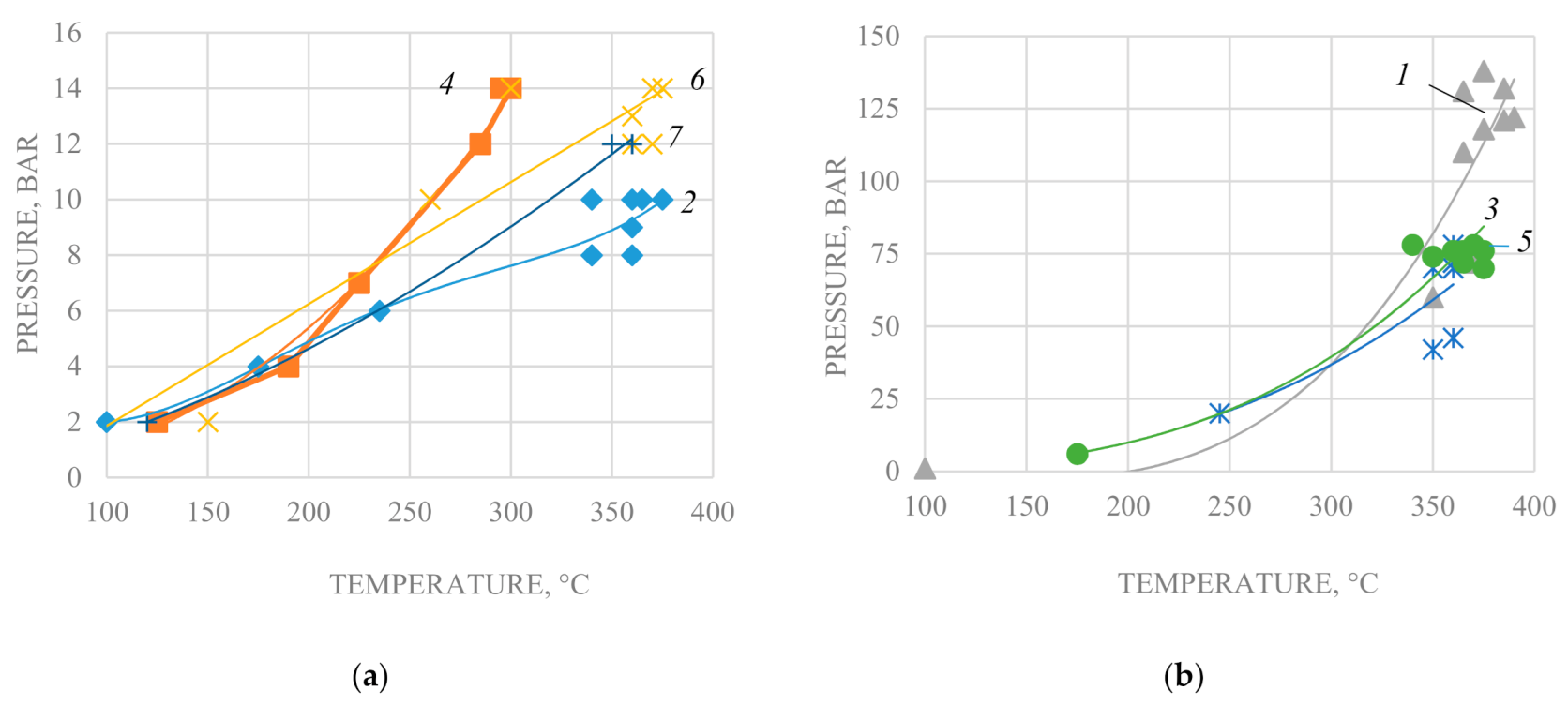
The experiments on hydrothermal catalytic conversion of heavy crude oil were carried out in a laboratory-scale plant of periodic action (
Figure 1) in the isochoric-isothermal mode at 290–375 °C and pressures ranging from 10 to 135 bar (
Figure 2), test duration being 2.5 h. Operating temperatures stabilization was carried out in a stream of nitrogen, initial pressure before heating corresponded to atmospheric. The installation is equipped with a heated reactor (T-101), electric drive, gas supply module (MFC-103), which provides measuring and controlling mass flow rates, a reservoir for collecting the final products (S-101). The initial mixture in the form of suspensions consisting of water–oil emulsions and mineral additives was loaded into an autoclave. Under the hydrothermal conditions, the water from the liquid state goes into a state of superheated steam, which ensures uniform distribution of heat throughout the reactor, slowing down polymerization and coke formation reactions by reducing partial pressure. Upon completion of experiments after cooling the reactor to the initial temperature, the presence of residual pressure of 1.5 and 2 bar was recorded, respectively, due to the presence of gaseous compounds in the final products.
During the hydrothermal transformations of hydrocarbons in a confined space, final products are involved in new reactions until an equilibrium is established, which is characterized by no pressure changes (constant pressure) in the reaction system. During the hydrothermal process, the hydrogen balance between the initial and converted oil is preserved, hydrogen deficiency of final products is not observed. The yield of gaseous products directly depends on the reactor free volume and does not exceed 1–2% wt., which leads to a low rate of reactions of formation of carbonaceous substances. With a temperature and pressure increase during the hydrothermal process, more intense dissolution of superheated steam in oil occurs, which results in uniform heating of the reaction mixture, reducing the partial pressure of hydrocarbons and, as a result, prevents the formation of carbonaceous substances. With a further increase in temperature and pressure, the water goes into a metastable state, where, in addition to the dissolving properties, it has a high concentration of hydrogen ions and hydroxide ions involved in the hydrolysis of heteroatomic oil compounds.
The water from the final liquid products of the experiments was removed by “Bottletest” method, being poured into volumetric flasks. The demulsifier was added with a microsyringe; then they were mixed in a shaking apparatus for 10–12 min and kept for 2 h at 80 °C. The particles of mineral additives from final products of experiments were extracted in Soxhlet apparatus by hydrocarbon tetrachloride.
2.3. Analysis of Products
Analysis of group composition of oil samples was carried out in accordance with the common technique (SARA-analysis). Asphaltenes were precipitated by 40-fold amount of aliphatic solvent—hexane. Maltenes were separated by liquid-adsorption chromatography on aluminum oxide. The saturated hydrocarbons were eluted with hexane as an adsorbent, aromatic compounds—with toluene, and resins were extruded from the adsorbent with the help of solvents, namely, benzene and isopropyl alcohol taken in equal proportions.
The study on the composition of paraffinonaphthenic and aromatic hydrocarbons of the crude oil and final products of hydrothermal treatment was carried out by PerkinElmer “AutoSystemXL” chromatograph with a flame ionization detector. A quartz column was used with a length of 25 m and an internal diameter of 0.2 mm, filled with the liquid phase SE-30, and the flow rate of the carrier gas was 2 mL/min. Temperature program: isothermal mode 1 min at initial temperature 60 °C, linear programming 10 °C/min to temperature 280 °C, isothermal mode 10 min. For the analysis, 5–6 mL of chloroform solution of the analyzed product were introduced through the evaporator into the column. The quantitative processing of chromatograms was carried out by internal normalization method using TotalChromNavigator program by calculating the area of each peak and their subsequent summation.
The elemental analysis of test products was carried out by burning sample plates on a CHN-3 analyzer at a temperature of 1000 °C. A weighed amount of 0.1 g was placed in special sockets—“carriages”, whence they were transported to a special glass tube filled with copper, and the sample was burned. The structural group composition of the initial oil and end products of hydrothermal treatment was determined by molecular infrared spectroscopy using a Fourier FTIR spectrophotometer “Spectrum Two FT-IR Spectrometer” from PerkinElmer with a universal diamond set-top box, having spectral range of 8300–350 cm−1.
Structural-rheological properties were studied on a Haake RheoStress 6000 rheometer. Rheological studies of oil samples were carried out using a cone-plane measuring cell in the temperature range from 10 to 80 °C. Based on measured shear stresses and shear rates, dynamic viscosity η (mPa·s) was calculated. The obtained values were used to construct rheological curves in the coordinates viscosity—shear stress. The following characteristics were determined for each temperature: structural viscosity (ηmax), viscosity of the Newtonian flow (ηmin), anomalous viscosity index characterizing the strength of structures to shear deformations and calculated as the ratio of the viscosity of the intact structure (structural viscosity) to viscosity of the destroyed structure (Newtonian viscosity) θ = ηmax/ηmin.
Scanning electron microscopy (HitachiTM-1000 scanning electron microscope) was used to study the changes occurring on the surface of the carbonate rock. Thermal analysis was performed on MOM Q-1500D derivatograph in the temperature range of 20–1000 °C with a furnace heating rate of 10 °C/min, the atmosphere in the furnace is air stationary, a platinum crucible was used in the experiments, aluminum oxide was used as an inert substance, the sample weight was 50 mg.
3. Results and Discussion
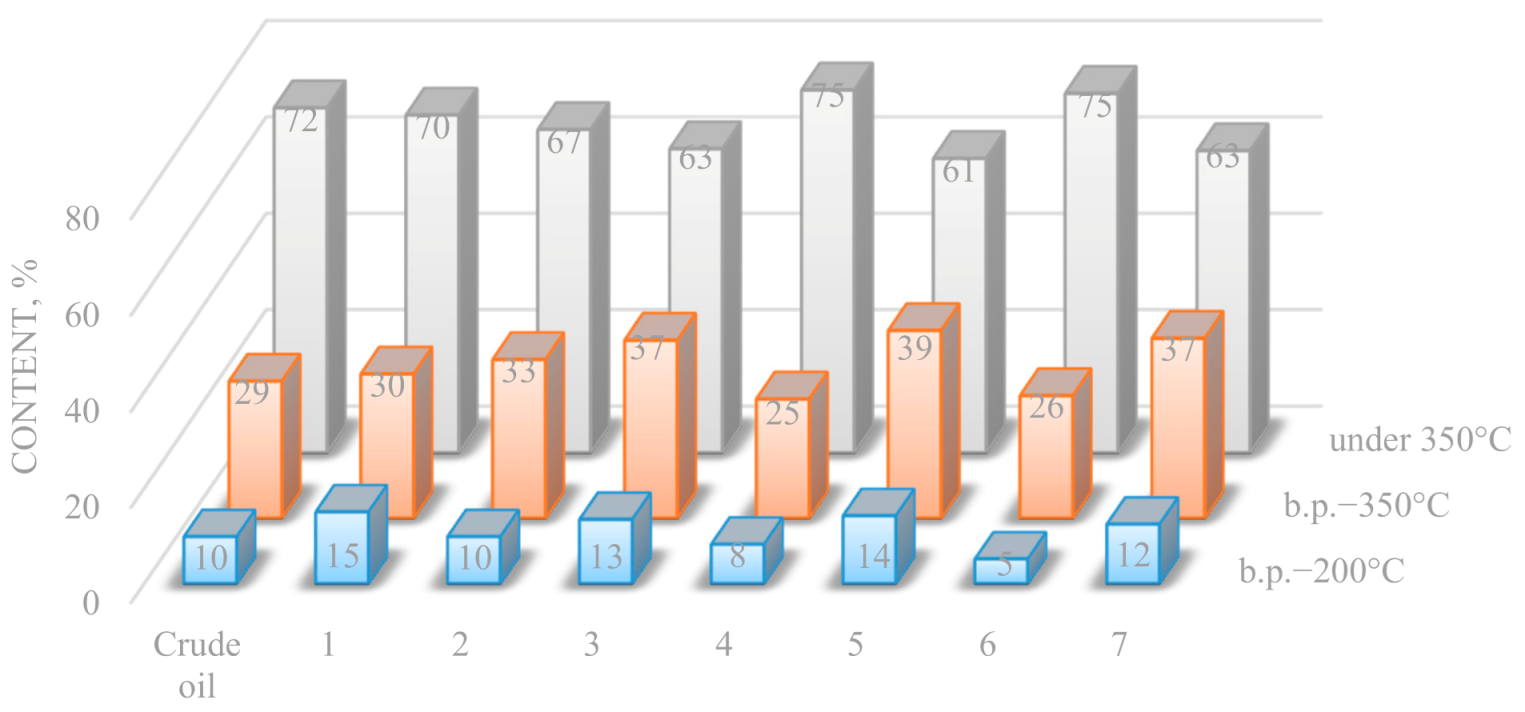
The content of hydrocarbons boiling up to 350 °C in the composition of the crude oil and end products of hydrothermal catalytic conversion is shown in
Figure 3. As a result of experiment 1, carried out in water vapor medium at 375 °C and 135 bar in the presence of carbonate and Al
2O
3, an increase in the content of low-boiling hydrocarbons i.b.p. 200 °C from 9.8% to 15% by weight is observed. When the temperature of hydrothermal catalytic conversion is reduced to 290 °C and pressure to 14 bar in experiment 4 in the presence of carbonate and Al
2O
3, the content of hydrocarbons boiling up to 200 °C in oil is reduced by 47% rel., hydrocarbons boiling up to 350 °C—13% rel. The same trend in the redistribution of low-boiling hydrocarbon fractions in oil is observed in experiment 2 at 375 °C and 10 bar in the presence of a carbonate compound in the hydrothermal environment. In the converted oil, the content of hydrocarbon compounds boiling up to 200 °C remains almost unchanged, while the yield of the fraction 200–350 °C increases by 20% rel.
The presence of a carbonate compound along with a tribasic weak carboxylic acid in heavy oil under the conditions of hydrothermal catalytic conversion in experiment 6 (at 360 °C and 14 bar) resulted in a decrease of light hydrocarbon fractions boiling up to 200 and 350 °C in the final product. On the contrary, during experiments with heavy oil in similar thermodynamic conditions of experiment 7, characterized by the presence of aluminosilicate and monobasic carboxylic acid in final products instead of the carbonate compound, the content of low-boiling hydrocarbons boiling up to 350 °C increased. In the final product of experiment 5 in the presence of Al2O3, NiO and poly-α-olefins the content of light fractions boiling up to 350 °C increases from 28.5% to 39% by weight. Such a redistribution of hydrocarbons indicates the involvement of high-boiling components in cracking reactions with further C—C bond breaking at tertiary carbon atoms with the formation of compounds concentrated in diesel fractions.
The analysis of the component composition of heavy crude oil and end products of hydrothermal catalytic conversion suggests a preferential redistribution of paraffinonaphthenic and aromatic tars with the formation of lighter hydrocarbons of lube (
Table 2). Superheated steam in the reaction mixture helps to reduce the partial pressure of the initial and final products, which prevents the formation of carbonaceous deposits. This is confirmed by the absence of carbonaceous deposits in the composition of final products of hydrothermal catalytic conversion of heavy crude oil.
The study of Al2O3 in experiment 1 at 375 °C: a slight increase in the asphaltene content from 6.5% to 6.9% may indicate the presence of polycondensation reactions that occur along with the destruction reactions of C—N, C—O, C—S, and C—C bonds of high molecular weight resin compounds.
An increase in the resin content from 35.6% to 37.6% in the final product of experiment 2 with a decrease in the proportion of asphaltenes may indicate the transformation of some asphaltene structures into resins.
The decrease in the amount of asphaltenes from 6.5% to 4.7% by weight in experiment 4 at 290 °C and 14 bar is due to their adsorption on the surface of the carbonate compound. The presence of Al2O3, NiO, poly-α-olefins in the reaction mixture of experiment 5 has an effect on thermodestruction of asphaltenes and the resin content increases from 35.6% to 38.7% accordingly.
Under the conditions of hydrothermal catalytic conversion of the experiment 3 (350 °C and 78 bar), the presence of kaolin, salts of Ni and Cu, provides the decrease in the resin content from 35.6% wt. up to 32.5% wt. and the increase in the amount of saturated and aromatic hydrocarbons by 6% rel. in the composition of heavy crude oil. Heavy Ashalcha crude oil at 360 °C and 12 bar in the presence of kaolin and monobasic carboxylic acid in experiment 7 is characterized by a reduced resin content (from 35.6% wt. up to 31.9% wt.) and an increased content of saturated and aromatic hydrocarbons (by ≈7% rel.) (
Table 2). In experiment 2 at 350 °C and 10 bar in the presence of a carbonate compound in the reaction mixture, the component composition of oil remained almost unchanged. The temperature decrease (to 290 °C, experiment 4) in the presence of Al
2O
3 results in decrease of asphaltene and increase in resin content. Similar increase in resin content also occurs in experiment 6 in the presence of tribasic carboxylic acid at 360 °C and 14 bar due to polymerization and polycondensation reactions of saturated and aromatic hydrocarbons. The analysis of the component and fractional composition of oil before and after experiments 2, 4, 6 indicates the prevalence of polycondensation reactions over reactions of thermal destruction of high molecular hydrocarbons.
The analysis of changes in the structural group and component compositions indicates the absence of a noticeable catalytic activity of a carbonate compound with a small specific surface area under thermodynamic conditions of hydrothermal catalytic conversion (290–360 °C and 10–14 bar). The situation changes when Al2O3, aluminosilicate compound, Ni and Cu salts are present in the reaction mixture. The reactions of destruction of resinous components of Ashalcha heavy crude are projected; the number and length of aliphatic substituents are reduced, naphthenic fragments are reduced, asphaltenes are reduced in size.
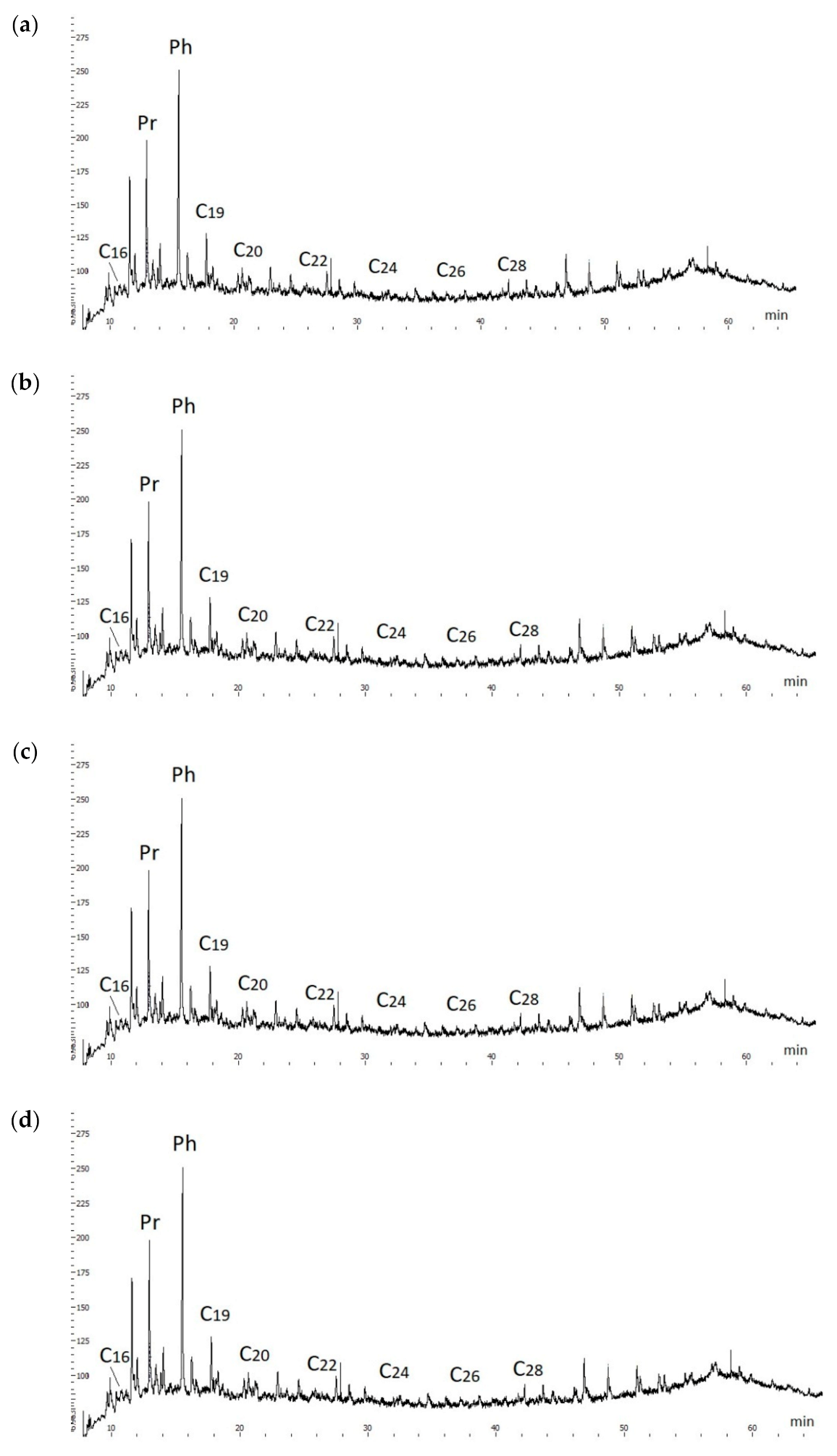
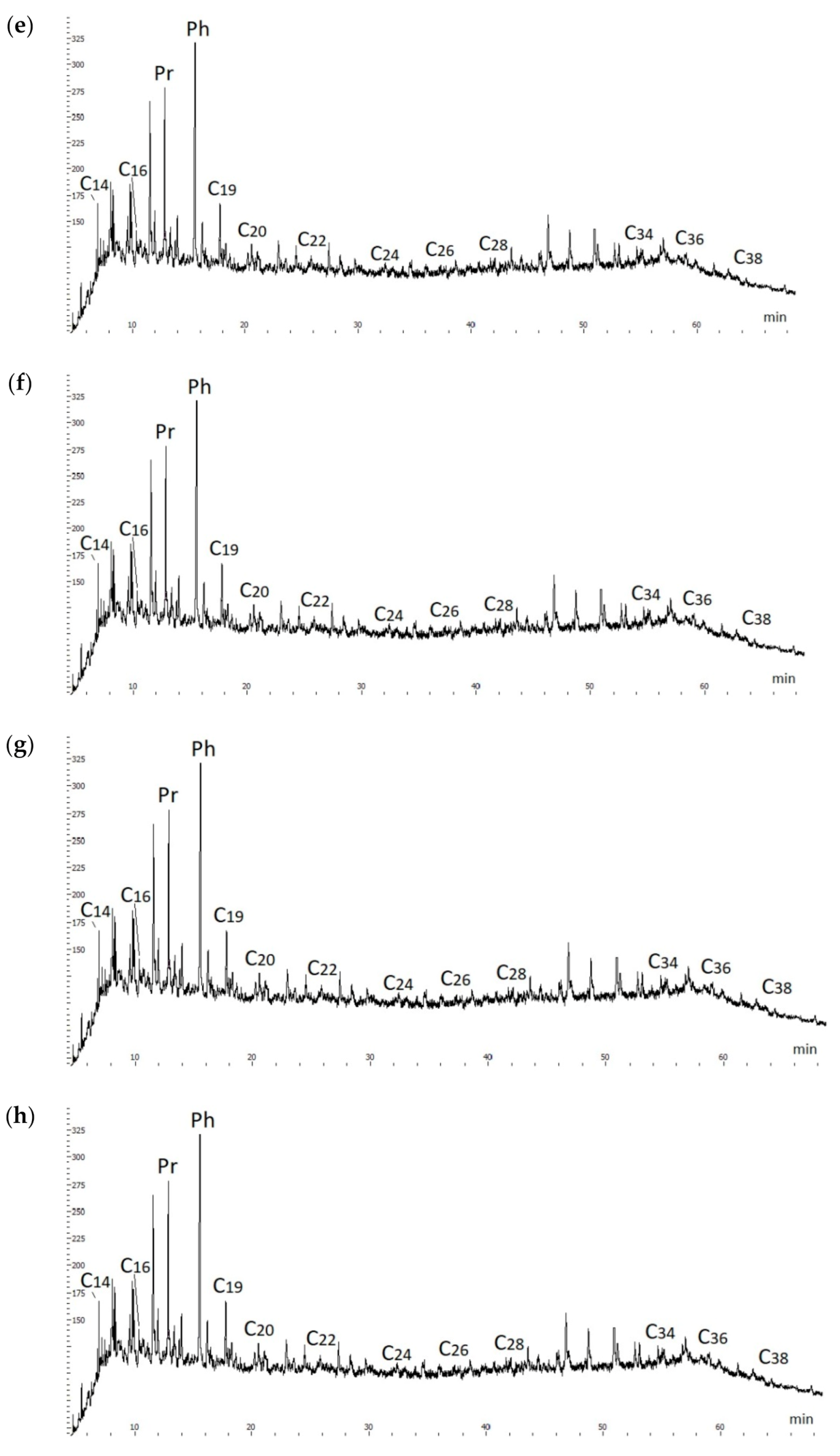
The chromatograms of the initial oil and end products of hydrothermal catalytic conversion of heavy oil demonstrate the prevalence of isoprenoid alkane peaks over the peaks of n-alkanes. The most intense peaks of isoprenoid alkanes occur on the pier and phytan, which are located next to n-alkanes C
17 and C
18 (
Figure 4). At the same time, there is no noticeable destruction of C
13–C
20 isoprenoid alkanes and formation of new low-boiling alkanes C10—C12 in chromatograms.
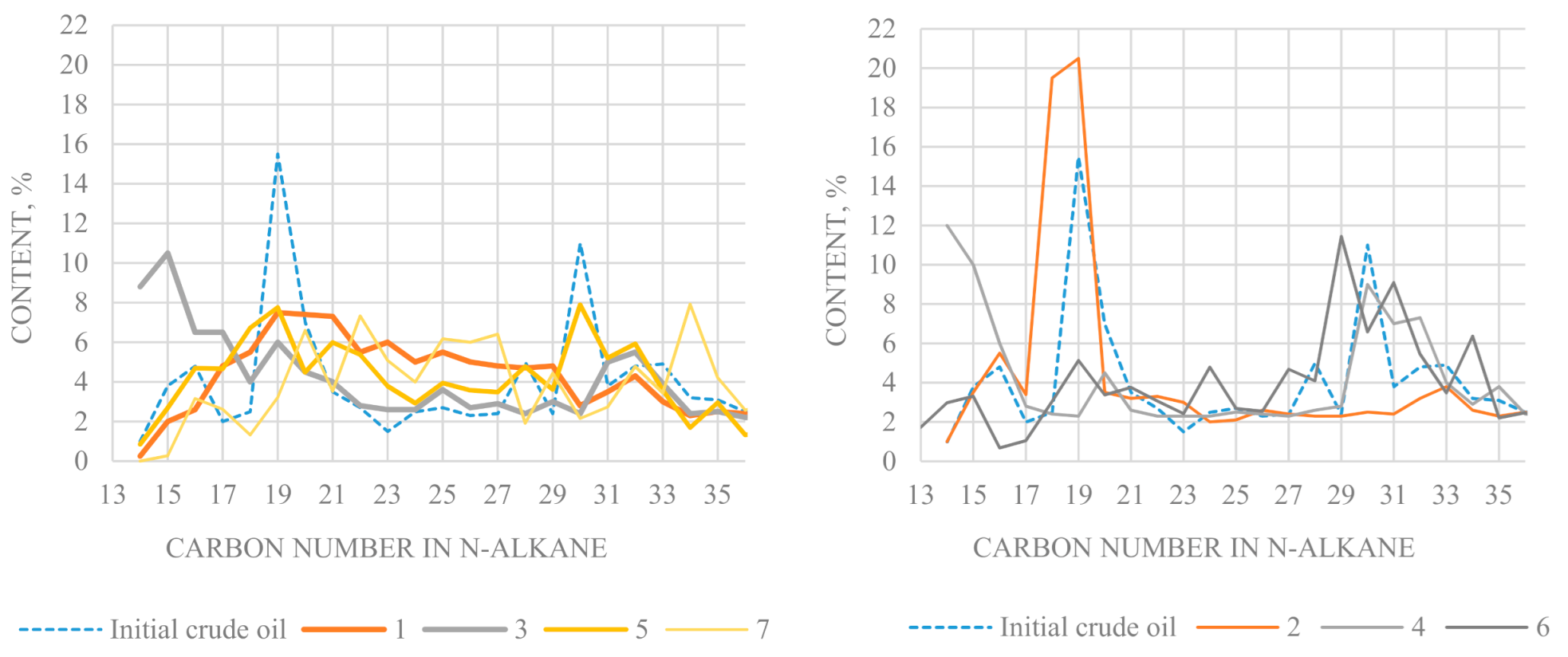
A bimodal distribution of n-alkanes per C
19 and C
29 is observed in the initial oil (
Figure 5). In general, final products of experiments 4, 5, 6, 7 differ from the original oil by a more uniform molecular mass distribution of n-alkanes, while their bimodal character is preserved. The final products of the hydrothermal treatment in the presence of Al
2O
3 particles, NiO and poly-α-olefins (at 350 °C and 78 bar) are characterized by an increased content of n-alkanes of C
27–C
29 and a reduced content of high-molecular n-alkanes of C
32–C
39. In the experiment 7, in the presence of aluminosilicate and monobasic carboxylic acid, the proportion of high molecular weight homologs with a carbon number of more than 21 increases noticeably in n-alkanes of oil.
In the converted oil of experiment 2 (350 °C and 10 bar), the content of C19 and C20 relative to C17–C18 decreases. In the converted oil of experiment 6 (in the presence of tribasic carboxylic acid and carbonate compound), the proportion of high molecular homologs with more than 22 carbon atoms is markedly increasing in n-alkanes. Compared to the initial oil, the final product of experiment 4, conducted in the presence of a carbonate compound and Al2O3, yield of the fraction boiling up to 200 °C decreases, while yield of the fraction 200–350 °C increases by 9% and the content of high-molecular n-alkanes in saturated hydrocarbons increases as well. In final products of experiments 1 and 5 (with Al2O3), n-alkanes of C30–C38 composition are almost absent.
The final products of hydrothermal catalytic conversion of heavy oil have rather close odd-odd (Odd/Even) values compared with the original oil (
Table 3 and
Table 4). The increase in the Σ(nC
27–nC
31)/Σ(nC
15–nC
19) ratio in the final products of experiments 6 and 7 reflects an increase in the share of high-molecular n-alkanes of C
27–C
31 in their composition, the reverse is observed in the final product of experiment 2, which is characterized by low values of ratio indicating a high content of low molecular weight n-alkanes C
12–C
19.
The most suitable criteria to estimate the degree of conversion of the original oil during hydrothermal experiments is isoprenoid coefficient (Pr + Ph)/(nC17 + nC18), which characterizes changes in the concentration of the basic isoprenoid alkanes Pr and Ph, boiling away in the range of 301 and 316 °C, as alkanes of the composition n-C17–n-C18. From a chemical point of view, this indicator is based on the known regularities of decay rates of isoprenoid alkanes, as a result, the numerical value of this ratio decreases with increasing the degree of oil conversion. Aside from the final product of experiment 6, a significant decrease of this parameter in the rest of converted oils, a decrease in the relative content of isoprenoid alkanes, and an increase in the share of n-alkanes.
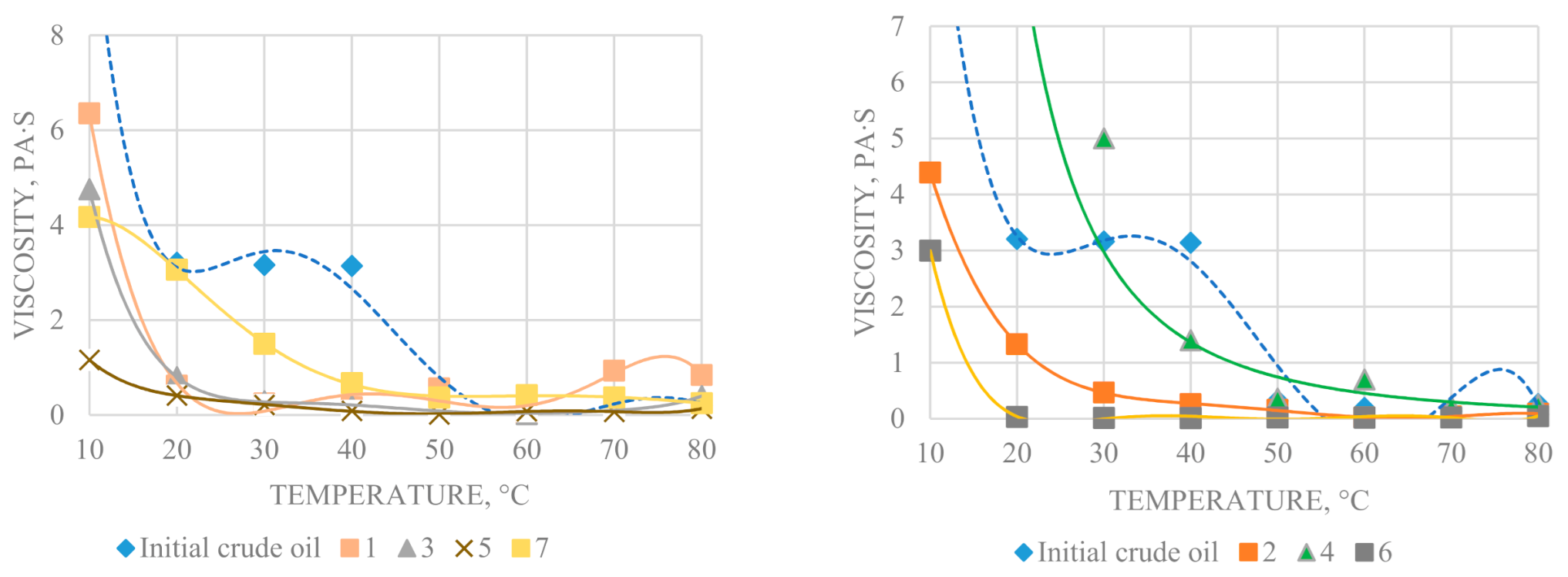
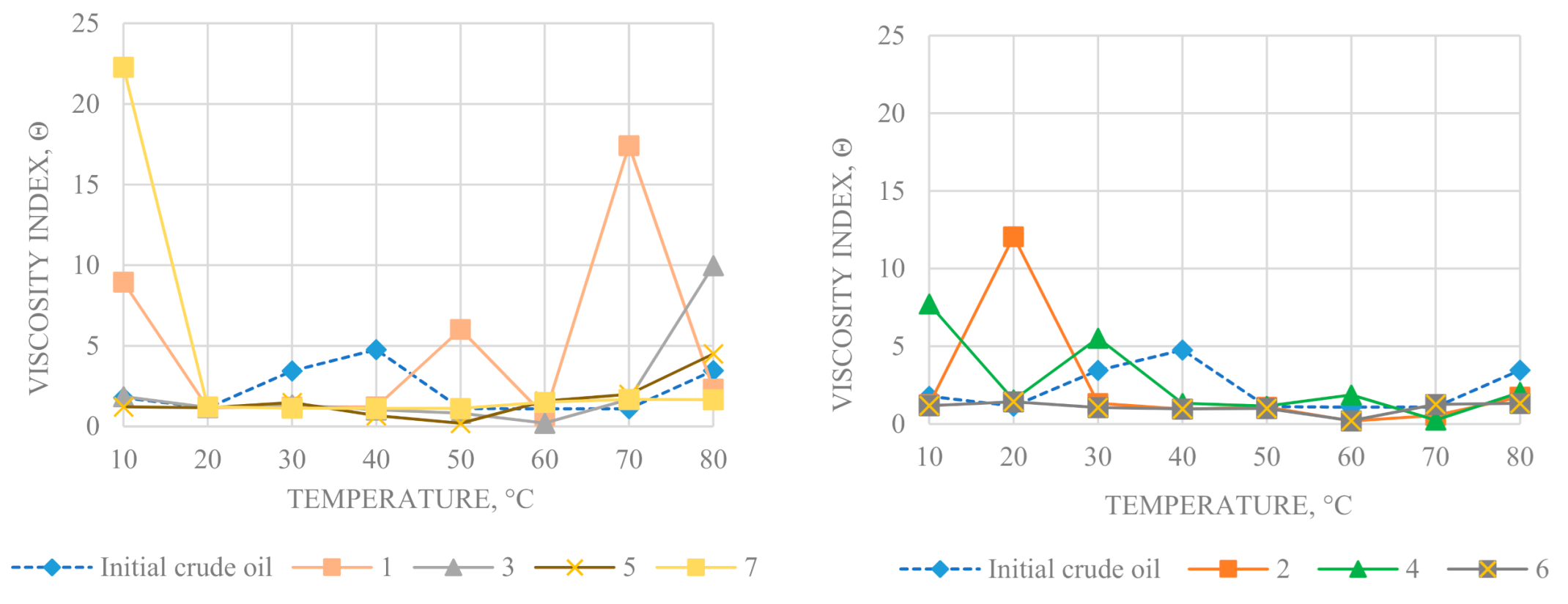
The flow curves at given temperature were used to determine structural viscosity η
max, Newtonian flow viscosity η
min, and the viscosity anomaly index θ (θ = η
max/η
min), which reflects the stability of structures to shear deformations (
Figure 6 and
Figure 7). The structural viscosity of samples is closely related to the content of resins and asphaltenes, capable of forming a coagulation type spatial structure. The structure of the oil dispersed system is formed by associates, whose strength depends on the balance of forces acting in the system. Over the entire temperature range for the converted oil, a decrease in the effective viscosity in the Newtonian flow region at a shear rate of 900 s
−1 is observed.
The following changes in oil composition affect the decrease in viscosity: an increase in the amount of low-boiling hydrocarbons, hydrocarbon oils, a decrease in heteroatomic high-molecular compounds, changes in the composition and content of resin-asphaltene compounds. The high viscosity of the original oil is due to the presence of native asphaltenes of “loose” structure, while the decrease in oil viscosity during the experiments can also be associated with the disintegration of their peripheral aliphatic structures with the formation of more compact secondary lower molecular weight asphaltenes. The presence of Al2O3 in oil in the amount of 2.3% by wt. under hydrothermal conditions (375 °C and 135 bar) leads to a decrease in viscosity from 6370 to 730 mPa at 10 °C. The final products of experiments 1, 3, 5, 6 have a lower viscosity of non-Newtonian flow in comparison with the original oil and are characterized by more gentle viscosity-temperature curves. Oil, transformed in the presence of aluminosilicate and carboxylic acid (experiment 7), has the highest viscosity, which can be attributed to the presence of kaolin in the final product. The rheological curves of final products of experiments in the temperature range from 50 to 80 °C differ as well.
The heavy oil after the experiment 4 (290 °C and 14 bar in the presence of Al2O3) is characterized by high viscosity, both structural and of Newtonian flow, high shear stress, specifically at low temperatures, which can be explained by the accumulation of resin in the final product, as a result, intermolecular interactions are enhanced with the formation of larger hydrodynamic particles compared to the original oil. The decrease in oil viscosity after experiment 2 by 55% rel. at 10 °C, may indicate the formation of oil dispersed system with a more compact, in comparison with the original oil, supramolecular structure, which creates less resistance during its movement.
The oil after hydrothermal experiment 6, conducted in the presence of tribasic carboxylic acid and carbonate compound, exhibits lower structural viscosity. Its Newtonian viscosity is lower than that of the original oil. It should be noted that converted oils differ in temperature, when viscosity index anomaly θ increases more than two times.
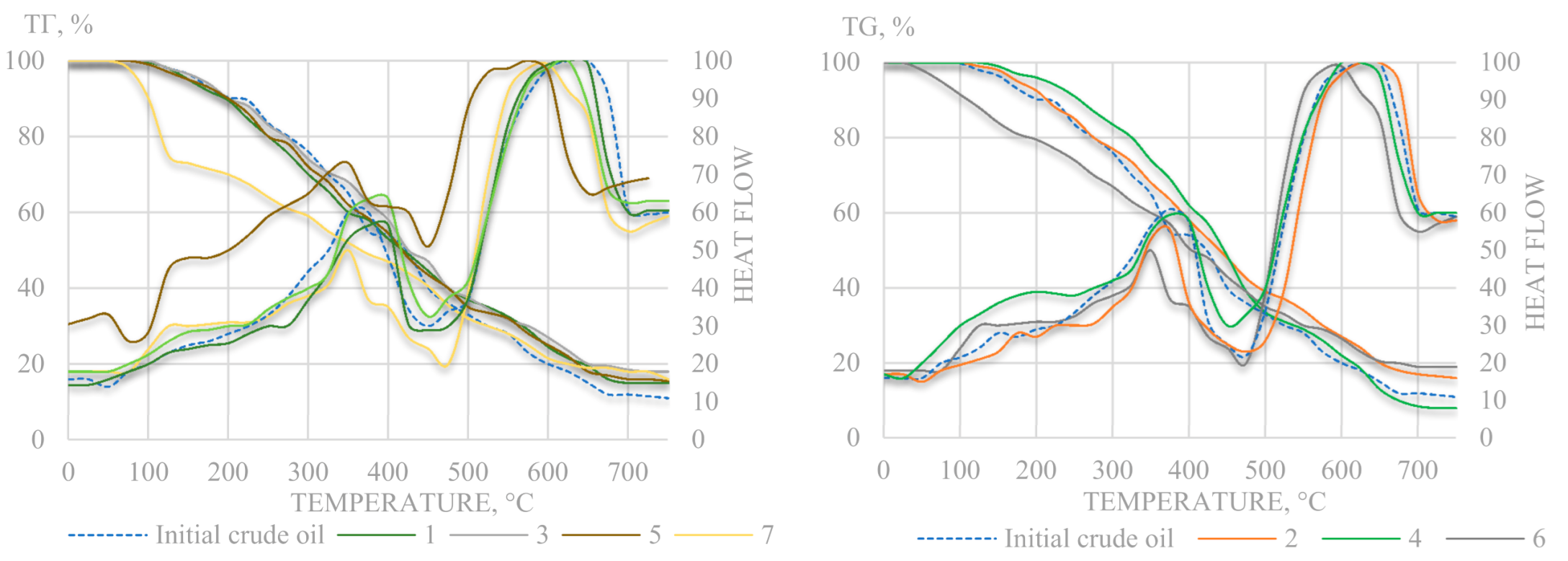
A sharp increase in θ indicates a decrease in the stability of oil to shear deformations, due to the presence of the coagulation-crystallization type spatial structure. The differences in temperatures with the maxima of viscosity anomalies are associated with changes in composition and content of saturated hydrocarbons in converted oils. The crude oil and end products of hydrothermal-catalytic experiments were studied by thermal analysis. The thermogravimetric curves and thermal analysis data are shown in
Figure 8 and
Table 5.
In terms of thermal stability, the end products of catalytic hydrothermal catalytic conversion of heavy oil range from larger to smaller in series: 4, 2, 3, 1, 5. The oil transformed in the course of experiment 4 at 290 °C and 14 bar in presence of Al2O3 has the greatest thermal stability with a higher content of oxidized structures and alkyl substituents, it is also characterized by a relatively higher density, as evidenced by the indicator F.
In contrast, the final products of experiments 1 and 5, also carried out with Al2O3, are characterized by the lowest density and low content of alkyl side or oxidized substituents. A distinctive feature in the conditions of experiments 1 and 5 from the 4th experiment is the temperature above 350 °C and pressure above 70 bar. Comparative analysis of data from thermal analysis of oil before and after experiments indicates that the original oil has the highest density (lowest F), and is the most thermally stable—it has a relatively low mass loss ∆m1, incl. relatively low losses up to 200 °C.
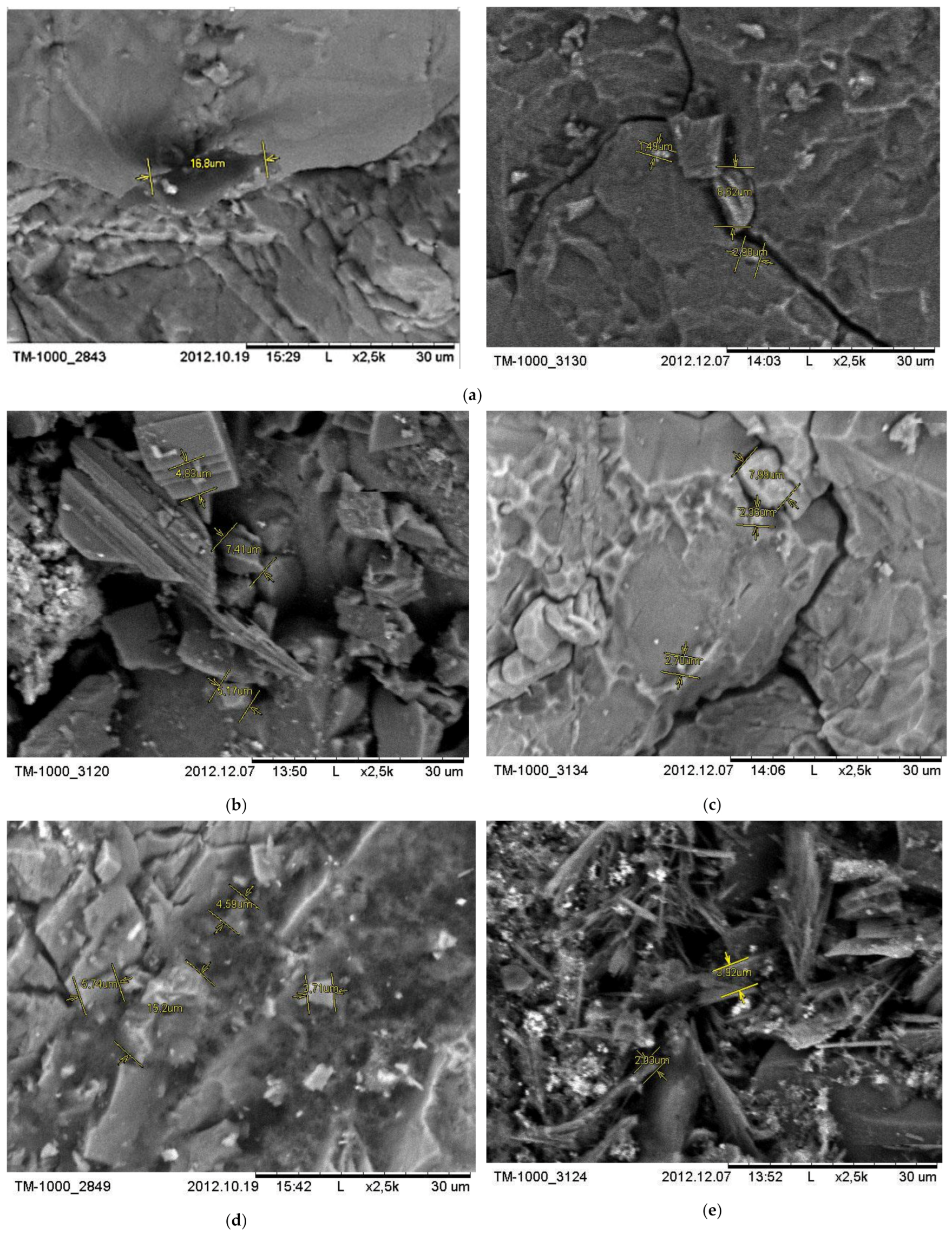
The hydrothermal catalytic conversion of heavy oil in experiments 1, 2, 4, 6 leads to the changes in the surface structure of the carbonate compound, consisting mainly of calcite and to a lesser extent dolomite (
Figure 9). Microstructural changes on the surface of the mineral compound are due to the presence of recrystallized calcite and dolomite structures.
The oil conversion during the hydrothermal catalytic conversion in the course of experiment 1 with the participation of Al
2O
3 is reflected on the morphology of the carbonate compound. The content of carbonaceous substances on the surface of the mineral compound increases, which will contribute to clogging of the communicating pores in the carbonate reservoir. The comparison of the data of the visual analysis of the carbonate surface with thermodynamic conditions of hydrothermal experiments indicates the intensity of the polycondensation reactions of petroleum hydrocarbons with the formation of carbonaceous substances clogging up the pore space of the mineral compound with increasing temperature and pressure. The presence of carboxylic acid in the hydrothermal environment (
Figure 9e) leads to the transformation of the original carbonate structure in the direction of the destruction of its pores and the formation of filtration channels that facilitate the extraction of oil. Carboxylic acids displace the weaker carbonic acid from the salts of Ca
2+ and Mg
2+ of the carbonate compound to form carbonic acid, which decomposes into water and carbon dioxide. The release of carbon oxides contributes to a more efficient recovery of oil from the reservoir.
In the hydrothermal environment of experiment 7 with kaolin, carboxylic acids are able to interact with the active metals of the mineral compound, hydrogen is evolved, and salts are formed (acetates capable of showing catalytic activity). In the course of experiment 4, the content of asphaltene substances clogging the interconnected pores, forming filtering channels promoting oil recovery, increases in carbonate.