The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications
Abstract
1. Introduction
2. Some Aspects about Microwave Heating Mechanisms during Enhanced Heavy Oil Thermal Recovery
3. Microwave Heating Impact on Hydrocarbons Structure
4. Nanoparticles Perspectives Application for Enhanced Oil Recovery by Microwave Electromagnetic Heating
5. Concluding Remarks
- The role of catalytic particles in absorbing microwave field energy;
- Studying the effect of particle dispersion on the process of microwave radiation conversion into thermal energy;
- Investigation of the electromagnetic field’s electric and magnetic components influence on the chemical transformation process of hydrocarbons;
- Developing efficient methods for injection of catalyst particles into the reservoir, such as injection through oil-soluble precursors which, in this case, allows for a significant catalyst dispersion throughout the reaction medium. Therefore, understanding the mechanism of in-situ formation of nanoparticles, as a result of oil-soluble catalyst precursor decomposition into a reservoir porous medium, under the influence of microwave heating would help us to understand and develop new innovative methods for enhancing unconventional oil recovery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Liu, X.; Oilfield, L.; Liu, Y.; Zhong, L. In Situ upgrading heavy oil by aquathermolytic treatment under steam injection conditions. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005; Society of Petroleum Engineers: Richardson, TX, USA, 2005; pp. 1–8. [Google Scholar]
- Montgomery, W.; Court, R.W.; Rees, A.C.; Sephton, M.A. High temperature reactions of water with heavy oil and bitumen: Insights into aquathermolysis chemistry during steam-assisted recovery. Fuel 2013, 113, 426–434. [Google Scholar] [CrossRef]
- Elahi, S.M.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. In-situ upgrading and enhanced recovery of heavy oil from carbonate reservoirs using nano-catalysts: Upgrading reactions analysis. Fuel 2019, 252, 262–271. [Google Scholar] [CrossRef]
- Lin, R.; Chen, K.; Miao, M.; Zhang, L.; Wang, X.; Jiang, Y.; Zhang, J.; Wang, Y.; Pan, H. Reaction Mechanism of H2S Generation during Tetrahydrothiophene Aquathermolysis Reaction. Energy Fuels 2020, 34, 2781–2789. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Korneev, D.S.; Pevneva, G.S.; Golovko, A.K. Thermal Transformations of Asphaltenes at a Temperature of 120 C. J. Sib. Fed. Univ. Chem. 2019, 12, 101–117. [Google Scholar]
- Gafurov, M.R.; Volodin, M.A.; Rodionov, A.A.; Sorokina, A.T.; Dolomatov, M.Y.; Petrov, A.V.; Vakhin, A.V.; Mamin, G.V.; Orlinskii, S.B. EPR study of spectra transformations of the intrinsic vanadyl-porphyrin complexes in heavy crude oils with temperature to probe the asphaltenes’ aggregation. J. Pet. Sci. Eng. 2018, 166, 363–368. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Vakhin, A.V.; Mukhamatdinov, I.I.; Onishchenko, Y.V.; Feoktistov, D.A. Effects of calcite and dolomite on conversion of heavy oil under subcritical condition. Pet. Sci. Technol. 2019, 37, 687–693. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Mukhamatdinov, I.I.; Feoktistov, D.A.; Vakhin, A.V. Comparative Kinetic Study on Heavy Oil Oxidation in the Presence of Nickel Tallate and Cobalt Tallate. Energy Fuels 2019, 33, 9107–9113. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Rakhmatullin, I.Z.; Sitnov, S.A.; Laikov, A.V.; Klochkov, V.V.; Vakhin, A.V. Influence of Co-based catalyst on subfractional composition of heavy oil asphaltenes during aquathermolysis. J. Pet. Sci. Eng. 2020, 186, 106721. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I.; et al. Hydrogen donating capacity of water in catalytic and non-catalytic aquathermolysis of extra-heavy oil: Deuterium tracing study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Nurgaliev, D.K.; Vakhin, A.V. Thermal study on stabilizing combustion front via bimetallic Mn@Cu tallates during heavy oil oxidation. In Proceedings of the 20th International Conference on Petroleum Phase Behavior & Fouling (PetroPhase 2019), Kanazawa, Japan, 2–6 June 2019; p. 20. [Google Scholar]
- Cao, Y.B.; Zhang, L.L.; Xia, D.H. Catalytic aquathermolysis of Shengli heavy crude oil with an amphiphilic cobalt catalyst. Pet. Sci. 2016, 13, 463–475. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic aquathermolysis of boca de jaruco heavy oil with nickel-based oil-soluble catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Khaidarova, A.R.; Zaripova, R.D.; Mukhamatdinova, R.E.; Sitnov, S.A.; Vakhin, A.V. The composition and structure of ultra-dispersed mixed oxide (Ii, iii) particles and their influence on in-situ conversion of heavy oil. Catalysts 2020, 10, 114. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Feoktistov, D.A.; Vakhin, A.V. Conversion of Heavy Oil with Different Chemical Compositions under Catalytic Aquathermolysis with an Amphiphilic Fe-Co-Cu Catalyst and Kaolin. Energy Fuels 2018, 32, 6488–6497. [Google Scholar] [CrossRef]
- Yusuf, A.; Al-Hajri, R.S.; Al-Waheibi, Y.M.; Jibril, B.Y. In-situ upgrading of Omani heavy oil with catalyst and hydrogen donor. J. Anal. Appl. Pyrolysis 2016, 121, 102–112. [Google Scholar] [CrossRef]
- Nassar, N.N.; Husein, M.M. Ultradispersed particles in heavy oil: Part I, preparation and stabilization of iron oxide/hydroxide. Fuel Process. Technol. 2010, 91, 164–168. [Google Scholar] [CrossRef]
- Leite, L.F.; Borschiver, S.; Canongia, C.; Antunes, A.M.S. Survey of microwave technology potential application in heavy crude oil upgrading. In Proceedings of the 2nd Mercosur Congress on Chemical Engineering and 4th Mercosur Congress on Process Systems Engineering, Rio de Janeiro, Brazil, 14–18 August 2005. [Google Scholar]
- Mokhlisse, A.; Chanâa, M.B.; Outzourhit, A. Pyrolysis of the Moroccan (Tarfaya) oil shales under microwave irradiation. Fuel 2000, 79, 733–742. [Google Scholar]
- Gunal, O.G.; Islam, M.R. Alteration of asphaltic crude rheology with electromagnetic and ultrasonic irradiation. J. Pet. Sci. Eng. 2000, 26, 263–272. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Awang Biak, D.R.; Zubaidi, S.L. Microwave-assisted pyrolysis of biomass waste: A mini review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Franus, M.; Panek, R.; Madej, J.; Franus, W. The properties of fly ash derived lightweight aggregates obtained using microwave radiation. Constr. Build. Mater. 2019, 227, 116677. [Google Scholar] [CrossRef]
- Ghasemi, H.; Mozaffari, S.; Mousavi, S.H.; Aghabarari, B.; Abu-Zahra, N. Decolorization of wastewater by heterogeneous Fenton reaction using MnO2-Fe3O4/CuO hybrid catalysts. J. Environ. Chem. Eng. 2021, 105091. [Google Scholar] [CrossRef]
- Ghasemi, H.; Aghabarari, B.; Alizadeh, M.; Khanlarkhani, A.; Abu-Zahra, N. High efficiency decolorization of wastewater by Fenton catalyst: Magnetic iron-copper hybrid oxides. J. Water Process Eng. 2020, 37, 101540. [Google Scholar] [CrossRef]
- Malinowski, S.; Presečki, I.; Jajčinović, I.; Brnardić, I.; Mandić, V.; Grčić, I. Intensification of Dihydroxybenzenes Degradation over Immobilized TiO2 Based Photocatalysts under Simulated Solar Light. Appl. Sci. 2020, 10, 7571. [Google Scholar] [CrossRef]
- Sajid, M.; Ayoub, M.; Yusup, S.; Abdullah, B.; Shamsuddin, R.; Bilad, R.; Chong, C.C.; Aqsha, A. Short-Chain Polyglycerol Production via Microwave-Assisted Solventless Glycerol Polymerization Process Over Lioh-Modified Aluminium Pillared Clay Catalyst: Parametric Study. Processes 2020, 8, 1093. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Y.; Sun, L.; Luo, S.; Kyzas, G.Z.; Fu, J. Residue Char Derived from Microwave-Assisted Pyrolysis of Sludge as Adsorbent for the Removal of Methylene Blue from Aqueous Solutions. Processes 2020, 8, 979. [Google Scholar] [CrossRef]
- Khelfa, A.; Rodrigues, F.A.; Koubaa, M.; Vorobiev, E. Microwave-Assisted Pyrolysis of Pine Wood Sawdust Mixed with Activated Carbon for Bio-Oil and Bio-Char Production. Processes 2020, 8, 1437. [Google Scholar] [CrossRef]
- Auwal, I.A.; Wong, K.-L.; Ling, T.C.; Ooi, B.S.; Ng, E.-P. Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition. Processes 2020, 8, 603. [Google Scholar] [CrossRef]
- Mozaffari, S.; Li, W.; Thompson, C.; Ivanov, S.; Seifert, S.; Lee, B.; Kovarik, L.; Karim, A.M. Colloidal nanoparticle size control: Experimental and kinetic modeling investigation of the ligand–metal binding role in controlling the nucleation and growth kinetics. Nanoscale 2017, 9, 13772–13785. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, G.D.; Muñoz, A.N.; Sturm, G.S.J.; Stankiewicz, A. A helicopter view of microwave application to chemical processes: Reactions, separations, and equipment concepts. Rev. Chem. Eng. 2014, 30, 233–259. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Frankevich, E.L. Chemical Generation and Reception of Radio-And Microwaves; John Wiley & Sons: Hoboken, NJ, USA, 1993; ISBN 047118859X. [Google Scholar]
- Buchachenko, A.L.; Berdinskii, V.L. Chemically induced radio-frequency emission and chemical radiophysics. Russ. Chem. Rev. 1983, 52, 1. [Google Scholar] [CrossRef]
- Berlinsky, V.; Yasina, L.L.; Buchachenko, A. Microwave magnetic isotope effect. Theory. Chem. Phys. 2005, 24, 35–41. [Google Scholar]
- Menéndez, J.A.; Juárez-Pérez, E.J.; Ruisánchez, E.; Bermúdez, J.M.; Arenillas, A. Ball lightning plasma and plasma arc formation during the microwave heating of carbons. Carbon N. Y. 2011, 49, 346–349. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Mitani, T.; Serpone, N. Control of microwave-generated hot spots. 6. Generation of hot spots in dispersed catalyst particulates and factors that affect catalyzed organic syntheses in heterogeneous media. Ind. Eng. Chem. Res. 2014, 53, 14941–14947. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave-generated hot spots. Part V. Mechanisms of hot-spot generation and aggregation of catalyst in a microwave-assisted reaction in toluene catalyzed by Pd-loaded AC particulates. Appl. Catal. A Gen. 2013, 460, 52–60. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, J.; Su, Z.; Ou, Y.; You, Z. Microwave catalytic effect: A new exact reason for microwave-driven heterogeneous gas-phase catalytic reactions. Catal. Sci. Technol. 2016, 6, 698–702. [Google Scholar] [CrossRef]
- Zhou, J.; You, Z.; Xu, W.; Su, Z.; Qiu, Y.; Gao, L.; Yin, C.; Lan, L. Microwave irradiation directly excites semiconductor catalyst to produce electric current or electron-holes pairs. Sci. Rep. 2019, 9, 1–7. [Google Scholar]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Effects of microwave dielectric heating on heterogeneous catalysis. Catal. Lett. 2003, 88, 33–38. [Google Scholar] [CrossRef]
- Wong, G.K.; Yen, T.F. An electron spin resonance probe method for the understanding of petroleum asphaltene macrostructure. J. Pet. Sci. Eng. 2000, 28, 55–64. [Google Scholar] [CrossRef]
- Liu, F.; Darjani, S.; Akhmetkhanova, N.; Maldarelli, C.; Banerjee, S.; Pauchard, V. Mixture effect on the dilatation rheology of asphaltenes-laden interfaces. Langmuir 2017, 33, 1927–1942. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, V.P. Possibilities of electron-stimulated cracking of high-molecular compounds in the oil and gas industry. In Proceedings of the 3rd International Forum on Nanotechnologies, Moscow, Russia, 1–3 November 2010. [Google Scholar]
- Maganov, R.; Sayakhov, F. High-frequency electromagnetic action for the recovery of high-viscosity heavy oils. Proc. High. Educ. Institutions. Oil Gas 2000, 45–51. [Google Scholar]
- Burkin, K.E.; Akhmedyanova, R.; Liakumovich, A. One-step method for producing isoprene from trimethylcarbinol and 1, 3, 5-trioxane on a cation-exchange resin using microwave radiation. Kazan Technol. Univ. Bull. 2013, 16, 568–572. [Google Scholar]
- Kovaleva, L.A.; Zinnatullin, R.R.; Mullayanov, A.I.; Shrubkovskii, I.I. Experimental studies of heating rheologically complex fluids with electromagnetic field. High Temp. 2016, 54, 612–614. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Konstantinov, G.I.; Chistyakov, A.V.; Arapova, O.V.; Perederii, M.A. Utilization of petroleum residues under microwave irradiation. Chem. Eng. J. 2016, 292, 315–320. [Google Scholar] [CrossRef]
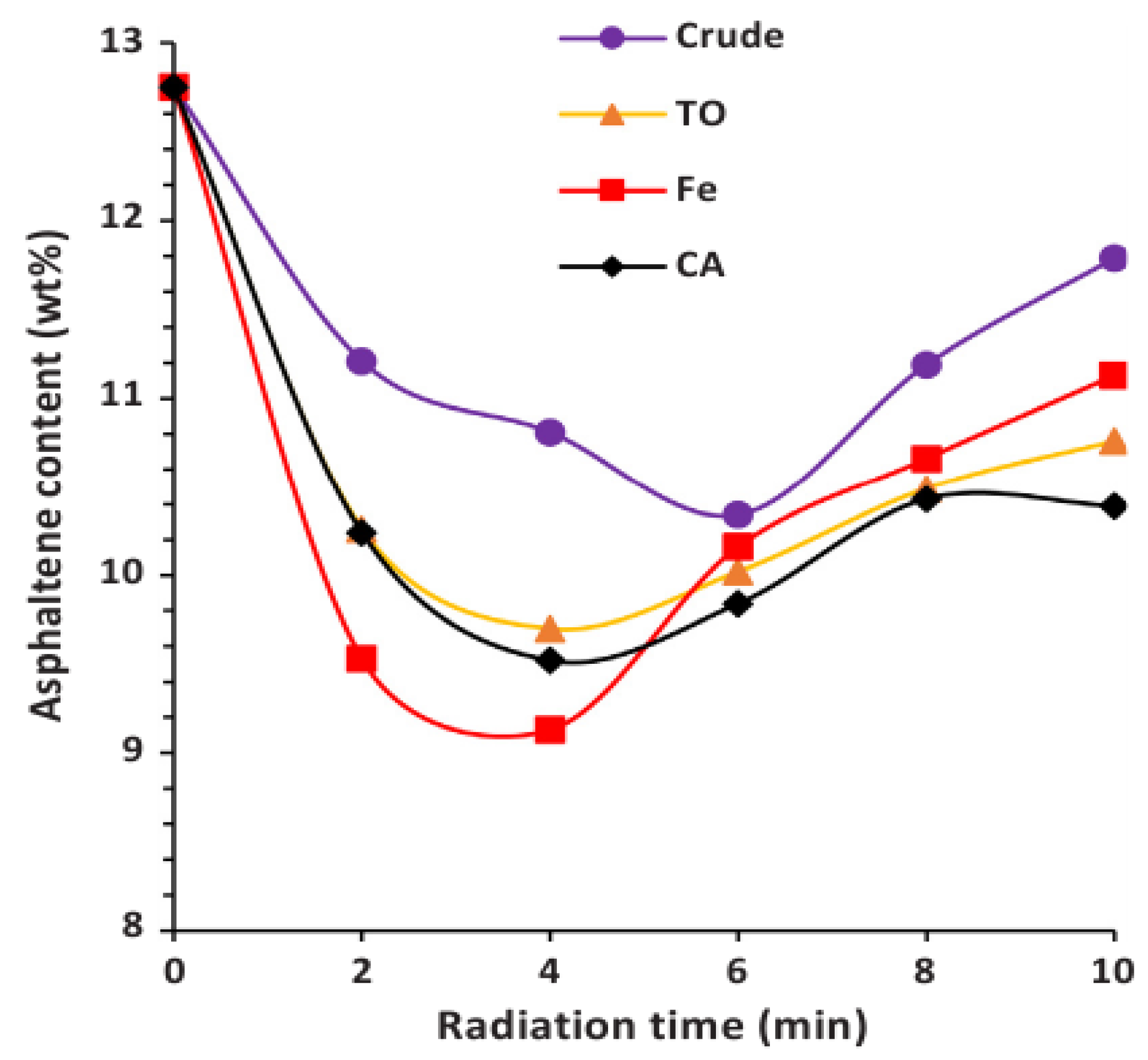
- Li, L.; Feng, H. Effect of microwave radiation time on the composition of gas produced from microwave assisted heavy oil upgrading. Pet. Sci. Technol. 2020, 38, 659–665. [Google Scholar] [CrossRef]
- Salihov, K.M. 10 Lectures about Spin Chemistry; Unipress: Kazan, Russia, 2000. [Google Scholar]
- White, O.; Iskanderov, Y.M.; Muramovich, V.; Tuev, S.; Anisimov, P. Molecular modification of liquid hydrocarbon fuels by electric fields. In Proceedings of the All-Russian Scientific and Practical Conference “Transport of Russia: Problems and prospects”, St. Petersburg, Russia, 1–2 November 2010. [Google Scholar]
- Zhang, Y.; Adam, M.; Hart, A.; Wood, J.; Rigby, S.P.; Robinson, J.P. Impact of Oil Composition on Microwave Heating Behavior of Heavy Oils. Energy Fuels 2018, 32, 1592–1599. [Google Scholar] [CrossRef]
- Adam, M.; Hart, A.; Wood, J.; Robinson, J.P.; Rigby, S.P. Microwave-assisted Catalytic Upgrading of Heavy Oil. In Proceedings of the 4th North American Symposium for Chemical Reaction Engineering (NASCRE 4), Houston, TX, USA, 10–13 March 2019. [Google Scholar]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Wan, J.K.S. Microwaves and chemistry: The catalysis of an exciting marriage. Res. Chem. Intermed. 1993, 19, 147. [Google Scholar] [CrossRef]
- Cundy, C.S. Microwave techniques in the synthesis and modification of zeolite catalysts. A review. Collect. Czechoslov. Chem. Commun. 1998, 63, 1699–1723. [Google Scholar] [CrossRef]
- Kustov, L.M.; Sinev, I.M. Microwave activation of catalysts and catalytic processes. Russ. J. Phys. Chem. A 2010, 84, 1676–1694. [Google Scholar] [CrossRef]
- Hayward, D. Apparent equilibrium shifts and hot-spot formation for catalytic reactions induced by microwave dielectric heating. Chem. Commun. 1999, 975–976. [Google Scholar]
- Cameron, K.L.; Depew, M.C.; Wan, J.K.S. Pulsed microwave catalytic decomposition of olefins. Res. Chem. Intermed. 1991, 16, 57–70. [Google Scholar] [CrossRef]
- Stuerga, D.; Gaillard, P. Microwave heating as a new way to induce localized enhancements of reaction rate. Non-isothermal and heterogeneous kinetics. Tetrahedron 1996, 52, 5505–5510. [Google Scholar] [CrossRef]
- Kim, D.-W.; Im, K.-K.; Kim, H.J.; Lee, D.H.; Kim, Y.A.; Choi, J.; Yang, K.S. Effects of electromagnetic irradiation on low-molecular-weight fraction of fluidized catalytic cracking decant oil for synthesis of pitch precursor. J. Ind. Eng. Chem. 2020, 82, 205–210. [Google Scholar] [CrossRef]
- Shang, H.; Yue, Y.; Zhang, J.; Wang, J.; Shi, Q.; Zhang, W.; Liu, L.; Omar, S. Effect of microwave irradiation on the viscosity of crude oil: A view at the molecular level. Fuel Process. Technol. 2018, 170, 44–52. [Google Scholar] [CrossRef]
- Ostapenko, A. Electroviscous effect in an alternating electric field. Tech. Phys. J. 2000, 70, 136. [Google Scholar] [CrossRef]
- Sheikh Ali, D. On the role of electrokinetic phenomena in the process of paraffin deposits during oil production. Nedra Mosc. 1965, 139–153. [Google Scholar]
- Sayakhov, F.L.; Maganov, R.U.; Kovaleva, L.A. Application of electromagnetic influence in the production of high-viscosity oils. Izv. Univ. Oil Gas 1998, 1, 35–39. [Google Scholar]
- Hot, D.; Kayukova, G.; Nigmedzyanova, L.; Kiyamova, A.; Ganeeva, Y. Influence of microwave heating on the yield and composition of natural bitumen from sandstones of the Shugurovskoye field. In Proceedings of the Materials of the International Scientific and Practical Conference, Moscow, Russia, 1–3 November 2007; pp. 178–183. [Google Scholar]
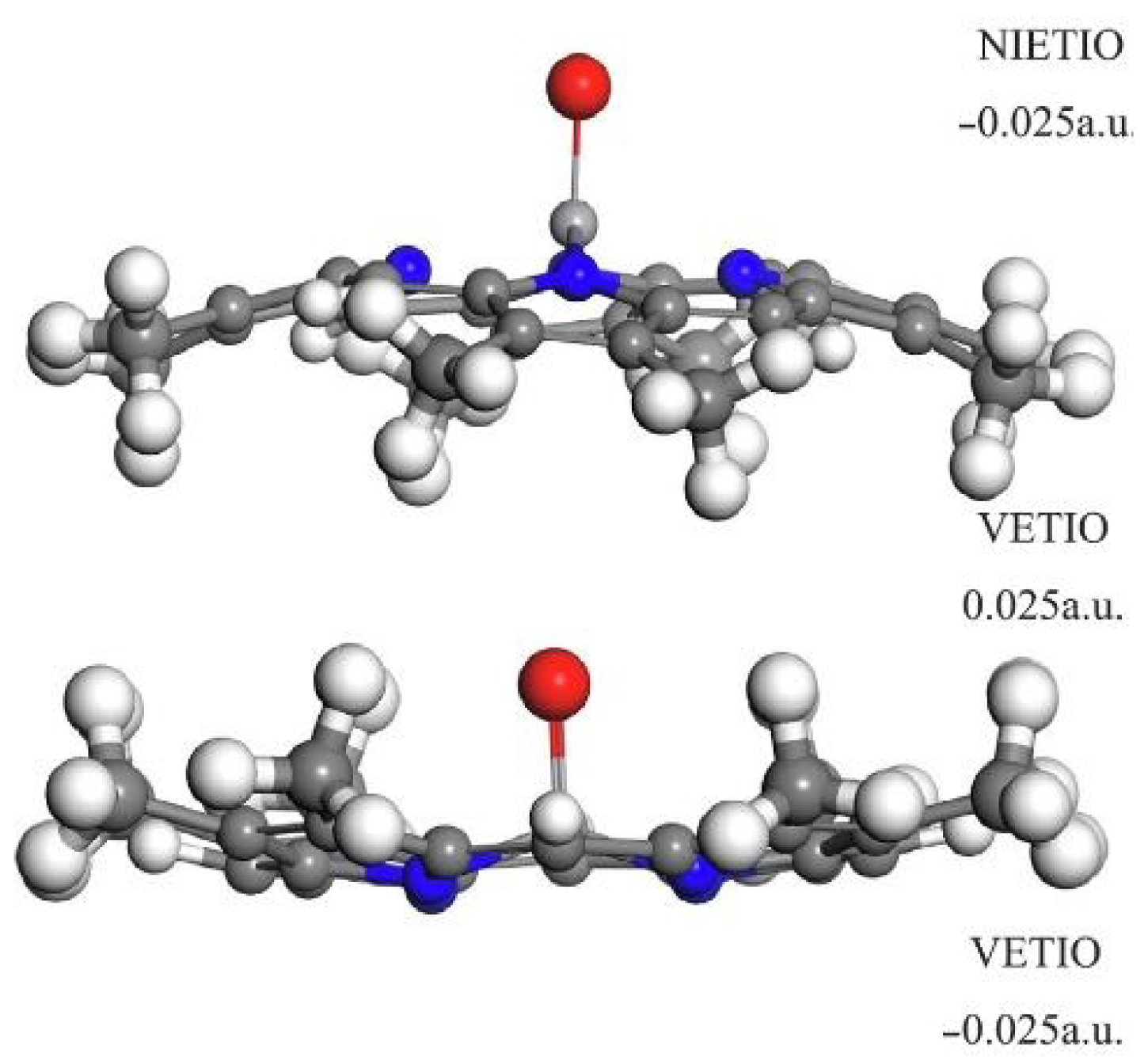
- Li, Y.; Shang, H.; Zhang, Q.; Elabyouki, M.; Zhang, W. Theoretical study of the structure and properties of Ni/V porphyrins under microwave electric field: A DFT study. Fuel 2020, 278, 118305. [Google Scholar] [CrossRef]
- Chemat, F.; Esveld, D.C.; Poux, M.; Di-Martino, J.L. The Role of Selective Heating in the Microwave Activation of Heterogeneous Catmnsis Reactions Using a Continuous Microwave Reactor. J. Microw. Power Electromagn. Energy 1998, 33, 88–94. [Google Scholar] [CrossRef]
- Arapova, O.V.; Chistyakov, A.V.; Palankoev, T.A.; Bondarenko, G.N.; Tsodikov, M.V. Microwave-Assisted Lignin Conversion to Liquid Products in the Presence of Iron and Nickel. Pet. Chem. 2020, 60, 1019–1025. [Google Scholar] [CrossRef]
- Wang, T.; Shang, H.; Zhang, Q. Adsorption behavior of thiophene on MoS2 under a microwave electric field via DFT and MD studies. Chem. Eng. Sci. 2020, 228, 115950. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, H.; Du, W.; Liu, Z. Development of microwave assisted oxidative desulfurization of petroleum oils: A review. J. Ind. Eng. Chem. 2013, 19, 1426–1432. [Google Scholar] [CrossRef]
- Hu, L.; Li, H.A.; Babadagli, T.; Ahmadloo, M. Experimental investigation of combined electromagnetic heating and solvent-assisted gravity drainage for heavy oil recovery. J. Pet. Sci. Eng. 2017, 154, 589–601. [Google Scholar] [CrossRef]
- Kovaleva, L.A.; Zinnatullin, R.R.; Valeev, M.D.; Minnigalimov, R.Z.; Fassahov, R.H. Laboratory investigations of the heating of high-viscosity oil in pipelines by a high frequency electromagnetic field (Russian). Oil Ind. J. 2019, 2019, 82–85. [Google Scholar]
- Kar, T.; Hascakir, B. The role of resins, asphaltenes, and water in water-oil emulsion breaking with microwave heating. Energy Fuels 2015, 29, 3684–3690. [Google Scholar] [CrossRef]
- Kovaleva, L.; Zinnatullin, R.; Mullayanov, A.; Blagochinov, V.; Valiev, S.M. Investigation of the integrated effect of microwave electromagnetic radiation and the field of centrifugal forces on water-oil emulsions. In Proceedings of the XI All-Russian Congress on Fundamental Problems of Theoretical and Applied Mechanics, Kazan, Russia, 20–24 August 2015; pp. 1831–1833. [Google Scholar]
- Kovaleva, L.A.; Zinnatullin, R.R.; Sultanguzhin, R.F.; Shrubkovski, I.I.; Myasnikov, A.V. Experimental studies on heating oil-saturated rocks by electromagnetic field. High Temp. 2017, 55, 837–839. [Google Scholar] [CrossRef]
- Aziz, H.; Tunio, S.Q. Enhancing oil recovery using nanoparticles—A review. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 33001. [Google Scholar] [CrossRef]
- Perry, W.L.; Cooke, D.W.; Katz, J.D.; Datye, A.K. On the possibility of a significant temperature gradient in supported metal catalysts subjected to microwave heating. Catal. Lett. 1997, 47, 1–4. [Google Scholar] [CrossRef]
- Thomas, J.R. Particle size effect in microwave-enhanced catalysis. Catal. Lett. 1997, 49, 137–141. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Chase, H.A. Catalytic microwave pyrolysis of waste engine oil using metallic pyrolysis char. Appl. Catal. B Environ. 2015, 176, 601–617. [Google Scholar] [CrossRef]
- Bregar, V.B. Advantages of ferromagnetic nanoparticle composites in microwave absorbers. IEEE Trans. Magn. 2004, 40, 1679–1684. [Google Scholar] [CrossRef]
- Neto, A.; Thomas, S.; Bond, G.; Thibault-Starzyk, F.; Ribeiro, F.; Henriques, C. The oil shale transformation in the presence of an acidic BEA zeolite under microwave irradiation. Energy Fuels 2014, 28, 2365–2377. [Google Scholar] [CrossRef]
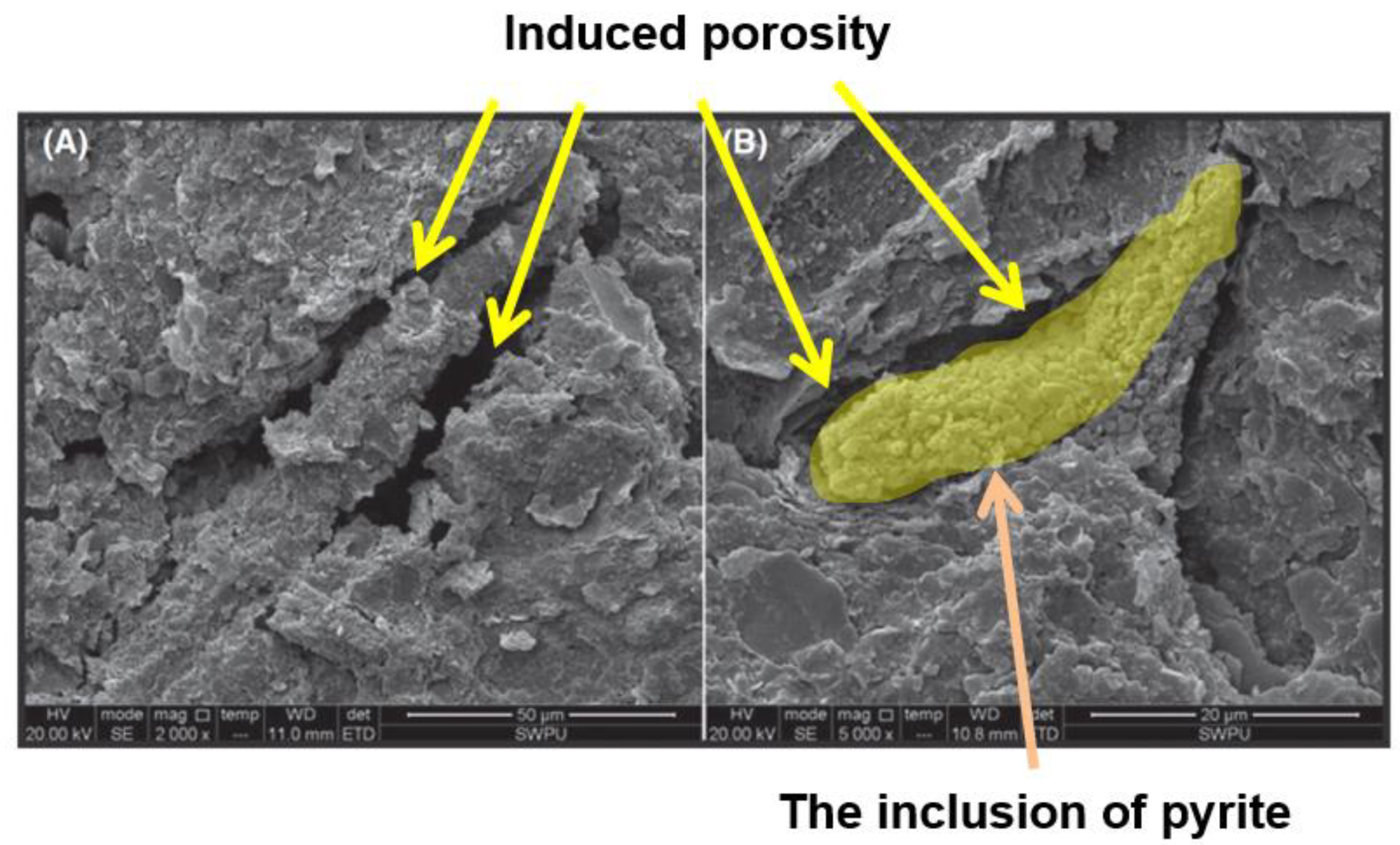
- Yang, Z.; Zhu, J.; Li, X.; Luo, D.; Qi, S.; Jia, M. Experimental investigation of the transformation of oil shale with fracturing fluids under microwave heating in the presence of nanoparticles. Energy Fuels 2017, 31, 10348–10357. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Z.; Li, X.; Qi, S.; Jia, M. Application of microwave heating with iron oxide nanoparticles in the in-situ exploitation of oil shale. Energy Sci. Eng. 2018, 6, 548–562. [Google Scholar] [CrossRef]
- Nasri, Z. Upgrading vacuum distillation residue of oil refinery using microwave irradiation. Chem. Eng. Process. Intensif. 2019, 146, 107675. [Google Scholar] [CrossRef]
- Nasri, Z.; Mozafari, M. Multivariable statistical analysis and optimization of Iranian heavy crude oil upgrading using microwave technology by response surface methodology (RSM). J. Pet. Sci. Eng. 2018, 161, 427–444. [Google Scholar] [CrossRef]
- Yan, S.J.; Zhen, L.; Xu, C.Y.; Jiang, J.T.; Shao, W.Z. Microwave absorption properties of FeNi3 submicrometre spheres and SiO2@ FeNi3 core–shell structures. J. Phys. D Appl. Phys. 2010, 43, 245003. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kwak, N.S.; Rho, N.S.; Ko, C.H.; Na, J.-G.; Yi, K.B.; Park, S. Bin Catalytic pyrolysis of Athabasca bitumen in H2 atmosphere using microwave irradiation. Chem. Eng. Res. Des. 2012, 90, 1292–1296. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, J.-T.; Guan, Z.-J.; Yan, S.-J.; Zhen, L.; Xu, C.-Y. Designing Co 7 Fe 3@ TiO 2 Core–Shell Nanospheres for Electromagnetic Wave Absorption in S and C Bands. Electron. Mater. Lett. 2020, 16, 1–11. [Google Scholar] [CrossRef]
- Depew, M.C.; Lem, S.; Wan, J.K.S. Microwve inducdd catalytic decomposition of some alberta oil sands and bitumens. Res. Chem. Intermed. 1991, 16, 213–223. [Google Scholar] [CrossRef]
- Hu, L.; Li, H.; Babadagli, T.; Xie, X.; Deng, H. Thermal stimulation of shale formations by electromagnetic heating: A clean technique for enhancing oil and gas recovery. J. Clean. Prod. 2020, 277, 123197. [Google Scholar] [CrossRef]
- Schieck, R.; Hartmann, A.; Fiechter, S.; Könenkamp, R.; Wetzel, H. Electrical properties of natural and synthetic pyrite (FeS 2) crystals. J. Mater. Res. 1990, 5, 1567–1572. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Z.; Li, X.; Qi, S.; Fang, Q.; Ding, Y. The experimental study of microwave heating on the microstructure of oil shale samples. Energy Sci. Eng. 2019, 7, 809–820. [Google Scholar] [CrossRef]
- Muradova, P.A.; Zulfugarova, S.M.; Graser, E.; Strekov, A.S.; Litvishkov, Y.N. Microwaves Induced Thermolysis of Petroleum under Contact with Heterogenous Catalysts. Chem. Ing. Tech. 2018, 90, 393–397. [Google Scholar] [CrossRef]
- Bera, A.; Babadagli, T. Effect of native and injected nano-particles on the efficiency of heavy oil recovery by radio frequency electromagnetic heating. J. Pet. Sci. Eng. 2017, 153, 244–256. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Siavashi, M. Application of SiO 2–water nanofluid to enhance oil recovery. J. Therm. Anal. Calorim. 2019, 135, 565–580. [Google Scholar] [CrossRef]
- Adil, M.; Lee, K.; Mohd Zaid, H.; Ahmad Latiff, N.R.; Alnarabiji, M.S. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR). PLoS ONE 2018, 13, e0193518. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Heavy crude oil upgrading using nanoparticles by applying electromagnetic technique. Fuel 2018, 232, 704–711. [Google Scholar] [CrossRef]
- Greff, J.H.; Babadagli, T. Catalytic effects of nano-size metal ions in breaking asphaltene molecules during thermal recovery of heavy-oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011; Society of Petroleum Engineers: Richardson, TX, USA, 2011; Volume 3, pp. 1903–1912. [Google Scholar]
- Li, K.; Hou, B.; Wang, L.; Cui, Y. Application of carbon nanocatalysts in upgrading heavy crude oil assisted with microwave heating. Nano Lett. 2014, 14, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Mahari, W.A.W.; Cheng, C.K.; Omar, R.; Chong, C.T.; Chase, H.A. Recovery of diesel-like fuel from waste palm oil by pyrolysis using a microwave heated bed of activated carbon. Energy 2016, 115, 791–799. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Gafurov, M.R.; Morozov, O.G.; Nasybullin, A.R.; Karandashov, S.A.; Ponomarev, A.A.; Krapivnitskaia, T.O.; Glyavin, M.Y.; et al. The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications. Processes 2021, 9, 420. https://doi.org/10.3390/pr9030420
Vakhin AV, Khelkhal MA, Tajik A, Gafurov MR, Morozov OG, Nasybullin AR, Karandashov SA, Ponomarev AA, Krapivnitskaia TO, Glyavin MY, et al. The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications. Processes. 2021; 9(3):420. https://doi.org/10.3390/pr9030420
Chicago/Turabian StyleVakhin, Alexey V., Mohammed Amine Khelkhal, Arash Tajik, Marat R. Gafurov, Oleg G. Morozov, Aydar R. Nasybullin, Sergey A. Karandashov, Andrey A. Ponomarev, Tatiana O. Krapivnitskaia, Mikhail Yu. Glyavin, and et al. 2021. "The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications" Processes 9, no. 3: 420. https://doi.org/10.3390/pr9030420
APA StyleVakhin, A. V., Khelkhal, M. A., Tajik, A., Gafurov, M. R., Morozov, O. G., Nasybullin, A. R., Karandashov, S. A., Ponomarev, A. A., Krapivnitskaia, T. O., Glyavin, M. Y., Slavkina, O. V., & Shchekoldin, K. A. (2021). The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications. Processes, 9(3), 420. https://doi.org/10.3390/pr9030420










