Abstract
Products of the refractory industry are key for the production of heavy industry goods such as steel and iron, cement, aluminum and glass. Corresponding industries are dependent on thermal processes to manufacture their products, which in turn would not be possible if there were no refractory materials, such as refractory bricks or refractory mixes. For the production of refractory materials, primary raw materials or semi-finished products such as corundum, bauxite or zircon are used. Industrial recycling of refractory raw materials would reduce dependencies, conserve resources and reduce global CO2 emissions. Today, only a small quantity of the refractory materials used can be recycled because raw materials (regenerates) obtained from end-of-life materials are of insufficient quality. In this study, regenerates from different refractory waste products could be obtained using the innovative processing method of electrodynamic fragmentation. It was shown that these regenerates have a high chemical purity and are therefore of high quality. It could be confirmed that the use of these regenerates in refractory materials does not affect the characteristic properties, such as refractoriness and mechanical strength. Thus, electrodynamic fragmentation is a process, which is able to provide high-quality raw materials for the refractory industry from used materials.
1. Introduction
Today, refractory materials are used in a wide variety of industrial sectors. These ceramic materials are products that are generally used under a high-temperature load of >1500 °C [1] for lining and delivery of thermotechnical facilities, such as blast furnaces or converters, as well as for transport devices in iron and steel, aluminum, cement and ceramic industries, incineration plants and refineries. The material separates the reaction zone from the outer parts of the process devices and are in contact with solid and liquid but also gaseous, partly very aggressive reaction components and reaction products [2]. Without these refractory materials there would be no technical thermal processes, which are fundamental to the production of steel, iron, aluminum, cement and glass. For example, in 2016 the German steel industry produced a total of 40.2 million t of steel products, requiring 0.5 million t of refractory materials [3]. This means approximately 10 kg of refractory materials were needed to produce 1 t of steel.
The necessary refractability as well as other important properties such as zero shrinkage, high thermal shock resistance, chemical resistance and mechanical or temperature-dependent strength are given to refractory materials by their non-metallic, inorganic composition. The main components consist of the six basic oxides SiO2, Al2O3, MgO, CaO, Cr2O3 and ZrO2, often in combination with carbon (e.g., SiC) [4]. These oxides form refractory compounds such as bauxite, corundum or white corundum, tabular alumina, zirconium (zirconium silicate), fireclay or silicon carbide through complex and high-emission thermal processes (dehydrogenation, sintering reactions or melt flow processes). These raw materials or semi-finished products are then used to produce the refractory materials. The largest raw material resources are located mainly in China, Russia, South Africa, South America and Australia. Accordingly, the German and European refractory industry is highly dependent on these imports. The refractory materials are exposed to high thermal, physical and chemical loads and serve as wear material during the use in industrial processes.
The average lifetime of refractory materials depends on the application and ranges from few days (e.g., purging plugs) to a few weeks (e.g., steel-casting ladles and converters) to long-term applications from one to several years (e.g., converters and heat-exchangers/preheaters). After the utilization phase, the refractory lining must be repaired or at least relined. The majority of the lining wears out completely and is no longer usable or is chemically contaminated such that it requires an extensive replacement. A smaller amount of the material (approximately 5–10% of the waste material) is currently recycled after stripping and used as so-called regenerates for the production of new refractory materials. For this purpose, the stripped material is pre-sorted and afterwards crushed in a jaw crusher, for example. The subsequently sieved material can partly be used as aggregate in new refractory products. However, a higher chemical purity of these regenerates required for the refractory industry cannot be achieved. For these reasons, little recycled material is used in the refractory industry today. Predominantly waste material and thus valuable secondary raw material for the refractory industry is deposited in special landfills for final disposal. A comprehensive industrial recycling process for refractory materials is a major challenge that offers the chance to reduce the dependency on primary raw material imports and the global CO2 emissions.
A suitable method for processing refractory waste material in order to enable a high rate of recycling could be electrodynamic fragmentation. This innovative method uses pulsed high voltage discharges to separate bulky multi-phase material selectively along grain boundaries. The discharge has to take place underwater to enable the solid material to be penetrated. Thus, the whole approach is a “wet process”. The potential of this pulsed power processing approach was demonstrated for the recycling of waste concrete [5] or the separation of municipal waste incineration ash [6,7]. Like refractory material, the investigated ash is a mineral-based compound material. Reusable components like iron metal, glass or ceramics are enclosed by a siliceous matrix. It was proved that after processing the ash with electrodynamic fragmentation the individual components of the ash could be recovered selectively.
The aim of the study presented here was to use the innovative process of electrodynamic fragmentation as a recycling strategy for refractory waste products in order to obtain regenerates with a high chemical purity. With these high-quality regenerates, new sources of raw materials for the refractory industry could be made accessible, and thus primary resources are conserved. The increased use of such high-purity refractory regenerates would lower the cost of refractory materials and could reduce dependencies on world markets.
2. Materials and Methods
2.1. Electrodynamic Fragmentation
Electrodynamic fragmentation is a technique using pulsed power discharges to separate compound materials selectively. The method itself was first investigated and described at the University of Tomsk in the late 1940s [8]. The efficiency of the method is the higher the more electrical discharges are generated over time. Usually, a so-called Marx generator is used to enable a high rate of high voltage discharges [9]. Besides a high voltage, a high slew rate is of special interest. The slew rate describes how fast the maximum voltage of a discharge can be achieved and thus determines the possible pulse length. With a Marx generator a slew rate that enables pulse lengths of a few nanoseconds can be realized. This is very important in order to force the electrical discharge into a solid material. Whether an electrical discharge can penetrate a material or not depends on the dielectric strength of the given material. The dielectric strength of a material is not a constant but varies in dependency with the slew rate and therefore the length of a discharge [10]. At a pulse rise time lower than 500 nanoseconds, the dielectric strength of water is higher than that of solid material (see Figure 1). Electrodynamic fragmentation utilizes this physical principle.
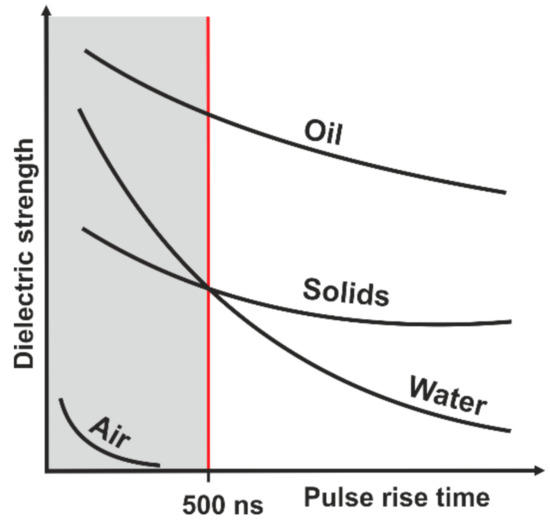
Figure 1.
Dielectric strength of different materials as a function of the pulse rise time (modified from [10]).
In the process vessel of the fragmentation unit, a solid material sample is placed underwater. The bottom of the process vessel serves as an electrode. A second electrode is placed on top of the sample. The distance between sample and upper electrode can be varied. After voltage application, a polarization of the sample takes place. This leads to electrostriction, meaning micro stress within the sample as the charge carrier (e.g., electrons) of the solid material are not freely movable [11]. As different components in a compound material, such as refractory waste material, differ in their dielectric strength, the grain boundaries represent the regions with the biggest contrast in polarization. The grain boundaries therefore are the path of least resistance for the discharge. Along the grain boundaries, so-called streamers infiltrate the material during a first stage of the pulse discharging. When the first streamer reaches the electrode at the bottom of the process vessel, the complete energy of the discharge runs along the corresponding path. A short-lived plasma channel is generated reaching temperatures of up to 104 K. During the collapse of the plasma channel, a shockwave of up to 10 GPa shatters the material having the strongest influence for the separation. The shockwave is reflected at the process vessel and penetrates the material a second time. As the described procedure occurs with each discharge, a fast and efficient separation of a solid material is achieved (Figure 2).
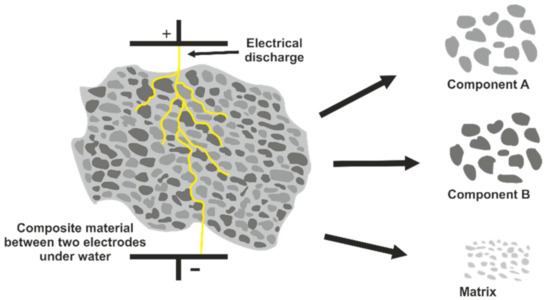
Figure 2.
Principle of electrodynamic fragmentation of a composite material.
In this study a laboratory plant was used, which works in batch mode with a five liter process vessel [12]. The general setup of the lab plant allows a processing of samples with a diameter of around 40 mm. The maximum volume of sample material for a single fragmentation step depends on the density of the material and is about several hundred grams of material generally. The default operating parameters for the lab plant are a pulse rate of 5 Hz and a voltage of 180 kV per pulse, though these parameters are adjusted to achieve an optimum in separation for each sample processed.
2.2. Sample Material
In this study, different refractory waste or break-out material was investigated and chosen from different industrial fields of application that were as varied as possible. It was also important that the selected materials were exposed to different temperatures during the manufacturing process or during use. In refractory materials, higher temperatures lead to a stronger sintering of the individual components, which makes it considerably more difficult to process or cleanly separate the individual components with conventional processing methods.
For this study, three different refractory waste materials were collected (Figure 3), from different fields of application and containing potential regenerates, such as bauxite or zircon. A brief description of the sample material with details of the potential regenerates is given in Table 1. All materials used in this study originate from shaped bricks.
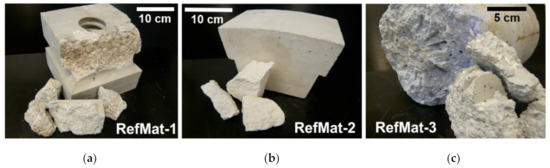
Figure 3.
Refractory waste materials from various applications. (a) Highly sintered brick for sintering or rotary kilns; (b) corundum stone for melting tanks; (c) functional refractory ceramic from the steel industry.

Table 1.
Brief description of the sample material used.
The sample material RefMat-1 (Figure 3a) consists of preformed bricks, which are fired at high temperatures (1250 °C) before being used in a sintering furnace. Only after the firing is the stone used for the kiln lining. These stones are mainly composed of bauxite, zirconia-alumina fused grain (ZAC), SiC and microsilica as well as a matrix of alumina-cement-based binder. Of this refractory material, there is interest above all in recovering bauxite and ZAC as regenerates.
The sample material RefMat-2 (Figure 3b) is a pure corundum stone, which is used at very high temperatures and has a high chemical load. The main components are white corundum and alumina-cement-based binder. These highly sintered bricks are used as lining for melting furnaces, e.g., for aluminum melts. The recovery of the white corundum as regenerate would considerably save primary resources.
The sample material RefMat-3 (Figure 3c) consists of functional refractory ceramics from the steel industry. These functional refractory ceramics have fine channels and are used in the furnace linings or at the bottom of the blast furnaces and ladle linings of steelworks to inject various gases into the molten steel via the channels. The main components of these functional refractory ceramics are tabular alumina and white corundum in a binder matrix of alumina cement. Potential regenerates with this sample material would be tabular alumina and the white corundum.
2.3. Fragmentation of the Sample Material
In order to obtain coarse sample fragments, prior to the fragmentation experiments, the three different blocks were crushed down using a sledgehammer. The resulting size of sample material was about 4 to 5 cm each. At the beginning of the fragmentation experiments (Figure 4), several small samples of each material were processed, whereby the parameters voltage, electrode-sample gap, number of pulses and pulse frequency were varied. The goal was to identify the optimal fragmentation parameters for each sample material in order to subsequently process a larger amount of sample material with the optimized parameters.
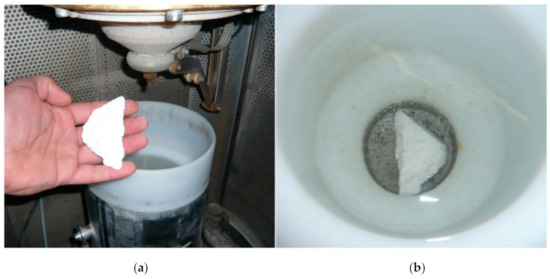
Figure 4.
Fragmentation of the RefMat-2 sample. Placing the sample in the process vessel (a) and underwater sample in the process vessel (b).
After the optimal fragmentation parameters for the individual sample materials had been determined in the preliminary tests, larger quantities of the individual materials could be processed in the next step in order to obtain sufficient material or regenerates for the subsequent recycling tests. Finally, between 20 and 32 kg of each of the three samples was fragmented in batches. After fragmentation, the separated material was dried and classified into different grain size fractions by means of sieve classification. In addition, the process water from the individual tests was filtered and the filter residue dried so that a fine fraction could be obtained.
In the next step, the fragmented and sieve-classified material was sorted in order to keep the separated aggregates, or regenerates, sorted by type. Therefore, the fragmented material was sorted by optical sorting, meaning by differences in color and translucency. The sorting was carried out with a laboratory system for optical bulk material sorting (TableSort), which was developed at the Fraunhofer IOSB [13,14]. Equipped with a RGB filter camera and a filigree blow-out device, this system is suitable for small amounts of material. The electro-optical sorting was carried out in several steps or passes for each individual grain size fraction. In each pass, the optical filter was adjusted in such a way that the desired material, meaning the regenerate in question, was blown out from the bulk mass flow.
2.4. Methods of Investigation
To characterize the collected material, all samples were analyzed before the fragmentation process by X-ray phase analysis (XRD) to determine the mineralogical phase composition and by X-ray fluorescence analysis (XRF) to investigate the chemical composition. For the XRD analysis, a D2 Phaser (Bruker) was employed, and for the XRF analysis, an Epsilon 3 XL (Panalytical) was used. The XRD analysis was performed using powder samples. For the XRF analysis, powder compacts were used. Before sample preparation, all materials were crushed and milled down. For the analysis before the fragmentation experiments, the fragments obtained from crushing the refractory blocks with a sledgehammer were milled in a two-step process. Firstly, a vibratory disc mill was used to achieve a grain size below 1 mm. Secondly, the material was milled down using a McCrone micronizing mill. With this wet-milling process a powder fineness was achieved suitable for XRD analysis. After the material was fragmented and sorted, the obtained regenerates were examined by optical microscopy to evaluate the degree of the achieved detachment. To determine the chemical purity of the recovered material, the regenerates were analyzed by XRD and XRF. Subsequently, the analytical results were compared with the analysis of primary aggregates (e.g., ZAC or tabular alumina).
The sample material obtained from the fragmentation experiments was milled down in a two-step process as well. The aforementioned McCrone mill was used after the material was pretreated in a ball mill to obtain the particle size required in the wet-milling process.
Furthermore, the recovered material was used to produce the refractory test specimen (prisms with a dimension 4 × 4 × 16 cm) in accordance with industrial requirements. The cold compressive strength as well as the cold bending tensile strength of all prisms was determined using a Z100 testing machine from ZwickRoell and an Alpha 3-3000 S testing machine from Form + Test, respectively.
3. Results
3.1. Characterization of the Refractory Waste Material
As expected, all samples show a very high Al2O3 content, which is mostly distributed between the mineralogical phases α-alumina (corundum) and β-alumina. Therefore, all samples are high alumina refractories.
In addition to the high Al2O3 content, the sample material RefMat-1 shows high contents of SiO2 and ZrO2 (Table 2). This chemism is reflected in the mineralogical phases corundum (Al2O3), baddeleyite (ZrO2) and mullite (3Al2O3-2SiO2). Minor phases are potassic and alkali feldspars, SiC and grossite (CaAl4O7) and some zirconium (ZrSiO4). The two mineral phases corundum and baddeleyite are components of the zirconia-alumina fused grain (ZAC), which is one of the main components of these refractory materials. Furthermore, the two highly refractory mineral phases corundum and mullite are components of calcined bauxite, which is also used as an important raw material in many shaped and unshaped refractory products.

Table 2.
Chemical and mineralogical composition of the sample material RefMat-1.
The sample material RefMat-2 is an almost pure Al2O3 product (Table 3). Besides the very high Al2O3 content, only very low contents of Fe2O3 and CaO could be detected. The Al2O3 is mainly found in the mineral phase corundum or in white corundum. White corundum, a chemically pure alumina, is extracted from the melt and is used both in unshaped and shaped refractory products. Figure 5 shows an example of the X-ray diffractogram of sample RefMat-2.

Table 3.
Chemical and mineralogical composition of the sample material RefMat-2.
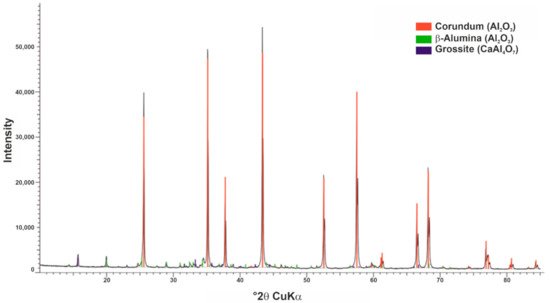
Figure 5.
X-ray diffractogram of sample RefMat-2.
Sample material RefMat-3 has a slightly lower Al2O3 content than sample RefMat-2. In addition, contents of MgO and traces of SiO2, Fe2O3, CaO and Cr2O3 were analyzed (Table 4). The two mineral phases α-alumina (corundum) and spinel (MgAl2O4) could be detected by phase analysis. The α-alumina can be attributed to the tabular alumina contained in the sample, but also to the white corundum. The two phases can hardly be distinguished by X-ray diffraction. Tabular alumina is chemically equivalent to the white corundum described above. The only difference is that white corundum is obtained from the melt and tabular alumina is recrystallized or sintered α-alumina with a high density, the morphology of which consists of large (50–200 µm), tabular corundum crystals. The two mineral phases catoite (Ca3Al2(OH)12) and grossite (CaAl4O7) are residual components of the alumina-cement-based binder.

Table 4.
Chemical and mineralogical composition of the sample material Ref-Mat-3.
3.2. Fragmentation of the Refractory Waste Material
Based on the fragmentation pre-tests, the individual parameters could be determined for each sample material, allowing a larger amount of material to be processed in the next step. The resulting material was then dried and subsequently screened into four grain size fractions (<3, 2–3, 1–2 and >1 mm; see Figure 6). In addition, a fine fraction was obtained from the process water by filtration.

Figure 6.
Sample material RefMat-3 before (central) and after fragmentation and subsequent classification by grain size.
Table 5 shows the mass balance of the individual grain size fractions. It can be seen from this table that the proportion of the fine fraction (filter residue) from the process water for sample MatRef-1 and sample MatRef-3 is almost 45 wt.% and for sample MatRef-2 even 60 wt.%. This high proportion is due to the relatively high number of pulses per material input (Table 6). In order to separate the individual components from the binder matrix, a high number of pulses with a high voltage had to be applied. This resulted in a lot of fine material. After drying, it was also observed that the fine material from the process water partially solidified. One reason for this is that a large amount of binder accumulates in the filter residue, and the binder also has a residual hydraulic activity.

Table 5.
Weight percentages of the individual fractions after fragmentation.

Table 6.
Process data.
The various aggregates of the fragmented refractory ceramics, which are to be recycled as regenerates, have mostly accumulated in the coarser fractions (1 to >3 mm). In addition to the very cleanly exposed aggregates, binder residues can also be detected in the coarser fractions.
3.3. Sorting of Fragmented Material
In the next step, the fragmented and screened materials were sorted according to optical criteria in order to obtain the exposed regenerates in the mono-fraction. Due to the significant color differences, the desired regenerates of the sample material RefMat-1 (Figure 7), the zirconia-alumina fused aggregate (ZAC) and the bauxite could be separated from the remaining material of the sample with high accuracy for all grain size fractions. The optical sorting was performed in several runs. In the first run, the yellow or flesh colored ZAC was sorted out with as little residual material as possible. Afterwards, the greyish brown to black colored bauxite was separated from the residual material. The remaining residual material consists mainly of coarse grains of light grey colored binder matrix and aggregates with residual material on its surface (ZAC and bauxite). The largest proportion of regenerates could be obtained from the fraction >3 mm. A total of approximately 5.3 kg (26 wt.%) of ZAC and approximately 2.7 kg (13 wt.%) of bauxite were exposed from 20 kg of the refractory material (RefMat-1). However, the results also showed that there was still a high proportion of residual material in the 2–3 mm fraction.
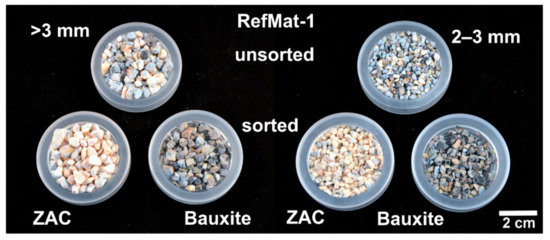
Figure 7.
Sample RefMat-1 before and after optical sorting.
In case of the sample material RefMat-2, it was possible to separate the desired regenerate white corundum (glassy transparent grains) for each grain size fraction in just one pass (Figure 8). However, the output in all three fractions was very low, and the proportion of residual material, i.e., white colored binder matrix and not cleanly exposed white corundum, was relatively high. In all three fractions, only approximately 3.3 kg (13.8 wt.%) could be separated cleanly from the binder matrix, whereby approximately 23.6 kg of sample material (RefMat-2) was fragmented. The largest proportion of white corundum could be found in the 1–2 mm fraction.
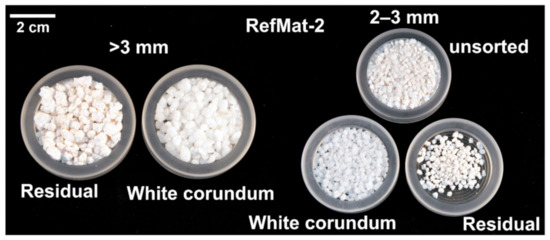
Figure 8.
Sample RefMat-2 before and after optical sorting.
From the RefMat-3 sample, two different regenerates (tabular alumina and white corundum) needed to be sorted out, as for the RefMat-1 sample. However, it was discovered that due to the optical properties, the glassy transparent white corundum could hardly be distinguished from the grayish colored binder grains by the laboratory sorting system and could therefore not be sorted out. The optical filters could not be adjusted accordingly on the laboratory equipment, so a small amount of white corundum from the 2–3 mm fraction was sorted out by hand. Nevertheless, it was possible to sort out the white colored tabular alumina from all fractions from the sample material RefMat-3. This regenerate could be easily distinguished from the remaining material by optical sorting and could be sorted out for all three fractions (Figure 9). In addition to the non-sortable white corundum, the remaining material consists mainly of binder matrix and not cleanly exposed tabular alumina. With this sample material, the yield of regenerates is not very high. From the total fragmented sample material (RefMat-3) of approximately 31.5 kg, only approximately 4.8 kg (15.3 wt.%) of regenerates distributed over three grain size fractions could be obtained.
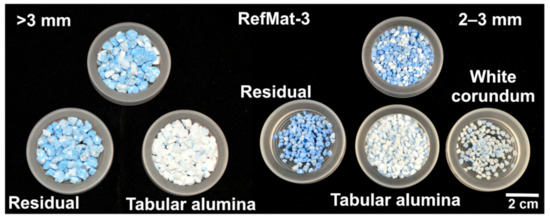
Figure 9.
Sample RefMat-3 before and after optical sorting.
It is obvious that the desired regenerates from the three different samples could be cleanly separated out of each sample material. However, it can also be seen that the yield of regenerates is not very high. Regenerates can still be found in the remaining materials that have been sorted out, but these have not been sufficiently liberated from the binder matrix by the fragmentation.
3.4. Characterization of the Fragmented Material
On the basis of the microscopic examinations, the extent of binder matrix adhesions to the regenerates obtained was investigated. In the recovered material from the sample material RefMat-1, namely the sorted out ZAC (Figure 10a) and bauxite (Figure 10b), binder adhesion could be detected on rare occasions. Generally, the individual grains were very effectively separated from the binder matrix using electrodynamic fragmentation.

Figure 10.
Micrographs of the fragmented sample RefMat-1, sorted out ZAC (a) and sorted out bauxite (b).
The sorted out white corundum regenerates, which were obtained from the sample material RefMat-2, also show hardly any binder residues on the grain surfaces (Figure 11a). Only sporadic white binder residues are still visible (Figure 11b). The degree of detachment for these regenerates is thus evaluated as very high in purely optical terms.

Figure 11.
Micrographs of the fragmented sample RefMat-2. Sorted white corundum (a), single grain of white corundum (b).
The microscopic images of the fragmented and unsorted RefMat-3 sample (Figure 12a) clearly show why the white corundum could not be clearly detected by optical sorting. Due to the different blue coloration of the binder, the glassy transparent white corundum cannot be clearly distinguished optically. On the other hand, the exposed white colored tabular alumina can be recognized and can therefore be sorted out without difficulty by optical sorting. The sorted out tabular alumina (Figure 12b) shows only slight binder adhesion.

Figure 12.
Micrographs of the fragmented sample RefMat-3. Unsorted material (a) and sorted out tabular alumina (b).
For a better evaluation of the chemical purity of the recovered regenerates, they were investigated in terms of their chemistry and mineralogical phase composition. Table 7 shows the results of the XRF measurement of the ZAC regenerates and bauxite regenerates from the sample material RefMat-1. For comparison, primary materials (ZAC and bauxite) were also analyzed and then cross-checked with the results of the recovered materials.

Table 7.
Comparison of the chemical composition (XRF) of ZAC regenerates and bauxite regenerates with primary material (b.d.l.—below determination limit).
It becomes clear that the chemical composition of the regenerates does not differ greatly from the primary material. There are no significant differences between the regenerate and the primary bauxite, so the chemical purity of the obtained bauxite regenerate can be confirmed. The recovered ZAC has a slightly higher Al2O3 content and slightly lower ZrO2 and SiO2 contents compared to the primary material. As these are the three main elements in ZAC, and no unusually high contents of CaO, Na2O or Fe2O3 or other impurities were measured, the deviating element contents are due to the inhomogeneity of the material. The material can therefore be regarded as a regenerate with a high chemical purity.
Additionally, the mineralogical phase composition does not show high impurities (Table 8). Only the grossite (CaAl4O7) detected in both regenerates, which is a relic of the hydrated alumina cement, indicates that there is a small amount of binder residue.

Table 8.
Mineralogical phase composition of the regenerates ZAC and bauxite without X-ray amorphous content.
The determined chemical composition of both regenerates, white corundum from sample RefMat-2 and tabular alumina from sample RefMat-3, show no impurities (Table 9). The chemical analysis of the tabular alumina obtained could be compared to an analysis of primary material. This confirms the very high chemical purity of the tabular alumina regenerates. The phase composition (Table 10) also shows no major impurities. As with the recovered material from the RefMat-1 sample, minimal contents of grossite and spinel are detectable, which indicate binder residues.

Table 9.
Chemical composition of regenerates white corundum from sample RefMat-2 and tabular alumina from sample RefMat-3 compared to a primary sample of tabular alumina (AlfaTab 30) (b.d.l.—below determination limit).

Table 10.
Mineralogical phase composition of the regenerates white corundum (RefMat-2) and tabular alumina (RefMat-3).
In summary, based on these results, it can be seen that the regenerates obtained by fragmentation have a high chemical purity, which is a basic prerequisite for a high-quality recycling.
3.5. Reuse of the Regenerates
Only after it was proved by analysis that the regenerates obtained possess a high chemical purity were the regenerates used in new refractory ceramics. The aim was to evaluate whether the recovered materials can substitute for the primary raw materials without disadvantages in terms of rheological, mechanical and refractory properties. A refractory concrete (mixture RC) and a refractory tamped concrete (mixture TC) were selected for the recycling tests, in which the primary raw materials were substituted by the regenerates obtained in this study. In the refractory concrete, the regenerates ZAC and bauxite were used, while in the refractory tamped concrete, the tabular alumina was used. In parallel, both mixtures were produced using primary raw materials, acting as reference samples for later performance comparisons. During the production of the different mixtures (RC and TC), it was found that the rheology, and thus the workability of the fresh mixtures, did not change and was absolutely comparable to the reference mixtures. The important factor here was to reach a comparable flow behavior and thus a comparable workability without changing the water demand. Regenerates of lower quality might require more water in order to achieve satisfactory workability. A higher water demand is known to have negative effects on the strength development of refractory concretes as well as tamped concretes. This is also known from concrete technology [15].
Several test specimen prisms were produced with the mixed concretes. All prisms were stripped after 24 h, stored for another 24 h at room temperature and then dried for another 24 h in a drying oven at 120 °C. It was found that the setting and drying behavior of the regenerate masses and reference masses is close to identical. After drying all prisms, some of the samples were burned at different temperatures in a high temperature furnace. This high-temperature treatment (sintering) can be used to check the refractoriness of the samples produced. The strength development by sintering at different temperatures is also an important characteristic value for refractory materials. The specimens of mixture RC were sintered at 1000 and 1450 °C, and the specimens of mixture TC were sintered at 1000 and 1500 °C.
As a result of this sintering, no negative changes were found in the regenerate samples. Thus, it could be proved that using the regenerates provided by the fragmentation results in a comparable refractoriness compared to the refractory material made of primary raw materials.
For a final evaluation of the quality and usability of the regenerates obtained, a test of cold compressive strength and cold bending tensile strength was carried out. By previously treating the samples at different temperatures, three different strength characteristics could be determined for each mixture: the test specimens that were dried in the drying oven at 120 °C only (mixture RC and TC) and the test specimens that were sintered at 1000 °C (mixture RC and TC) as well as at 1450 °C (mixture RC) and 1500 °C (mixture TC).
The test results for the samples of mixture RC are shown in Figure 13. The direct comparison of the achieved strengths of the regenerate samples and the reference samples shows that there are no significant differences in strength. The achieved strengths of the regenerate samples are at least as high as the strengths of the reference samples. Some of the regenerate samples even show slightly higher strengths.
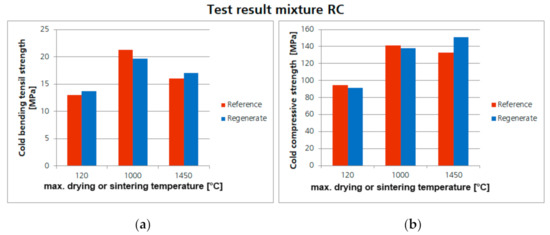
Figure 13.
Comparison of the achieved cold bending tensile strength (a) and cold compressive strengths (b) of the refractory concrete (mixture RC) with regenerates (ZAC and bauxite) and the corresponding reference samples.
After testing the cold bending tensile strength, the fracture surfaces of the individual test specimens could also be checked (Figure 14). It is clear that the microstructure of the refractory concrete with regenerates is completely comparable to the microstructure of the refractory concrete with primary raw materials. Thus it is shown that the regenerates used have no negative influence on the quality of the refractory concrete and can therefore substitute for primary raw materials equally.
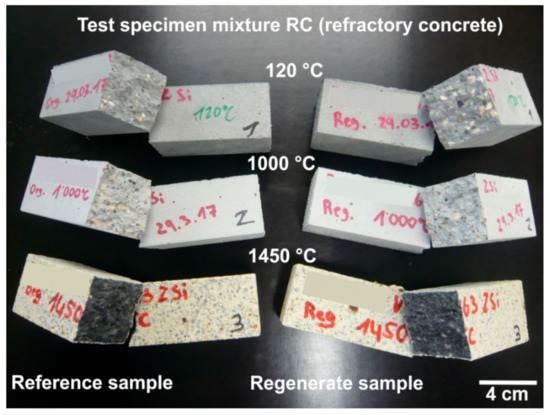
Figure 14.
Test specimen of mixture RC after the cold bending tensile strength test.
A similar result was found for the samples of mixture TC with the tabular alumina. The determined strengths of the regenerate samples and the reference samples are absolutely comparable (Figure 15). The strengths achieved in the regenerated sample are even slightly higher than in the reference samples. When examining the fracture surfaces of the test specimens (Figure 16), no differences can be found. Thus, the suitability of the recovered tabular alumina used could also be proven on the basis of these results.
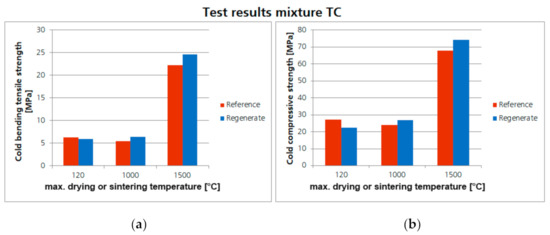
Figure 15.
Comparison of the achieved cold bending tensile strength (a) and cold compressive strength (b) of the refractory concrete (mixture TC) with regenerates (ZAC and bauxite) and the corresponding reference samples.
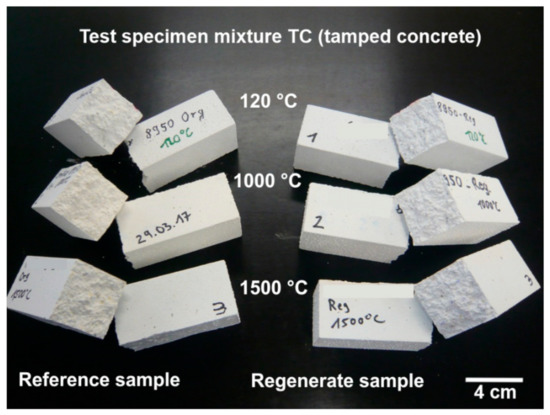
Figure 16.
Test specimen of mixture TC after the cold bending tensile strength test.
4. Discussion
The described results of this study prove that the technology of electrodynamic fragmentation is suitable for breaking down the composite material refractory into its individual components. While a conventional jaw crusher can only crush composite materials to a small size but still maintain the bond, electrodynamic fragmentation allows the valuable aggregates or regenerates respectively to be separated from the composite or binder matrix selectively. The advantage of the technology used is primarily that the electrical impulses run along the grain boundaries of the material, and the composite is torn from the inside to the outside by an expanding plasma channel. Another advantage of the fragmentation technology is that it can be considered as a dust-free and, above all, contamination-free process. Metallic abrasion, as is the case with jaw crushers, cannot take place.
After fragmentation, the various regenerates could be sorted by optical sorting, which is a necessary requirement for a high-quality reuse. Only the white corundum from sample material RefMat-3 could not be sorted out because the color differences compared to the matrix material are too small. In order to optically separate these materials with slight color differences, more powerful sorting approaches must be developed and used.
It could also be shown that the separated and sorted regenerates, namely tabular alumina, bauxite, ZAC and white corundum, have a very clean surface and are almost completely free of binder residues and other adhesions. By means of chemical–mineralogical analyses, a very high chemical purity of the recovered regenerates could be achieved. A comparison of the chemical composition with original raw materials clearly showed that there are almost no differences observable.
Finally, the recovered regenerates could be reused in refractory concrete or refractory tamped concrete without sophisticated post-treatment, thus replacing primary raw materials without affecting the properties of the refractory products. The processing properties of the fresh masses as well as the mechanical test results of the sintered samples using regenerates do not show any adverse effects, as is usually the case when using conventionally crushed material as aggregate material. However, no testing of the newly developed refractory material under working conditions (e.g., lining in an aluminum melting furnace) has been taken place so far. Thus, the material must be checked for further aspects such as corrosion resistance, thermal shock resistance and abrasion resistance.
The technology of electrodynamic fragmentation can be a promising alternative to existing recycling technologies. It is possible to recover high-quality secondary raw materials for the refractory industry and thus realize a high recycling rate for the material itself. However, further research and development work is needed to make a recycling process for refractory materials economically realizable on an industrial scale. This is especially true for the fragmentation process. For this purpose, the plant must be designed in such a way that a continuous throughput of material for an industrial viable throughput is possible.
5. Patents
Based on this study, a patent was applied for with the title “Method for recycling ceramics, regenerated materials obtained thereby, and use of the regenerated materials for manufacturing ceramics” (DE 10 2017 217 611).
Author Contributions
Conceptualization, S.S., S.D. and J.B.; methodology, S.S. and S.D.; validation, S.S., S.D. and J.B.; investigation, S.S. and S.D.; resources, J.B.; data curation, S.S. and S.D.; writing—original draft preparation, S.S.; writing—review and editing, S.D. and J.B.; visualization, S.S.; supervision, S.S.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to restrictions e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing patent process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DIN 51060:2000-06, Refractory Ceramic Raw Materials and Refractories—Definitions of the Terms Refractory, High Refractory; Beuth Verlag GmbH: Berlin, Germany, 2000.
- Schulle, W. Feuerfeste Werkstoffe: Feuerfestkeramik; Eigenschaften, Prüftechnische Beurteilung, Werkstofftypen, 1st ed.; Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1990; ISBN 3342003065. [Google Scholar]
- Wirtschaftsvereinigung Stahl. Fakten zur Stahlindustrie in Deutschland 2017; Wirtschaftsvereinigung Stahl: Düsseldorf, Germany, 2017. [Google Scholar]
- Taschenbuch Feuerfeste Werkstoffe, 3rd ed.; Routschka, G., Ed.; Vulkan-Verl.: Essen, Germany, 2001; ISBN 9783802731501. [Google Scholar]
- Thome, V. Cementing Emissions: Recycling waste concrete with lightning bolts. AWE Int. 2013, 2013, 18–25. [Google Scholar]
- Dittrich, S.; Thome, V.; Seifert, S.; Maier, M. Effektive Aufbereitung von Müllverbrennungsschlacken mittels Hochspannungsimpulsen. Chem. Ing. Tech. 2016, 88, 461–468. [Google Scholar] [CrossRef]
- Elektrodynamische Fragmentierung von MVA-Schlacken—Zerlegung der Schlacken und Abscheidung von Chloriden und Sulfaten//Aschen, Schlacken, Stäube. Aus Abfallverbrennung und Metallurgie; Thomé-Kozmiensky, K.J., Ed.; Berliner Schlackenkonferenz: Berlin, Germany; TK Verlag: Neuruppin, Germany, 2013. [Google Scholar]
- Semkin, B.V.; Usov, A.F.; Kurets, V.I. The principles of electric impulse destruction of materials. Kola Sci. Cent. Russ. Acad. Sci. 1995, 8. [Google Scholar]
- Marx, E. Versuche über die Prüfung von Isolatoren mit Spannungsstößen. Elektrotechnische Z. 1924, 1924, 652–654. [Google Scholar]
- Bluhm, H.; Frey, W.; Giese, H.; Hoppe, P.; Schultheiss, C.; Strassner, R. Application of pulsed HV discharges to material fragmentation and recycling. IEEE Trans. Dielect. Electr. Insul. 2000, 7, 625–636. [Google Scholar] [CrossRef]
- Sundar, V.; Newnham, R.E. Electrostriction and polarization. Ferroelectrics 1992, 135, 431–446. [Google Scholar] [CrossRef]
- Hoppe, P.; Singer, J.; Giese, H.; Stemmermann, P.; Schweike, U.; Edinger, W. Prozessreaktor und Betriebsverfahren für die Elektrodynamische Fragmentierung. European Patent 1673172, 6 August 2004. [Google Scholar]
- Robben, C.; Wotruba, H. Sensors-Based Ore Sorting Technology in Mining—Past, Present and Future. Minerals 2019, 9, 523. [Google Scholar] [CrossRef]
- Maier, G.; Pfaff, F.; Pieper, C.; Gruna, R.; Noack, B.; Kruggel-Emden, H.; Langle, T.; Hanebeck, U.D.; Wirtz, S.; Scherer, V.; et al. Experimental Evaluation of a Novel Sensor-Based Sorting Approach Featuring Predictive Real-Time Multiobject Tracking. IEEE Trans. Ind. Electron. 2021, 68, 1548–1559. [Google Scholar] [CrossRef]
- Nobis, C.; Vollpracht, A. Verwendung von Recyclingmaterial in der Betonproduktion—Sachstand. 2015. Available online: https://www.transportbeton.org (accessed on 13 November 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).