Pathways of Mycotoxin Occurrence in Meat Products: A Review
Abstract
:1. Introduction
2. Major TMP Mycotoxins
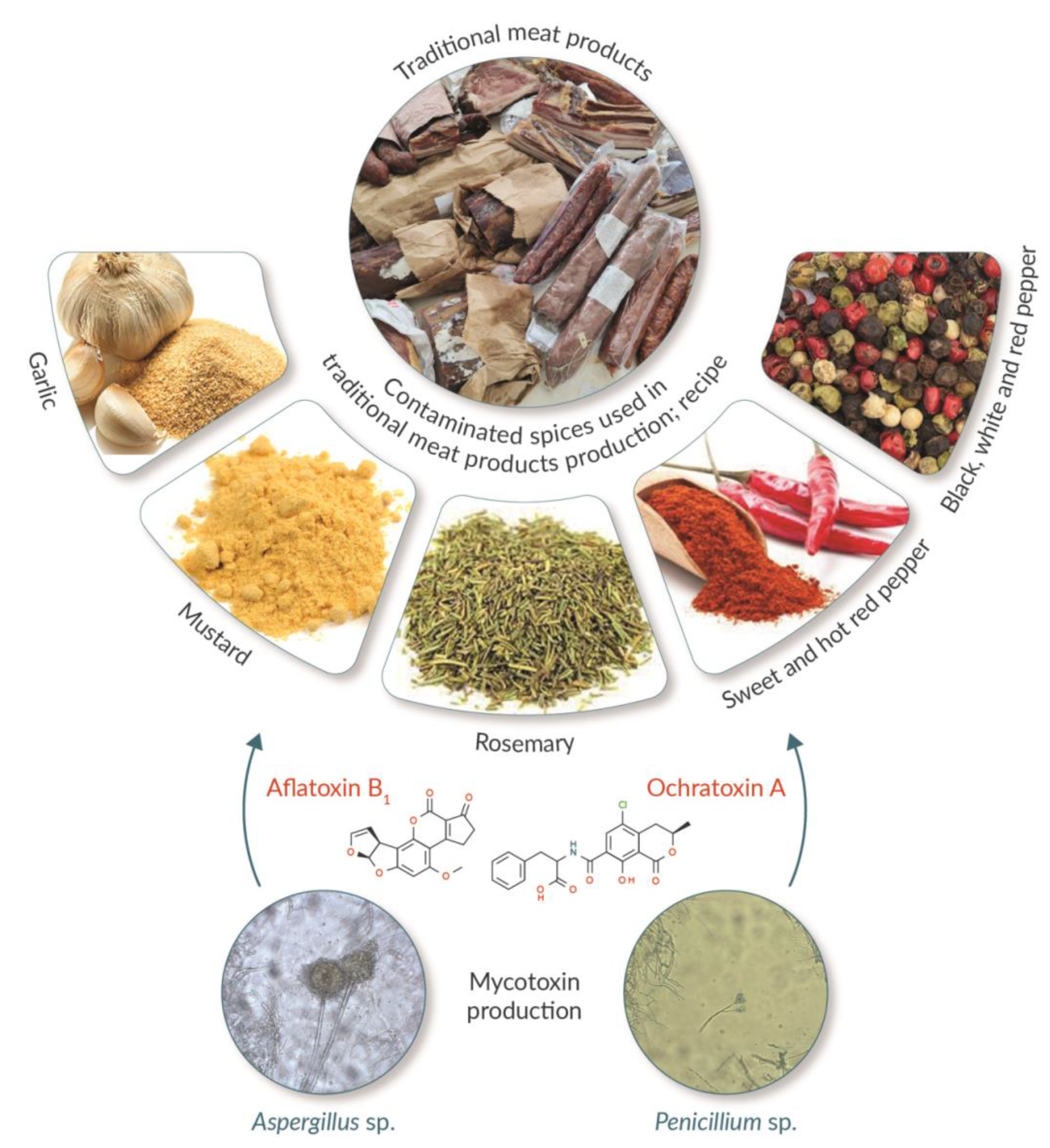
3. Mycotoxin Contamination through Spices
4. Transfer of Mycotoxins by Carry-Over Effect
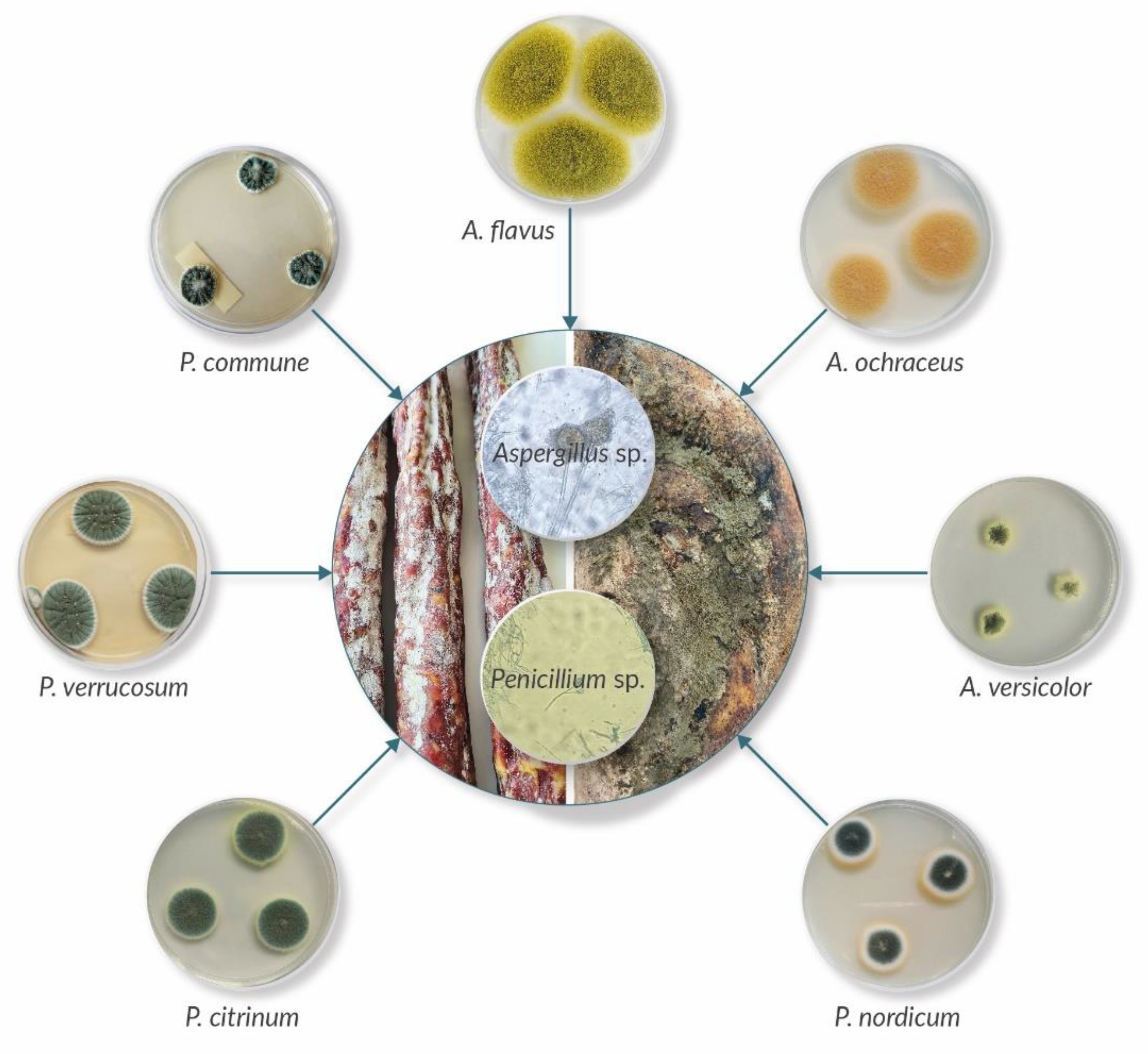
5. Mycotoxin Production by Surface Moulds
6. Occurrence of Mycotoxins in Meat Products
7. Control and Prevention
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in Food and Feed. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Elsevier: Cambrigde, UK, 2019; pp. 297–345. [Google Scholar]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res. Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ruyck, K.; Huybrechts, I.; Yang, S.; Arcella, D.; Claeys, L.; Abbeddou, S.; De Keyzer, W.; De Vries, J.; Ocke, M.; Ruprich, J.; et al. Mycotoxin exposure assessments in a multi-center European validation study by 24-hour dietary recall and biological fluid sampling. Environ. Int. 2020, 137, 105539. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Langsrud, S.; Omer, M.K.; Nesbakken, T.; Skaar, I. A HACCP plan for mycotoxigenic hazards associated with dry-cured meat production processes. Food Control 2011, 22, 831–837. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Gualla, A.; Morlacchini, M.; Pietri, A. Direct and indirect contamination with ochratoxin A of ripened pork products. Food Control 2013, 34, 79–83. [Google Scholar] [CrossRef]
- Pleadin, J.; Perši, N.; Kovačević, D.; Vahčić, N.; Scortichini, G.; Milone, S. Ochratoxin A in traditional dry-cured meat products produced from subchronic-exposed pigs. Food Addit. Contam. Part A 2013, 30, 1827–1836. [Google Scholar] [CrossRef]
- Perši, N.; Pleadin, J.; Kovačević, D.; Scortichini, G.; Milone, S. Ochratoxin A in raw materials and cooked meat products made from OTA-treated pigs. Meat Sci. 2014, 96, 203–210. [Google Scholar] [CrossRef]
- Pleadin, J.; Kovačević, D.; Perković, I. Impact of casing damaging on aflatoxin B1 concentration during the ripening of dry-fermented sausages. J. Immunoass. Immunochem. 2015, 36, 655–666. [Google Scholar] [CrossRef]
- Pleadin, J.; Zadravec, M.; Brnić, D.; Perković, I.; Škrivanko, M.; Kovačević, D. Moulds and mycotoxins detected in the regional speciality fermented sausage “slavonski kulen” during a 1-year production period. Food Addit. Contam. Part A 2017, 34, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M.; Scheuer, R. Ochratoxin A in meat and meat products. Arch. Leb. 2000, 51, 102–104. [Google Scholar]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicosis—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.C.; Pena, A.; Lino, C.M. Ochratoxin A in Portugal: A review to assess human exposure. Toxins 2010, 2, 1225–1249. [Google Scholar] [CrossRef]
- Pleadin, J.; Malenica Staver, M.; Vahčić, N.; Kovačević, D.; Milone, S.; Saftić, L.; Scortichini, G. Survey of aflatoxin B1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control 2015, 52, 71–77. [Google Scholar] [CrossRef]
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Omer, M.K.; Langsrud, S.; Nesbakken, T.; Skaar, I. Fungal growth pattern, sources and factors of mould contamination in a dry-cured meat production facility. Int. J. Food Microbiol. 2010, 140, 131–135. [Google Scholar] [CrossRef]
- Zadravec, M.; Vahčić, N.; Brnić, D.; Markov, K.; Frece, J.; Beck, R.; Lešić, T.; Pleadin, J. A study of surface moulds and mycotoxins in Croatian traditional dry-cured meat products. Int. J. Food Microbiol. 2020, 317, 108459. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1987; Volume 1–42, pp. 83–87. [Google Scholar]
- International Agency for Research on Cancer (IARC). Aflatoxins. In Chemical Agents and Related Occupations: A Review of Human Carcinogens; IARC Press: Lyon, France, 2012; Volume 100F, pp. 225–248. [Google Scholar]
- International Agency for Research on Cancer (IARC). Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1993; Volume 56. [Google Scholar]
- Iacumin, L.; Chiesa, L.; Boscolo, D.; Manzano, M.; Cantoni, C.; Orlić, S.; Comi, G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009, 26, 65–70. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; Van der Merwe, D. Mycotoxins in the food chain: Contamination of foods of animal origin. In Chemical Hazards in Foods of Animal Origin; Fink-Gremmels, J., Van der Merwe, D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 1190–1198. [Google Scholar] [CrossRef]
- Milićević, D.; Nedeljković-Trailović, J.; Mašić, Z. Mycotoxins in food chain—Risk assessment and importance for public health. Tehnol. Mesa 2014, 55, 22–38. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Contaminants in the Food Chain (EFSA CONTAM Panel). Scientific Opinion on the risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Martín, A.; Nuñez, F.; Córdoba, J.J. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control 2012, 27, 118–126. [Google Scholar] [CrossRef]
- Rodríguez, A.; Capela, D.; Medina, Á.; Córdoba, J.J.; Magan, N. Relationship between ecophysiological factors, growth and ochratoxin A contamination of dry-cured sausage based matrices. Int. J. Food Microbiol. 2015, 194, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bailly, J.D.; Guerre, P. Mycotoxins in meat and processed meat products. In Food Microbiology and Food Safety—Safety of Meat and Processed Meat; Toldrá, F., Ed.; Springer: New York, NY, USA, 2009; pp. 83–124. [Google Scholar]
- European Food Safety Authority Panel on Contaminants in the Food Chain (EFSA CONTAM Panel). Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- Ostry, V.; Toman, J.; Grosse, Y.; Malir, F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018, 11, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Frisvald, J.C.; Samson, A. Mycotoxins produced by species of Penicillium and Aspergillus occurring in cereals. In Cereal Grain; Chelkwski, J., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 441–476. [Google Scholar]
- Pitt, J.I.; Leistner, L. Toxigenic Penicillium species. In Mycotoxins and Animal Foods; Smith, J.E., Henderson, R.S., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1991; pp. 81–100. [Google Scholar]
- López-Díaz, T.M.; Santos, J.A.; García-López, M.L.; Otero, A. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium isolates. Int. J. Food Microbiol. 2001, 68, 69–74. [Google Scholar] [CrossRef]
- Andersen, S.J. Compositional changes in surface mycoflora during ripening of naturally fermented sausages. J. Food Prot. 1995, 58, 426–429. [Google Scholar] [CrossRef]
- Núñez, F.; Rodríguez, M.M.; Bermúdez, M.E.; Córdoba, J.J.; Asensio, M.A. Composition and toxigenic potential of the mould population on dry-cured Iberian ham. Int. J. Food Microbiol. 1996, 32, 185–197. [Google Scholar] [CrossRef]
- Peintner, U.; Geiger, J.; Pöder, R. The mycobiota of speck, a traditional tyrolean smoked and cured ham. J. Food Prot. 2000, 63, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority Panel on Contaminants in the Food Chain (EFSA CONTAM Panel). Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 2605. [Google Scholar] [CrossRef]
- Bailly, J.D.; Tabuc, C.; Quérin, A.; Guerre, P. Production and stability of patulin, ochratoxin A, citrinin, and cyclopiazonic acid on dry cured ham. J. Food Prot. 2005, 68, 1516–1520. [Google Scholar] [CrossRef]
- Alapont, C.; López-Mendoza, M.C.; Gil, J.V.; Martínez-Culebras, P.V. Mycobiota and toxigenic Penicillium species on two Spanish dry-cured ham manufacturing plants. Food Addit. Contam. Part A 2014, 31, 93–104. [Google Scholar] [CrossRef]
- Vulić, A.; Lešić, T.; Kudumija, N.; Zadravec, M.; Kiš, M.; Vahčić, N.; Pleadin, J. The development of LC-MS/MS method of determination of cyclopiazonic acid in dry-fermented meat products. Food Control 2021, 123, 107814. [Google Scholar] [CrossRef]
- Plavsic, D.; Okanovic, D.; Gubic, J.; Njezic, Z. Microbiological and chemical evaluation of dried smoked meat product. Procedia Food Sci. 2015, 5, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Milićević, D.R.; Stefanović, S.; Janković, S.; Radičević, T. Risk analysis and exposure assessment of Ochratoxin A in Serbia. Vet. World 2012, 5, 412–416. [Google Scholar] [CrossRef]
- Little, C.L.; Omotoye, R.; Mitchell, R.T. The microbiological quality of ready-to-eat foods with added spices. Int. Environ. Health Res. J. 2003, 13, 31–42. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Malir, J.; Toman, J.; Malir, F. Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years. Toxins 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Tawab, A.A.; El-Diasty, E.M.; Khater, D.F.; Al-baaly, Y.M. Mycological identification of some fungi isolated from meat products and spices with molecular identification of some Penicillium isolates. Adv. Anim. Vet. Sci. 2020, 8, 124–129. [Google Scholar] [CrossRef]
- Mandeel, Q.A. Fungal contamination of some imported spices. Mycopathologia 2005, 159, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, R.M. Occurrence and significance of moulds and their mycotoxins in spices as meat additives. Ben. Vet. Med. J. 2006, 17, 35–46. [Google Scholar]
- Bokhari, F.M. Spices mycobiota and mycotoxins available in Saudi Arabia and their abilities to inhibit growth of some toxigenic fungi. Myco J. 2007, 35, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocic´-Tanackov, S.D.; Dimic´, G.R.; Karalic, D. Contamination of spices with moulds potential producers of sterigmatocystine. Acta Period. Technol. 2007, 38, 29–35. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin producing fungi. Saudi Biol. Sci. J. 2010, 17, 167–175. [Google Scholar] [CrossRef]
- Janković, V.; Borović, B.; Velebit, B.; Mitrović, R.; Lakićević, B.; Spirić, D.; Baltić, T. Comparative mycological analysis of spices used in meat industry. Tehnol. Mesa 2013, 54, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.L.; Martins, H.M.; Bernardo, F. Aflatoxins in spices marketed in Portugal. Food Addit. Contam. 2001, 18, 315–319. [Google Scholar] [CrossRef]
- Jalili, M.; Jinap, S. Natural occurrence of aflatoxins and ochratoxin A in commercial dried chili. Food Control 2012, 24, 160–164. [Google Scholar] [CrossRef]
- Gambacorta, L.; Magistà, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J. 2018, 11, 159–174. [Google Scholar] [CrossRef]
- Zahra, N.; Khan, M.; Mehmood, Z.; Saeed, M.; Kalim, I.; Ahmad, I.; Malik, K. Determination of aflatoxins in spices and dried fruits. J. Sci. Res. 2018, 10, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Jacxsens, L.; Yogendrarajaha, P.; Meulenaer, B. Risk assessment of mycotoxins and predictive mycology in Sri Lankan spices: Chilli and pepper. Procedia Food Sci. 2016, 6, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Pleadin, J.; Kovačević, D.; Perši, N. Ochratoxin A contamination of the autochthonous dry-cured meat product “Slavonski Kulen” during a six-month production process. Food Control 2015, 57, 377–384. [Google Scholar] [CrossRef]
- Karan, D.D.; Vukojević, J.B.; Ljaljević-Grbić, M.V.; Milićević, D.R.; Janković, V.V. Presence of moulds and mycotoxins in spices. Proc. Nat. Sci. Matica Srpska 2005, 108, 77–84. [Google Scholar] [CrossRef]
- Hamad, S.H. Factors Affecting the Growth of Microorganisms in Food. In Progress in Food Preservation; Bhat, R., Alias, A.K., Paliyath, G., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 405–427. [Google Scholar]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Völkel, I.; Schröer-Merker, E.; Czerny, C.P. The Carry-Over of Mycotoxins in Products of Animal Origin with Special Regard to Its Implications for the European Food Safety Legislation. Food Nutr. Sci. 2011, 2, 852–867. [Google Scholar] [CrossRef] [Green Version]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.M.Y.; Rivas-Caceres, R.R.; Salem, A.Z.M. Mycotoxin toxicity and residue in animal products: Prevalence, consumer exposure and reduction strategies—A review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef]
- Deutsche Forschungsgemeinschaft (DFG). Ochratoxin A: Vorkommen und Toxikologische Bewertung; VCH: Weinheim, Germany, 1990. [Google Scholar]
- Duarte, S.C.; Lino, C.M.; Pena, A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbiol. 2011, 154, 1–13. [Google Scholar] [CrossRef]
- Milicevic, D.; Jovanovic, M.; Matekalo-Sverak, V.; Radicevic, T.; Petrovic, M.M.; Lilic, S. A Survey of Spontaneous Occurrence of Ochratoxin a Residues in Chicken Tissues and Concurrence With Histopathological Changes in Liver and Kidneys. J. Environ. Sci. Health C 2011, 29, 159–175. [Google Scholar] [CrossRef]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Jørgensen, K. Occurrence of ochratoxin A in commodities and processed food—A review of EU occurrence data. Food Addit. Contam. 2005, 22, 26–30. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request from the Commission Related to Ochratoxin A in food. EFSA J. 2006, 365, 1–56. [Google Scholar] [CrossRef]
- Rosi, A.; Sardi, L.; Zaghini, A.; Rizzi, L. Dietecontaminate de micotossine nel suino: Effetti in vivo e al macello. Suinicoltura 2006, 10, 131–134. [Google Scholar]
- Lusky, K.; Tesch, D.; Gobel, R. Influence of the mycotoxin ochratoxin A on animal health and formation of residues in pigs and different types of sausages derived from these animals. Arch. Lebensmittelhyg. 1993, 44, 131–134. [Google Scholar]
- Dall’Asta, C.; Galaverna, G.; Bertuzzi, T.; Moseriti, A.; Pietri, A.; Dossena, A.; Marchelli, R. Occurrence of ochratoxin A in raw ham muscle, salami and dry-cured ham from pigs fed with contaminated diet. Food Chem. 2010, 120, 978–983. [Google Scholar] [CrossRef]
- Beaver, R.W.; Wilson, D.M.; James, M.A.; Haydon, K.D.; Colvin, B.M.; Sangster, L.T.; Pikul, A.H.; Groopman, J.D. Distribution of Aflatoxins in Tissues of Growing Pigs Fed an Aflatoxin-Contaminated diet amended with a High-Affinity Aluminosilicate Sorbent. Vet. Hum. Toxicol. 1990, 32, 16–18. [Google Scholar]
- Meerpoel, C.; Vidal, A.; Tangni, E.K.; Huybrechts, B.; Couck, L.; De Rycke, R.; De Bels, L.; De Saeger, S.; Van den Broeck, W.; Devreese, M.; et al. A Study of Carry-Over and Histopathological Effects after Chronic Dietary Intake of Citrinin in Pigs, Broiler Chickens and Laying Hens. Toxins 2020, 12, 719. [Google Scholar] [CrossRef]
- Sørensen, L.M. Filamentous Fungi on Meat Products, their Ability to Produce Mycotoxins and a Proteome Approach to Study Mycotoxin Production. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, June 2009. [Google Scholar]
- Zadravec, M.; Markov, K.; Frece, J.; Perković, I.; Jakopović, Ž.; Lešić, T.; Mitak, M.; Pleadin, J. Toxicogenic fungi and the occurrence of mycotoxins in traditional meat products. Croat. J. Food Sci. Technol. 2019, 11, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Comi, G.; Orlić, S.; Redžepović, S.; Urso, R.; Iacumin, L. Moulds isolated from Istrian dried ham at the pre-ripening and ripening level. Int. J. Food Microbiol. 2004, 96, 29–34. [Google Scholar] [CrossRef]
- Iacumin, L.; Milesi, S.; Pirani, S.; Comi, G.; Chiesa, L.M. Ochratoxigenic mold and ochratoxin A in fermented sausages from different areas in Northern Italy: Occurrence, reduction or prevention with ozonated air. J. Food Saf. 2011, 31, 538–545. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Battilani, P.; Pietri, V.A.; Giorni, P.; Formenti, S.; Bertuzzi, T.; Toscani, T.; Virgili, R.; Kozakiewicz, Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food Prot. 2007, 70, 975–980. [Google Scholar] [CrossRef]
- Sørensen, L.M.; Jacobsen, T.; Nielsen, P.V.; Frisvad, J.C.; Koch, A.G. Mycobiota in the processing areas of two different meat products. Int. J. Food Microbiol. 2008, 124, 58–64. [Google Scholar] [CrossRef]
- Asefa, D.T.; Gjerde, R.O.; Sidhu, M.S.; Langsrud, S.; Kure, C.F.; Nesbakken, T.; Skaar, I. Moulds contaminants on Norwegian dry-cured meat products. Int. J. Food Microbiol. 2009, 128, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Sonjak, S.; Ličen, M.; Frisvad, J.C.; Gunde-Cimerman, N. The mycrobiota of three dry-cured meat products from Slovenia. Food Microbiol. 2011, 28, 373–376. [Google Scholar] [CrossRef]
- Nielsen, K.F. Mycotoxin production by indoor molds. Fungal Genet. Biol. 2003, 39, 103–117. [Google Scholar] [CrossRef]
- Ferrara, M.; Perrone, G.; Gallo, A.; Epifani, F.; Visconti, A.; Susca, A. Development of loop-mediated isothermal amplification (LAMP) assay for the rapid detection of Penicillium nordicum in dry-cured meat products. Int. J. Food Microbiol. 2015, 202, 42–47. [Google Scholar] [CrossRef]
- Perrone, G.; Samson, R.A.; Frisvad, J.C.; Susca, A.; Gunde-Cimerman, N.; Epifani, F.; Houbraken, J. Penicillium salamii, a new species occurring during seasoning of dry-cured meat. Int. J. Food Microbiol. 2015, 193, 91–98. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Pleadin, J.; Zadravec, M.; Lešić, T.; Vahčić, N.; Frece, J.; Mitak, M.; Markov, K. Co-occurrence of ochratoxin A and citrinin in unprocessed cereals established during a three-year investigation period. Food Addit. Contam. 2018, 11, 20–25. [Google Scholar] [CrossRef]
- Matrella, R.; Monaci, L.; Milillo, M.A.; Palmisano, F.; Tantillo, M.G. Ochratoxin A determination in paired kidneys and muscle samples from swines slaughtered in southern Italy. Food Control 2006, 17, 114–117. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T.; Gualla, A.; Piva, G. Occurrence of ochratoxin A in raw ham muscles and in pork products from Northern Italy. Ital. J. Food Sci. 2006, 18, 99–106. [Google Scholar]
- Roncada, P.; Altafini, A.; Fedrizzi, G.; Guerrini, A.; Polonini, G.L.; Caprai, E. Ochratoxin A contamination of the casing and the edible portion of artisan salamis produced in two Italian regions. World Mycotoxin J. 2020, 13, 553–562. [Google Scholar] [CrossRef]
- Pleadin, J.; Markov, K.; Frece, J.; Vulić, A.; Perši, N. Bio-Prevalence, Determination and Reduction of Aflatoxin B1 in Cereals. In Aflatoxins: Food Sources, Occurrence and Toxicological Effects; Faulkner, A.G., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 1–34. [Google Scholar]
- Pleadin, J.; Perši, N.; Kovačević, D.; Vulić, A.; Frece, J.; Markov, K. Ochratoxin A reduction in meat sausages using processing methods practiced in households. Food Addit. Contam. B 2014, 7, 239–246. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulić, A.; Perši, N.; Škrivanko, M.; Capek, B.; Cvetnić, Ž. Aflatoxin B1 occurrence in maize sampled from Croatian farms and feed factories during 2013. Food Control 2014, 40, 286–291. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulić, A.; Perši, N.; Škrivanko, M.; Capek, B.; Cvetnić, Ž. Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control 2015, 47, 221–225. [Google Scholar] [CrossRef]
- Domijan, A.M.; Pleadin, J.; Mihaljević, B.; Vahčić, N.; Frece, J.; Markov, K. Reduction of ochratoxin A in dry-cured meat products using gamma irradiation. Food Addit. Contam. A 2015, 32, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Markov, K.; Pleadin, J.; Bevardi, M.; Vahčić, N.; Sokolić-Mihalek, D.; Frece, J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control 2013, 34, 312–317. [Google Scholar] [CrossRef]
- Aziz, N.H.; Youssef, A.Y. Occurrence of aflatoxins and aflatoxin-producing moulds in fresh and processed meat in Egypt. Food Addit. Contam. 1991, 8, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Zaky, Z.M. Evaluation of the mycological status of luncheon meat with special reference to aflatoxigenic moulds and aflatoxin residues. Mycopathologia 1999, 146, 147–154. [Google Scholar] [CrossRef]
- Bernáldez, V.; Córdoba, J.J.; Rodríguez, M.; Cordero, M.; Polo, L.; Rodríguez, A. Effect of Penicillium nalgiovense as protective culture in processing of dry-fermented sausage “salchichón”. Food Control 2015, 32, 69–76. [Google Scholar] [CrossRef]
- Abd-Elghany, S.M.; Sallam, K.I. Rapid determination of total aflatoxins and ochratoxins A in meat products by immuno-affinity fluorimetry. Food Chem. 2015, 179, 253–256. [Google Scholar] [CrossRef]
- Sørensen, L.M.; Mogensen, J.; Nielsen, K.F. Simultaneous determination of ochratoxin A, mycophenolic acid and fumonisin B2 in meat products. Anal. Bioanal. Chem. 2010, 398, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Kudumija, N.; Vulić, A.; Lešić, T.; Vahčić, N.; Pleadin, J. Aflatoxins and ochratoxin A in dry-fermented sausages in Croatia, by LC-MS/MS. Food Addit. Contam. A. 2020, 13, 225–232. [Google Scholar] [CrossRef]
- Atherstone, C.; Grace, D.; Waliyar, F.; Lindahl, J.; Osiru, M. Aflatoxin Literature Synthesis and Risk Mapping: Special Emphasis on Sub-Saharan Africa; International Livestock Research Institute: Nairobi, Kenya, 2014. [Google Scholar]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364/5, 5–24.
- Commission Regulation (EC) No 165/2010 of 26 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, L50/8, 8–12.
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Amézqueta, S.; Peñas, G.E.; Arbizu, M.M.; De Certain, A.L. Ochratoxin A decontamination: A review. Food Control 2009, 20, 326–333. [Google Scholar] [CrossRef]
- Toldrá, F. Proteolysis and lipolysis in flavour development of dry cured meat products. Meat Sci. 1998, 49, 101–110. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Céspedes Sánchez, F.J.; León Crespo, F. Influence of molds on flavor quality of Spanish ham. J. Muscle Foods 2000, 11, 247–259. [Google Scholar] [CrossRef]
- Kovačević, D. Tehnologija Kulena I Drugih Fermentiranih Kobasica; Prehrambeno—tehnološki fakultet Osijek: Osijek, Hrvatska, 2014. [Google Scholar]
- Hanssen, H.P. Mould control in the meat processing industry: Using a BioRid coating system. Fleischwirtschaft 1995, 75, 52. [Google Scholar]
- Rodríguez, A.; Medina, A.; Córdoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119. [Google Scholar] [CrossRef]

| Mould Species | Mycotoxin | aw (Range) | T/°C (Range) |
|---|---|---|---|
| Aspergillus flavus | AFB1 | ≥0.84; ≥0.80 | 12–35 |
| Aspergillus parasiticus | AFB1 | ≥0.84 | 12–35 |
| Aspergillus ochraceus | OTA | ≥0.87 | 12–35 |
| Penicillium verrucosum | OTA | ≥0.85 | 2–34 |
| Penicillium nordicum | OTA | - | 15–30 |
| Penicillium commune | CPA | ≥0.90 | 12–30 |
| Product | Mycotoxin | N | % Positive Samples | Range (μgkg−1) | Country | Reference |
|---|---|---|---|---|---|---|
| Beef luncheon, burger and sausages | AFs | 150 | 0.6 | 2–7 | Egypt | [99] |
| 50 | 14 | 11.1 | Egypt | [100] | ||
| 10 | 0 | <LOD | Spain | [101] | ||
| 25 | 100 | 0.47–9 | Egypt | [102] | ||
| Blood sausages | OTA | 620 | 77 | 3.2 | German | [13] |
| Liver-type sausage | 620 | 68 | 5 | German | [13] | |
| Sausages | 100 | 45 | 7–8 | Italy | [23] | |
| 10 | 0 | <LOD/LOQ | Spain | [101] | ||
| Parma (retail product) | 22 | 4 | 56–158 | Denmark | [103] | |
| Dry-cured Iberian ham | 20 | Deep portion 15 | Deep portion 2–160 | Spain | [27] | |
| 20 | Superficial portion 25 | Superficial portion > 15 | Spain | [27] | ||
| 45 | 13 | 1.9–6.3 | Spain | [27] | ||
| Beef luncheon | 25 | 100 | 0.56–8.5 | Egypt | [102] | |
| Beef burger | 25 | 100 | 2.7–7.6 | Egypt | [102] | |
| Fermented meat products | OTA | 90 | 64.44 | 1.23–7.83 | Croatia | [98] |
| CIT | 4.44 | 1.0–1.3 | ||||
| AFB1 | 10 | 1.0–3.0 | ||||
| Traditional meat products | AFB1 | 410 | up to 11.1 | up to 1.69 | Croatia | [17] |
| OTA | up to 20.0 | up to 9.95 | ||||
| Traditional meat products | AFB1 | 160 | 8 | up to 1.92 | Croatia | [19] |
| OTA | 14 | up to 6.86 | ||||
| Dry-fermented sausages | OTA | 88 | 14.8 | up to 0.48 | Croatia | [104] |
| Dry-fermented sausages | CPA | 47 | 14.9 | 2.55–59.80 | Croatia | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleadin, J.; Lešić, T.; Milićević, D.; Markov, K.; Šarkanj, B.; Vahčić, N.; Kmetič, I.; Zadravec, M. Pathways of Mycotoxin Occurrence in Meat Products: A Review. Processes 2021, 9, 2122. https://doi.org/10.3390/pr9122122
Pleadin J, Lešić T, Milićević D, Markov K, Šarkanj B, Vahčić N, Kmetič I, Zadravec M. Pathways of Mycotoxin Occurrence in Meat Products: A Review. Processes. 2021; 9(12):2122. https://doi.org/10.3390/pr9122122
Chicago/Turabian StylePleadin, Jelka, Tina Lešić, Dragan Milićević, Ksenija Markov, Bojan Šarkanj, Nada Vahčić, Ivana Kmetič, and Manuela Zadravec. 2021. "Pathways of Mycotoxin Occurrence in Meat Products: A Review" Processes 9, no. 12: 2122. https://doi.org/10.3390/pr9122122
APA StylePleadin, J., Lešić, T., Milićević, D., Markov, K., Šarkanj, B., Vahčić, N., Kmetič, I., & Zadravec, M. (2021). Pathways of Mycotoxin Occurrence in Meat Products: A Review. Processes, 9(12), 2122. https://doi.org/10.3390/pr9122122










