Removal of Heavy Metals from Wastewater Using Novel Polydopamine-Modified CNTs-Based Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PD-CNT-Based Composite Membranes
2.1.1. Nanotube Surface Cleaning and Washing
2.1.2. Oxidation of Carbon Nanotubes
2.1.3. Dopamine-Coated Carbon Nanotubes
2.1.4. Membranes Casting PD-CNTs/SPS (PDCPS) Nanocomposite
2.2. Characterization of PD-CNT-Based Composite Membranes
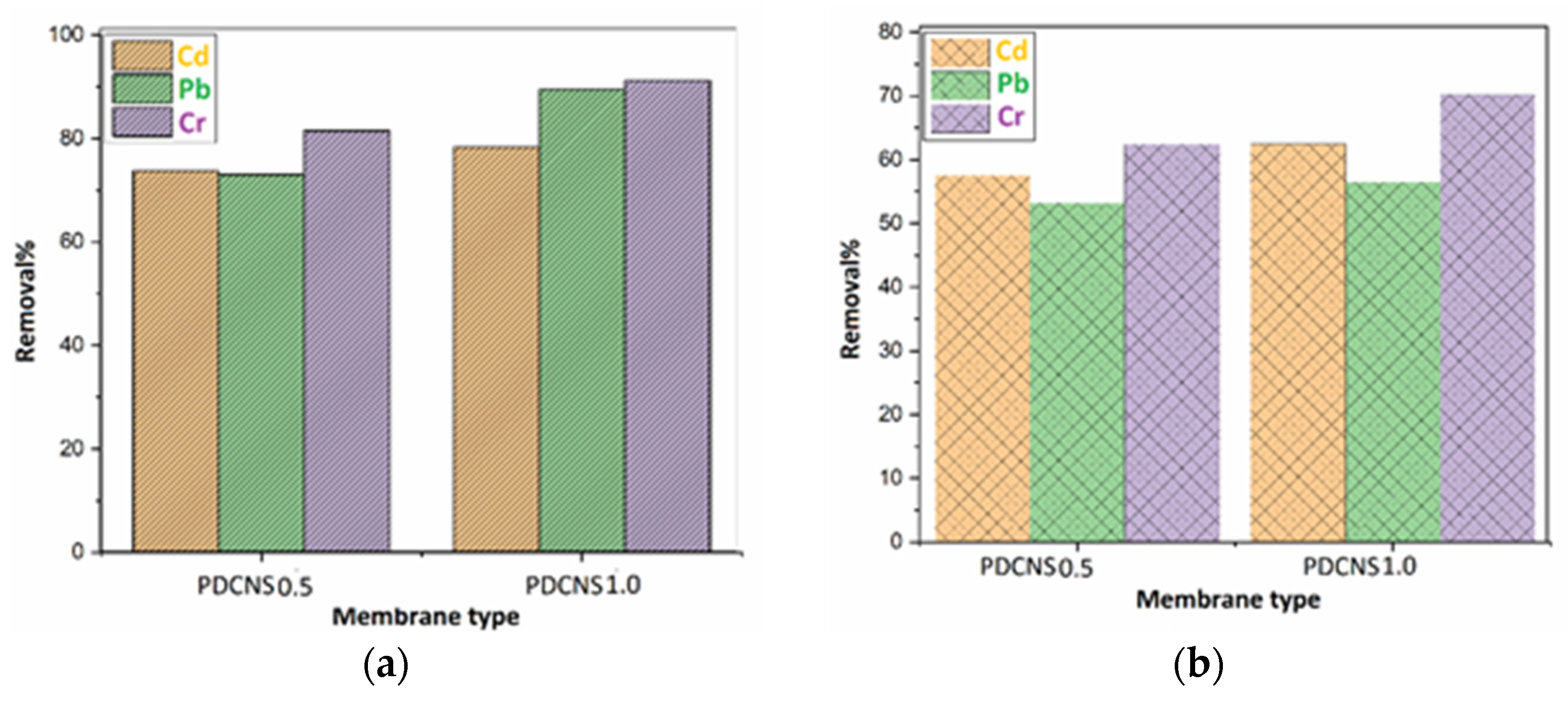
2.3. Removal Efficiency Determination by Atomic Absorption Spectrometry
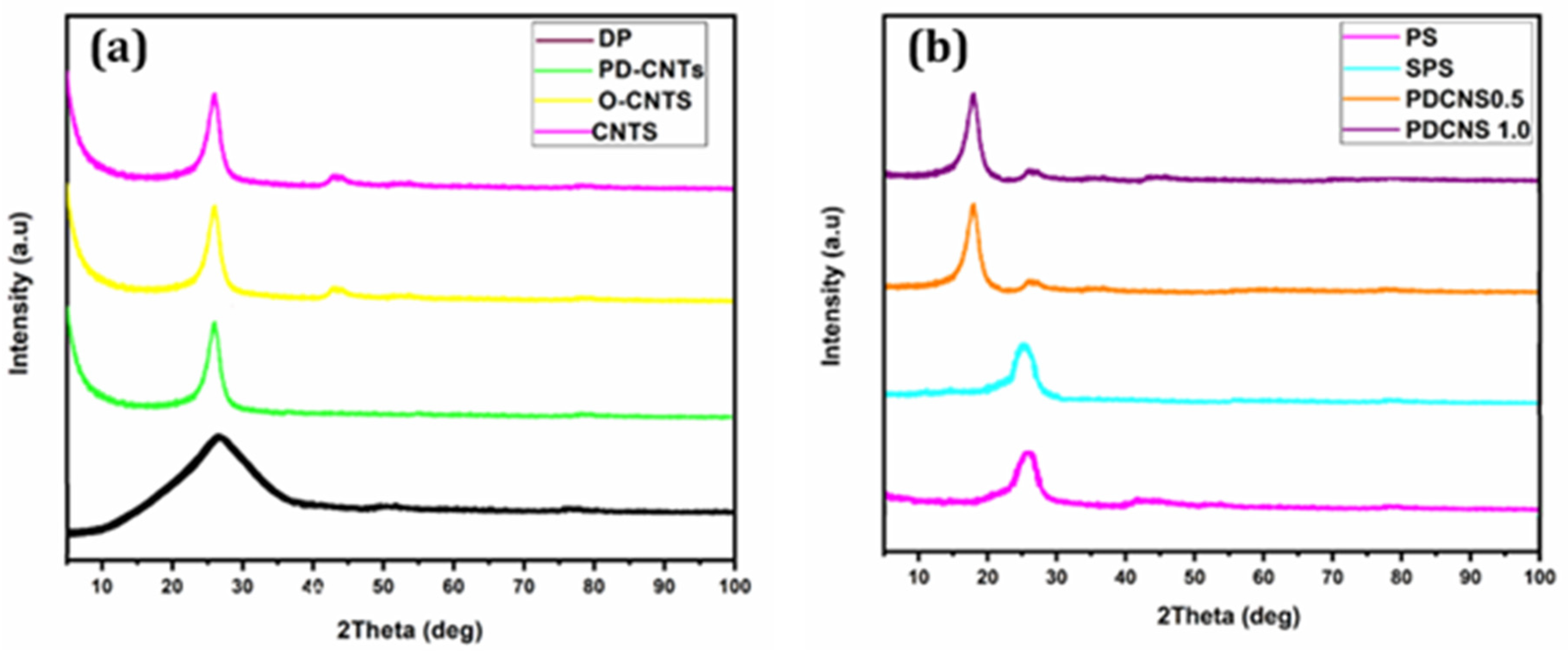
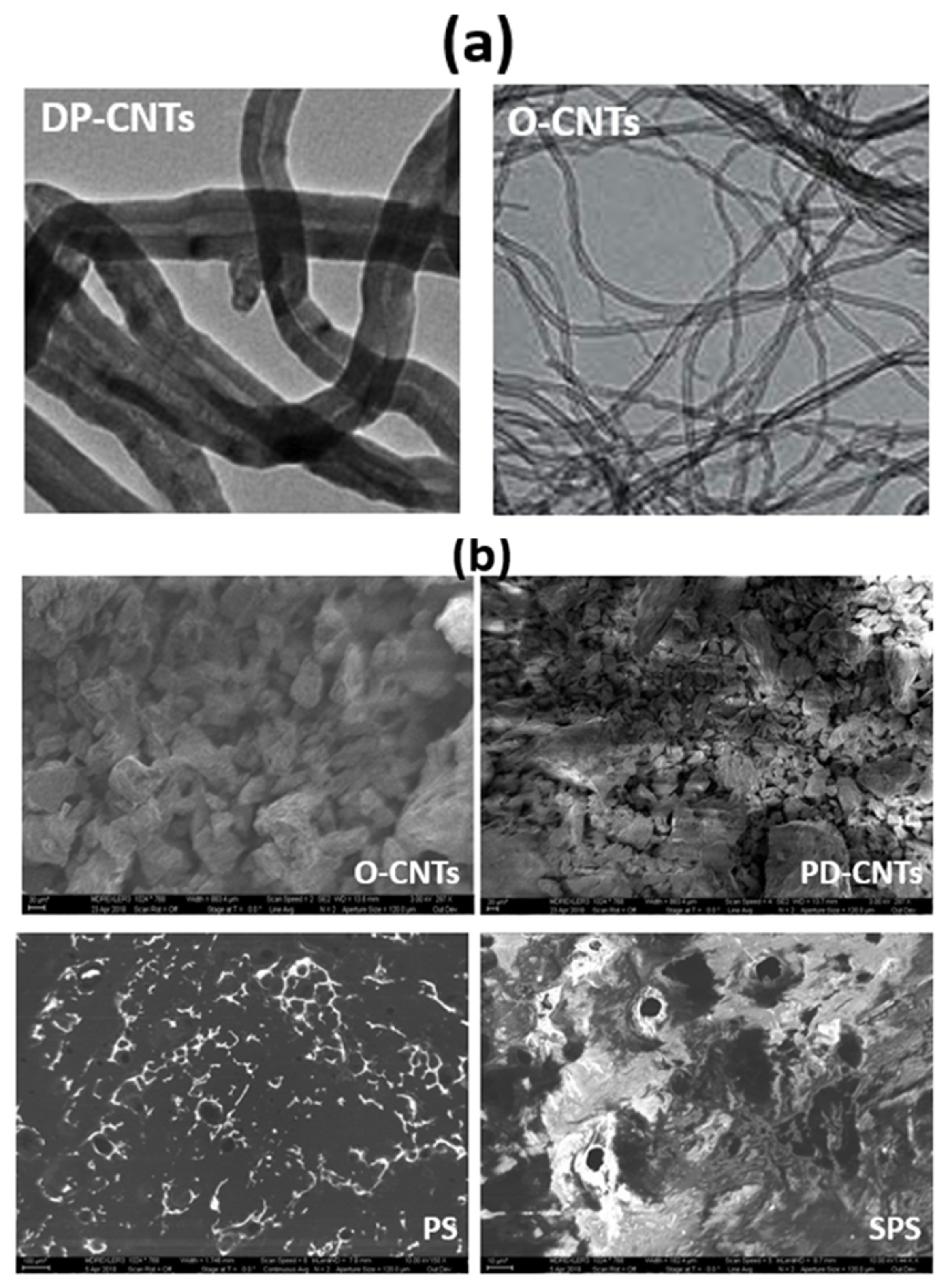
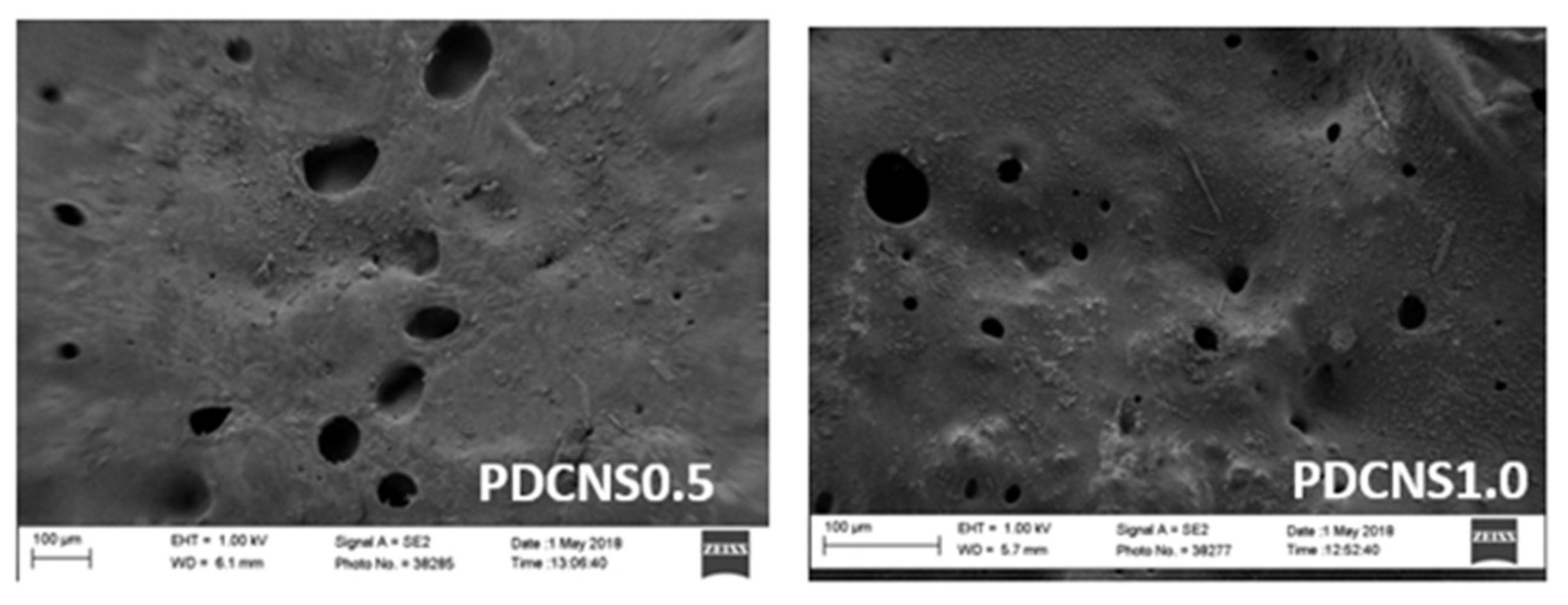
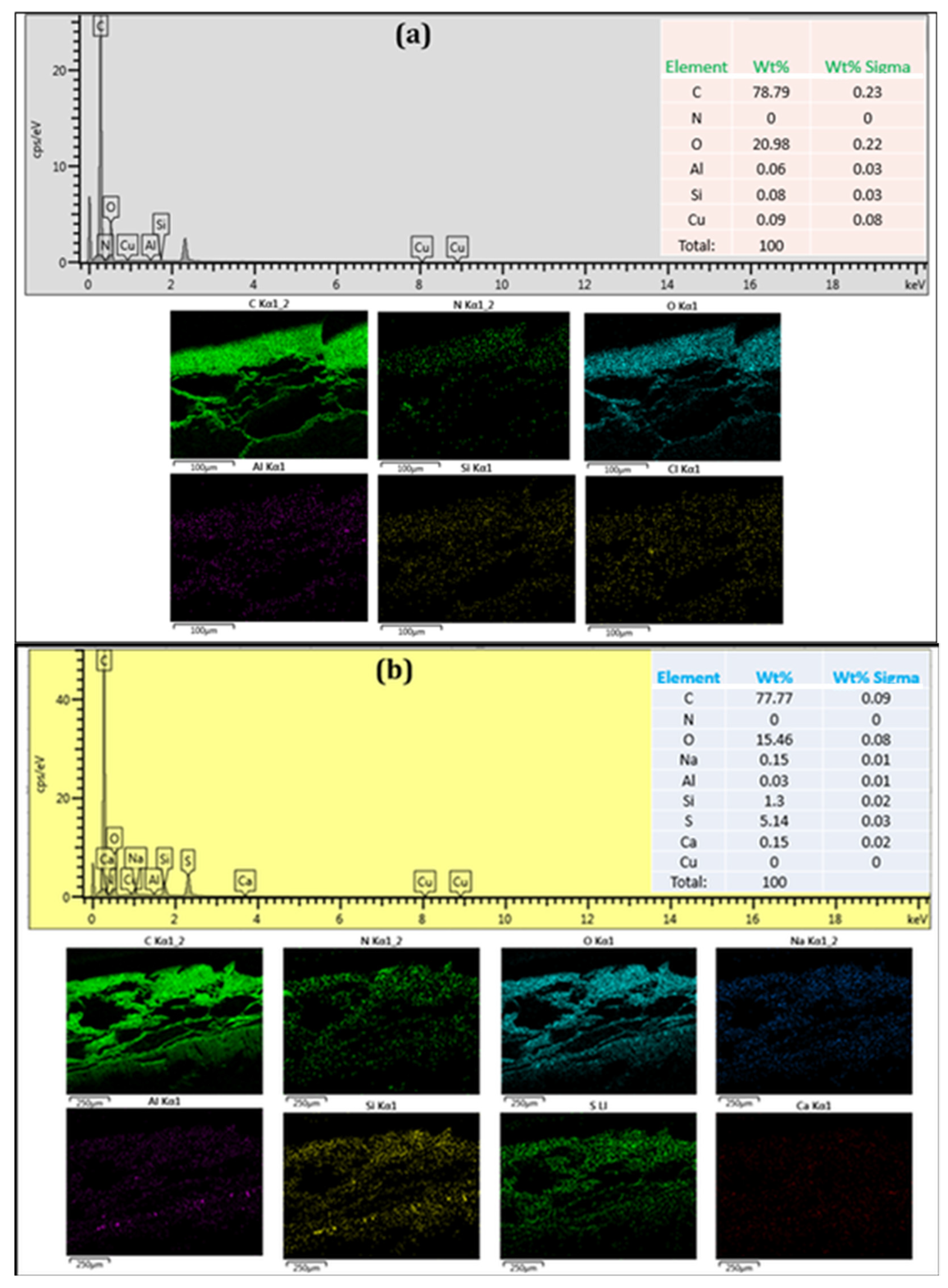
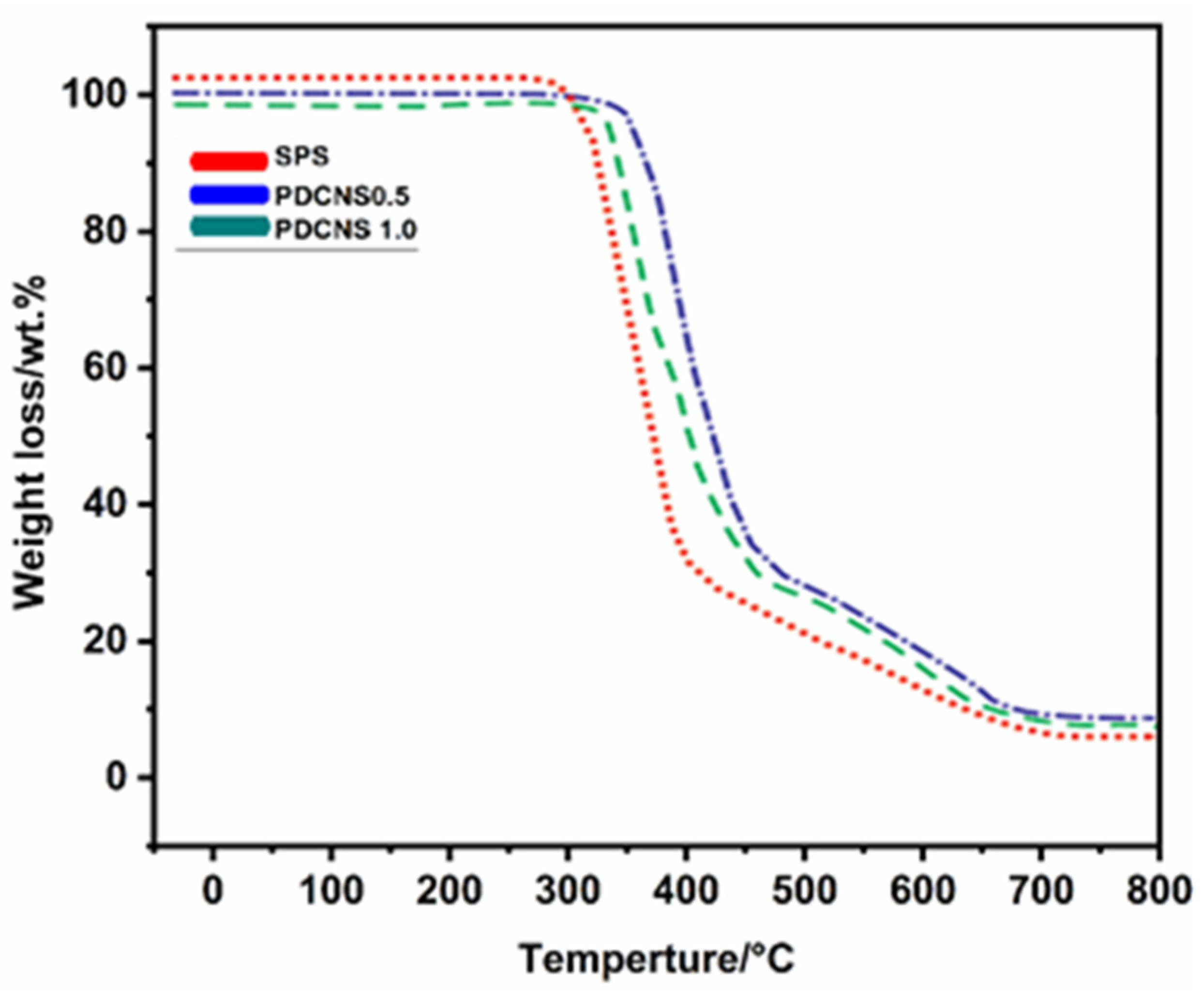
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, B.; Yao, J.; Knudsen, T.Š.; Chen, Z.; Liu, B.; Zhao, C.; Zhu, X. Simultaneous removal of typical flotation reagent 8-hydroxyquinoline and Cr (VI) through heterogeneous Fenton-like processes mediated by polydopamine functionalized ATP supported nZVI. J. Hazard. Mater. 2021, 126698, in press. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Salem Alsaiari, N.; Mnif, W.; Ben Rebah, F. Effective heavy metals removal from water using nanomaterials: A review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Qureshi, A.S. Challenges and prospects of using treated wastewater to manage water scarcity crises in the Gulf Cooperation Council (GCC) countries. Water 2020, 12, 1971. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Aldawsari, A.; Alsohaimi, I.; Hassan, H.; Al-Abduly, A.; Hassan, I.; Saleh, E. Multiuse silicon hybrid polyurea-based polymer for highly effective removal of heavy metal ions from aqueous solution. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Composite of Natural Polymers and Their Adsorbent Properties on the Dyes and Heavy Metal Ions. J. Polym. Environ. 2021, 29, 3477–3496. [Google Scholar] [CrossRef]
- Kour, G.; Kothari, R.; Azam, R.; Majhi, P.K.; Dhar, S.; Pathania, D.; Tyagi, V. Conducting Polymer Based Nanoadsorbents for Removal of Heavy Metal Ions/Dyes from Wastewater. In Advances in Hybrid Conducting Polymer Technology; Springer: Cham, Switzerland, 2021; pp. 135–157. [Google Scholar]
- Abd Hamid, S.; Shahadat, M.; Ismail, S. Zeolite-polysulfone-based adsorptive membrane for removal of metal pollutants. Chem. Pap. 2021, 75, 4479–4492. [Google Scholar] [CrossRef]
- Shaari, N.Z.K.; Rozlee, L.H.; Basri, M.F. Synthesis and Evaluation of Polysulfone/Chitosan/Polyvinyl Alcohol Integral Composite Membranes for the Removal of Mercury Ion. Transdiscipl. Res. Educ. Cent. Green Technol. Kyushu Univ. 2021, 8, 484–491. [Google Scholar] [CrossRef]
- Benkhaya, S.; Lgaz, H.; Chraibi, S.; Alrashdi, A.A.; Rafik, M.; Lee, H.-S.; El Harfi, A. Polysulfone/Polyetherimide Ultrafiltration composite membranes constructed on a three-component Nylon-fiberglass-Nylon Support for azo dyes removal: Experimental and Molecular dynamics simulations. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126941. [Google Scholar] [CrossRef]
- Alosaimi, A.M. Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg (II) Ions. Polymers 2021, 13, 2792. [Google Scholar] [CrossRef] [PubMed]
- Donga, C.; Mishra, S.B.; Abd-El-Aziz, A.S.; Mishra, A.K. Advances in graphene-based magnetic and graphene-based/TiO2 nanoparticles in the removal of heavy metals and organic pollutants from industrial wastewater. J. Inorg. Organomet. Polym. Mater. 2021, 31, 463–480. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Yin, J.; Wang, W.; Huang, G.; Qiu, X.; Schwingenschlögl, U.; Alshareef, H.N. Rational design of carbon anodes by catalytic pyrolysis of graphitic carbon nitride for efficient storage of Na and K mobile ions. Nano Energy 2021, 87, 106184. [Google Scholar] [CrossRef]
- Tsoeu, S.E. First-Principles Design of Hybrid Carbon Nitride (C2n) with Gallium Sulphide and Gallium Selenide Two-Dimensional (2D) Materials as High-Performance Photovoltaic Cells; University of Johannesburg: Johannesburg, South Africa, 2021. [Google Scholar]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; AlOtaibi, B. Enhanced heavy metals removal by a novel carbon nanotubes buckypaper membrane containing a mixture of two biopolymers: Chitosan and i-carrageenan. Sep. Purif. Technol. 2021, 276, 119300. [Google Scholar] [CrossRef]
- Cha, J.; Jin, S.; Shim, J.H.; Park, C.S.; Ryu, H.J.; Hong, S.H. Functionalization of carbon nanotubes for fabrication of CNT/epoxy nanocomposites. Mater. Des. 2016, 95, 1–8. [Google Scholar] [CrossRef]
- Sun, H.; Wang, T.; Xu, Y.; Gao, W.; Li, P.; Niu, Q.J. Fabrication of polyimide and functionalized multi-walled carbon nanotubes mixed matrix membranes by in-situ polymerization for CO2 separation. Sep. Purif. Technol. 2017, 177, 327–336. [Google Scholar] [CrossRef]
- McCollum, M. The Ultrasonic Polymerization of Isophorone Diisocyanate Based Polyurethane and Polyacrylamide with the In-Situ Sonication of Multi-Walled Carbon Nanotubes. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2017. [Google Scholar]
- Park, M.; Jang, J.-U.; Park, J.H.; Yu, J.; Kim, S.Y. Enhanced Tensile Properties of Multi-Walled Carbon Nanotubes Filled Polyamide 6 Composites Based on Interface Modification and Reactive Extrusion. Polymers 2020, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Pandele, A.M.; Serbanescu, O.S.; Voicu, S.I. Polysulfone composite membranes with carbonaceous structure. Synthesis and applications. Coatings 2020, 10, 609. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef]
- Guan, J.-F.; Zou, J.; Liu, Y.-P.; Jiang, X.-Y.; Yu, J.-G. Hybrid carbon nanotubes modified glassy carbon electrode for selective, sensitive and simultaneous detection of dopamine and uric acid. Ecotoxicol. Environ. Saf. 2020, 201, 110872. [Google Scholar] [CrossRef]
- Hansen, R.V.; Yang, J.; Zheng, L. Flexible electrochromic materials based on CNT/PDA hybrids. Adv. Colloid Interface Sci. 2018, 258, 21–35. [Google Scholar] [CrossRef]
- Wang, J.; Huang, T.; Zhang, L.; Yu, Q.J.; Hou, L.A. Dopamine crosslinked graphene oxide membrane for simultaneous removal of organic pollutants and trace heavy metals from aqueous solution. Environ. Technol. 2018, 39, 3055–3065. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S. Highly efficient as-synthesized and oxidized multi-walled carbon nanotubes for copper (II) and zinc (II) ion adsorption in a batch and fixed-bed process. J. Mater. Res. Technol. 2021, 15, 2848–2872. [Google Scholar] [CrossRef]
- Abuilaiwi, F.A.; Laoui, T.; Al-Harthi, M.; Atieh, M.A. Modification and functionalization of multiwalled carbon nanotube (MWCNT) via fischer esterification. Arab. J. Sci. Eng. 2010, 35, 37–48. [Google Scholar]
- Sianipar, M.; Kim, S.H.; Min, C.; Tijing, L.D.; Shon, H.K. Potential and performance of a polydopamine-coated multiwalled carbon nanotube/polysulfone nanocomposite membrane for ultrafiltration application. J. Ind. Eng. Chem. 2016, 34, 364–373. [Google Scholar] [CrossRef]
- Majeed, S.; Fierro, D.; Buhr, K.; Wind, J.; Du, B.; Boschetti-de-Fierro, A.; Abetz, V. Multi-walled carbon nanotubes (MWCNTs) mixed polyacrylonitrile (PAN) ultrafiltration membranes. J. Membr. Sci. 2012, 403, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wang, Z.; Wang, J.; Wang, S. A novel method of surface modification to polysulfone ultrafiltration membrane by preadsorption of citric acid or sodium bisulfite. Memb. Water Treat. 2012, 3, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Smitha, B.; Devi, D.A.; Sridhar, S. Proton-conducting composite membranes of chitosan and sulfonated polysulfone for fuel cell application. Int. J. Hydrog. Energy 2008, 33, 4138–4146. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zadehnazari, A. Rapid and green functionalization of multi-walled carbon nanotubes by glucose: Structural investigation and the preparation of dopamine-based poly (amide-imide) composites. Polym. Bull. 2014, 71, 2523–2542. [Google Scholar] [CrossRef]
- Hu, S.Y.; Zhang, Y.; Lawless, D.; Feng, X. Composite membranes comprising of polyvinylamine-poly (vinyl alcohol) incorporated with carbon nanotubes for dehydration of ethylene glycol by pervaporation. J. Membr. Sci. 2012, 417, 34–44. [Google Scholar] [CrossRef]
- Lee, M.; Ku, S.H.; Ryu, J.; Park, C.B. Mussel-inspired functionalization of carbon nanotubes for hydroxyapatite mineralization. J. Mater. Chem. 2010, 20, 8848–8853. [Google Scholar] [CrossRef]
- Shah, P.; Murthy, C. Studies on the porosity control of MWCNT/polysulfone composite membrane and its effect on metal removal. J. Membr. Sci. 2013, 437, 90–98. [Google Scholar] [CrossRef]
- Blanco, J.-F.; Sublet, J.; Nguyen, Q.T.; Schaetzel, P. Formation and morphology studies of different polysulfones-based membranes made by wet phase inversion process. J. Membr. Sci. 2006, 283, 27–37. [Google Scholar] [CrossRef]
- Benally, C.; Li, M.; El-Din, M.G. The effect of carboxyl multiwalled carbon nanotubes content on the structure and performance of polysulfone membranes for oil sands process-affected water treatment. Sep. Purif. Technol. 2018, 199, 170–181. [Google Scholar] [CrossRef]
- Guo, H.; Yao, Z.; Wang, J.; Yang, Z.; Ma, X.; Tang, C.Y. Polydopamine coating on a thin film composite forward osmosis membrane for enhanced mass transport and antifouling performance. J. Membr. Sci. 2018, 551, 234–242. [Google Scholar] [CrossRef]
- Tunckol, M.; Fantini, S.; Malbosc, F.; Durand, J.; Serp, P. Effect of the synthetic strategy on the non-covalent functionalization of multi-walled carbon nanotubes with polymerized ionic liquids. Carbon 2013, 57, 209–216. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altaf, F.; Ahmed, S.; Usman, M.; Batool, T.; Shamshad, J.; Bocchetta, P.; Batool, R. Removal of Heavy Metals from Wastewater Using Novel Polydopamine-Modified CNTs-Based Composite Membranes. Processes 2021, 9, 2120. https://doi.org/10.3390/pr9122120
Altaf F, Ahmed S, Usman M, Batool T, Shamshad J, Bocchetta P, Batool R. Removal of Heavy Metals from Wastewater Using Novel Polydopamine-Modified CNTs-Based Composite Membranes. Processes. 2021; 9(12):2120. https://doi.org/10.3390/pr9122120
Chicago/Turabian StyleAltaf, Faizah, Shakeel Ahmed, Muhammad Usman, Tahira Batool, Jaweria Shamshad, Patrizia Bocchetta, and Rida Batool. 2021. "Removal of Heavy Metals from Wastewater Using Novel Polydopamine-Modified CNTs-Based Composite Membranes" Processes 9, no. 12: 2120. https://doi.org/10.3390/pr9122120
APA StyleAltaf, F., Ahmed, S., Usman, M., Batool, T., Shamshad, J., Bocchetta, P., & Batool, R. (2021). Removal of Heavy Metals from Wastewater Using Novel Polydopamine-Modified CNTs-Based Composite Membranes. Processes, 9(12), 2120. https://doi.org/10.3390/pr9122120






