Influence of MAP on the Postharvest Quality of Glehnia littoralis Fr. Schmidt ex Miq.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Weight Loss and Color Characteristics
2.3. Total Chlorophyll Content
2.4. Electrolyte Leakage
2.5. Malondialdehyde Content
- ε (molar extinction coefficient): 155 mM−1 cm−1 for MDA; and
- DF: dilution factor.
2.6. HPLC Analysis of the Phenolic Compounds
2.7. Statistical Analysis
3. Results and Discussion
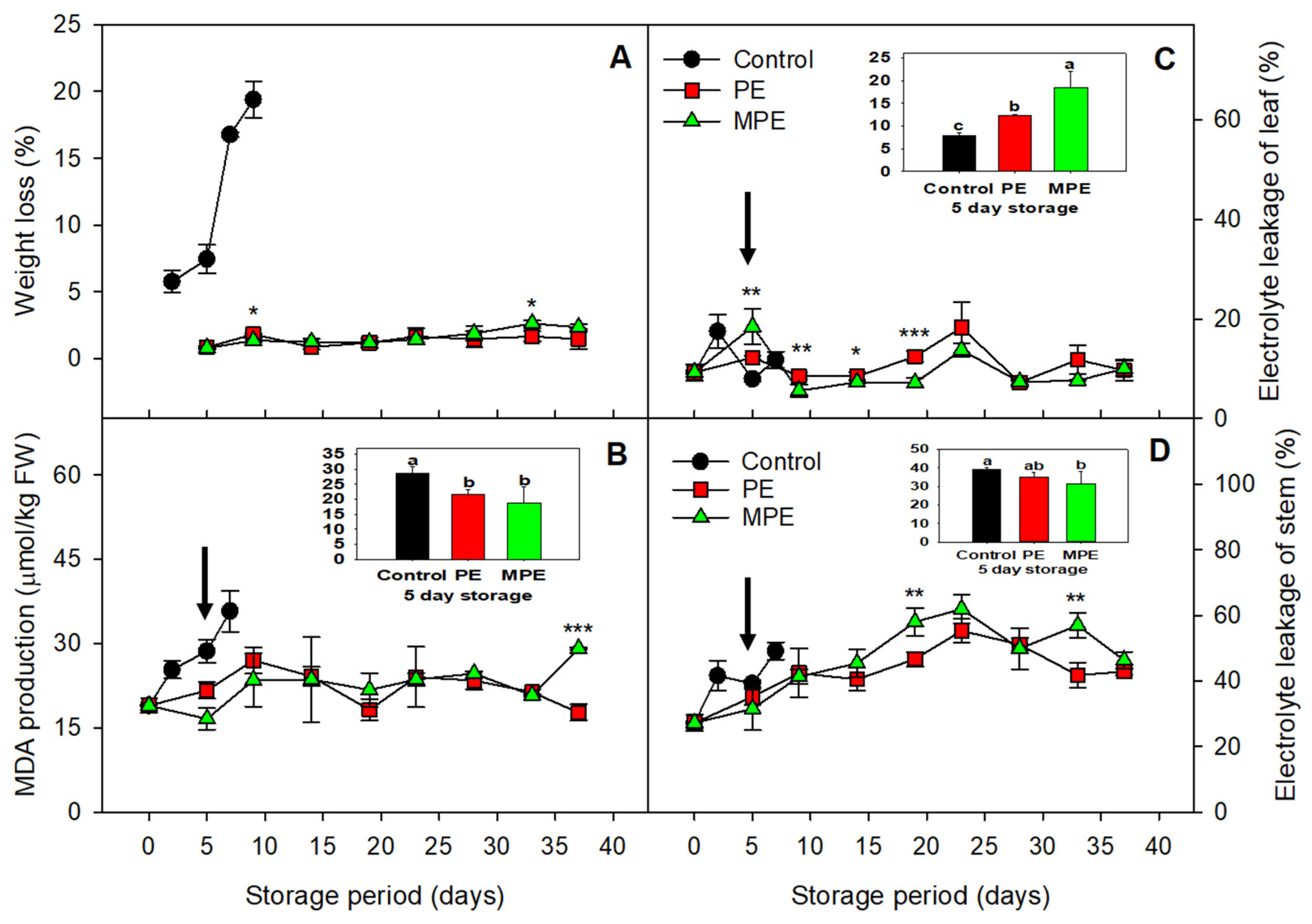
3.1. Changes in Weight Loss, MDA, and Electrolyte Leakage
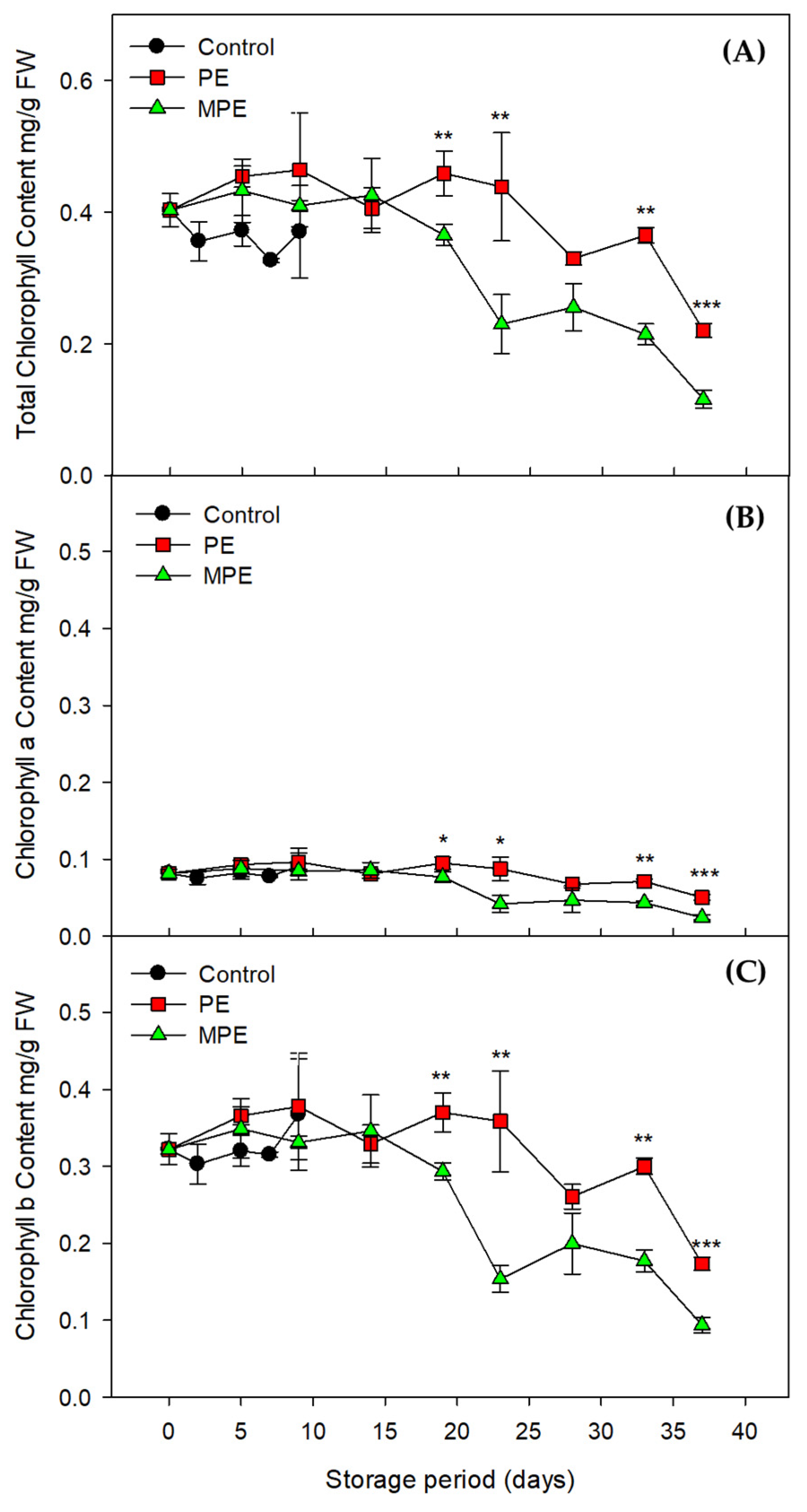
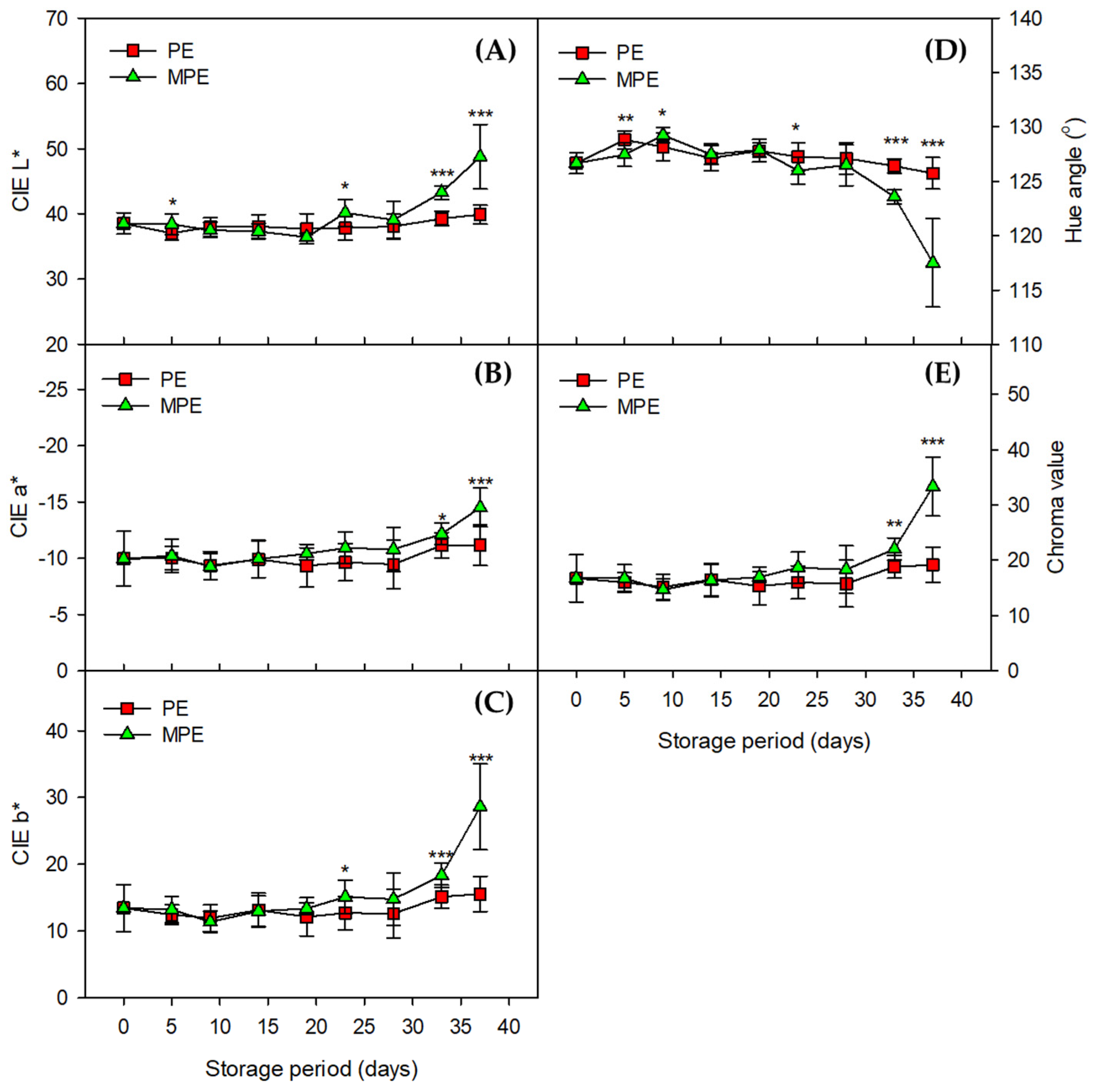
3.2. Changes in Total Chlorophyll Content and Color Values
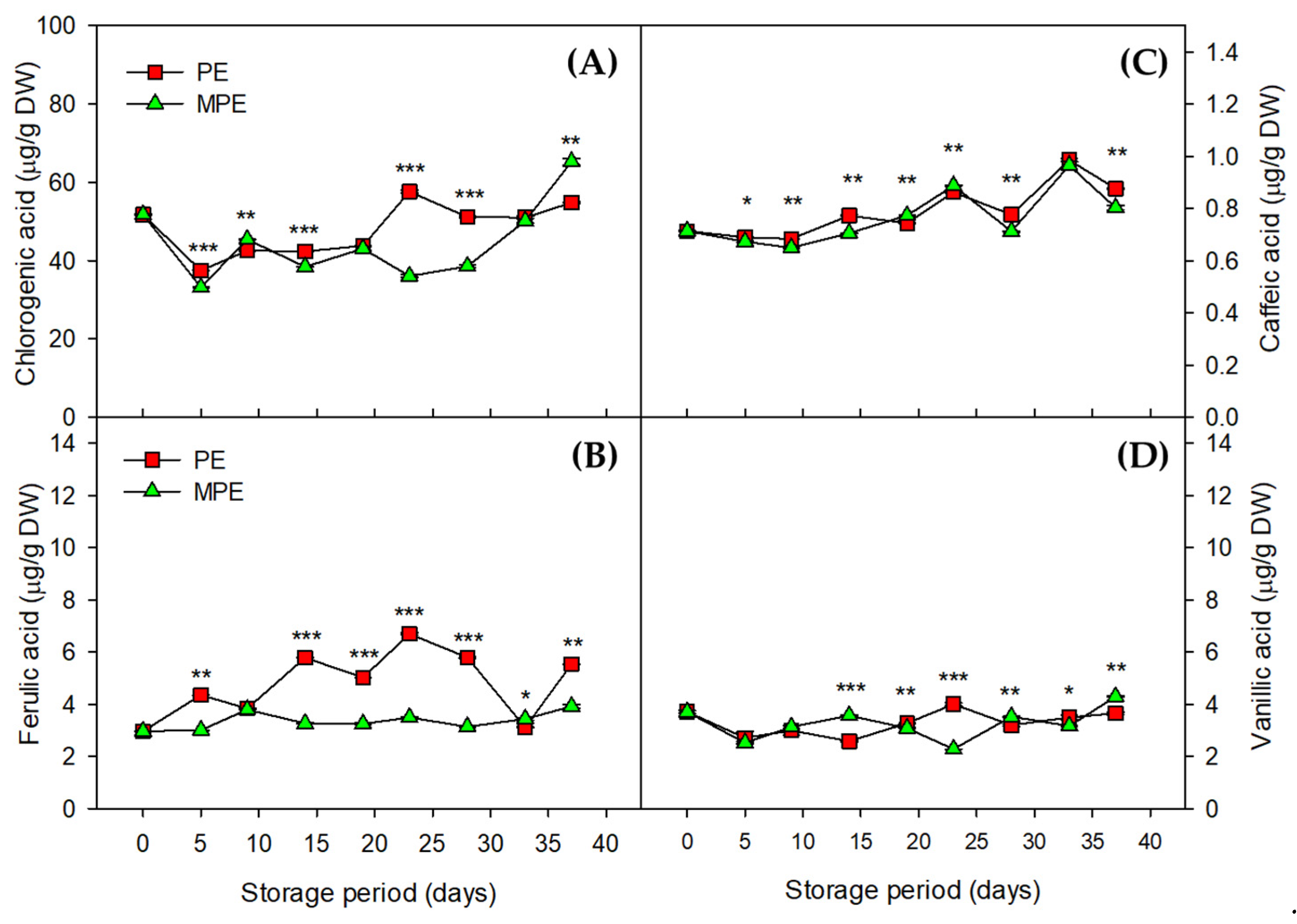
3.3. Changes in Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babich, O.; Dyshlyuk, L.; Sukhikh, S.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Chaplygina, T.; Kriger, O. Effects of biopreservatives combined with modified atmosphere packaging on the quality of apples and tomatoes. Pol. J. Food Nutr. Sci. 2019, 69, 289–296. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; University of California, Agriculture and Natural Resources: Los Angeles, CA, USA, 2002. [Google Scholar]
- Hong, S.J.; Yeoung, Y.R.; Eum, H.L. Phytochemical composition of everbearing strawberries and storage quality of strawberry fruit treated by precooling. Food Sci. Biotechnol. 2018, 27, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.R.; Panda, M.K.; Bal, L.M.; Pal, U.S.; Sahoo, D. Comparative study of MAP and shrink wrap packaging techniques for shelf life extension of fresh guava. Sci. Hortic. 2015, 182, 1–7. [Google Scholar] [CrossRef]
- Rai, D.R.; Paul, S. Packaging requirements of highly respiring produce under modified atmosphere packaging: A review. J. Food Sci. Technol. 2007, 44, 10–15. [Google Scholar]
- Toivonen, P.M.A. Effects of storage conditions and postharvest procedures on oxidative stress in fruits and vegetables. In Postharvest Oxidative Stress in Horticultural Crops; Hodges, D.M., Ed.; Food Products Press: New York, NY, USA, 2003; pp. 69–90. [Google Scholar]
- Meitha, K.; Pramestim, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Yan, J.Q.; Wang, J.; Tissue, D.; Holiday, A.S.; Allen, R.; Zhang, H. Photosynthesis and seed production under water-deficit condition in transgenic tobacco plants that over-express an Arabidopsis ascorbate peroxidase gene. Crop Sci. 2003, 43, 1477–1483. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. Changes in antioxidant activity and postharvest quality of sweet pomegranates cv. Hicrannar under modified atmosphere packaging. Postharvest Biol. Technol. 2014, 92, 29–36. [Google Scholar] [CrossRef]
- Kader, A.A.; Zagory, D.; Kerbel, E.L. Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1989, 28, 1–30. [Google Scholar] [CrossRef]
- Islam, M.Z.; Mele, M.A.; Lee, H.J.; Lee, K.S.; Hong, S.M.; Jeong, M.J.; Kim, I.-S.; Hong, S.-K.; Choi, I.-L.; Kang, H.M. Selection of non-perforated breathable film to enhance storability of cherry tomato for modified atmosphere storage at different temperatures. J. Bio-Environ. Control 2014, 23, 116–122. [Google Scholar] [CrossRef]
- Islam, M.Z.; Lee, Y.T.; Mele, M.A.; Choi, I.-L.; Jang, D.C.; Ko, Y.W.; Kim, Y.D.; Kang, H.M. Effect of modified atmosphere packaging on quality and shelf life of baby leaf lettuce. Qual. Assur. Saf. Crop. Foods 2019, 11, 749–756. [Google Scholar] [CrossRef]
- Lester, G.E. Oxidative stress affecting fruit senescence. In Postharvest Oxidative Stress in Horticultural Crops; Hodges, D.M., Ed.; Food Products Press: New York, NY, USA, 2003; pp. 113–129. [Google Scholar]
- Kwon, S.M.; Jeong, H.W.; Lee, H.R.; Jo, H.G.; Hwang, H.S.; Hwang, S.J. Growth and bioactive compound contents of Glehnia littoralis Fr. Schmidt ex Miquel as affected by different NaCl concentrations and treatment timing on nutrient solution of hydroponic system. J. Bio-Environ. Control 2021, 30, 101–109. [Google Scholar] [CrossRef]
- Lee, B.K.; Han, M.S.; Jung, Y.K.; Rha, E.S.; Yun, S.J.; Yoo, N.H. Comparative effect of plant growth regulators on callus induction and plant regeneration in Glehnia littoralis Schmidt et Miquel. Korean J. Plant Res. 2004, 17, 153–160. [Google Scholar]
- Jang, J.H.; Shin, H.I.; Park, J.S. Analysis of growth and functional substance for Cyperus rotundus and Glehnia littoralis by EC treatment in reclaimed soil conditions. J. Bio-Environ. Control 2019, 28, 411–419. [Google Scholar] [CrossRef]
- Hong, H.; dela Cruz, J.F.; Kim, W.S.; Yoo, K.; Hwang, S.G. Glehnia littoralis root extract inhibits fat accumulation in 3T3-L1 cells and high-fat diet-induced obese mice by down regulating adipogenic gene expression. Evid. Based Complement. Altern. Med. 2018, 2018, 1243049. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, A.R.; Ellis, S.M.; Hancock, R.E.W.; Towers, G.H.N. Antifungal screening of medicinal plants of British Columbian native peoples. J. Ethnopharmacol. 1994, 44, 157–169. [Google Scholar] [CrossRef]
- Yuan, Z.; Tezuka, Y.; Fan, W.; Kadota, S.; Li, X. Constituents of the underground parts of Glehnia littoralis. Chem. Pharm. Bull. 2002, 50, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crop Prod. 2016, 82, 9–12. [Google Scholar] [CrossRef] [Green Version]
- González-Romero, J.; Arranz-Arranz, S.; Verardo, V.; García-Villanova, B.; Guerra-Hernández, E.J. Bioactive compounds and antioxidant capacity of Moringa leaves grown in Spain versus 28 leaves commonly consumed in pre-packaged salads. Processes 2020, 8, 1297. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Bammou, M.; Hmidani, A.; Sellam, K.; Alem, C. Bioactive compounds and antioxidant, antiperoxidative, andantihemolytic properties investigation of three Apiaceae species grown in the southeast of Morocco. Scientifica 2020, 2020, 3971041. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int. J. Biol. Macromol. 2007, 41, 42–48. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.; Sunpapao, A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Campos, P.S.; Quartin, V.; Ramalho, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Lim, S.; Kim, J.; Oh, M. Heat shock treatments induce the accumulation of phytochemicals in kale sprouts. J. Hortic. Sci. Technol. 2012, 30, 509–518. [Google Scholar]
- Kaniuga, Z.; Michalski, W. Photosynthetic apparatus in chilling-sensitive plants. II. Changes in free fatty acid composition and photoperoxidation in chloroplasts following cold storage and illumination of leaves in relation to hill reaction activity. Planta 1978, 140, 129–136. [Google Scholar] [CrossRef]
- Dipierro, S.; Leonardis, S.D. The ascorbate system and lipid peroxidation in stored potato (Solanum tuberosum L.) tubers. J. Exp Bot. 1997, 48, 779–783. [Google Scholar] [CrossRef]
- Hong, S.J.; Park, N.I.; Kim, B.S.; Eum, H.L. Postharvest application of 1-MCP to maintain quality during storage on kimchi cabbage ‘Choongwang’. Hortic. Sci. Technol. 2018, 36, 215–223. [Google Scholar]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water loss of fresh fruit: Influencing preharvest, harvest and Postharvest factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Pongprasert, N.; Sekozawa, Y.; Sugaya, S.; Gemma, H. A novel postharvest UV-C treatment to reduce chilling injury (membrane damage, browning and chlorophyll degradation) in banana peel. Sci. Hortic. 2011, 130, 73–77. [Google Scholar] [CrossRef]
- Shao, H.B.; Liang, Z.S.; Shao, M.A.; Wang, B.C. Changes of anti-oxidative enzymes and membrane peroxidation for soil water deficits among 10 wheat genotypes at seedling stage. Colloids Surf. B 2005, 42, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M.A. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Barth, M.M.; Zhuang, H. Packaging design affects antioxidant vitamin retention and quality of broccoli florets during postharvest storage. Postharvest Biol. Technol. 1996, 9, 141–150. [Google Scholar] [CrossRef]
- Fukushima, T.; Yamazaki, M.; Tsugiyama, T. Chilling injury in cucumber fruits. I. Effects of storage temperature on symptoms and physiological changes. Sci. Hortic. 1977, 6, 185–197. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2002, 15, e0244365. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Incrocci, L.; Maggini, R.; Serra, G.; Tognoni, F. Colour changes of fresh-cut leafy vegetables during storage. J. Food Agric. Environ. 2004, 2, 40–44. [Google Scholar]
- Manolopoulou, E.; Varzakas, T. Effect of temperature in color change of green vegetables. Curr. Res. Nutr. Food Sci. 2016, 4, 10–17. [Google Scholar] [CrossRef]
- Taylor, A.O.; Graig, A.S. Plants under climatic stress, II. Low temperature, high light effects on chloroplast ultrastructure. Plant Physiol. 1971, 47, 719–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Eum, H.L.; Yi, T.G.; Hong, S.J.; Park, N. Variations of bioactive compound contents and antioxidant capacity of Asparagus seedlings in 23 varieties. Hortic. Sci. Technol. 2020, 38, 291–302. [Google Scholar]
- Przeor, M.; Flaczyk, E.; Kmiecik, D.; Buchowski, M.S.; Staniek, H.; Tomczak-Graczyk, A.; Kobus-Cisowska, J.; Gramza-Michałowska, A.; Foksowicz-Flaczyk, J. Functional Properties and Antioxidant Activity of Morus alba L. Leaves var. Zolwinska Wielkolistna (WML-P)—The Effect of Controlled Conditioning Process. Antioxidants 2020, 9, 668. [Google Scholar] [CrossRef]
- Hiraoka, N.; Chang, J.I.; Bohm, L.R.; Bohm, B.A. Furanocoumarin and polyacetylenic compound composition of wild Glehnia littoralis in North America. Biochem. Syst. Ecol. 2002, 30, 321–325. [Google Scholar] [CrossRef]
- Yang, W.; Ye, M.; Liu, M.; Kong, D.; Shi, R.; Shi, X.; Zhang, K.; Wang, Q.; Lantong, Z. A practical strategy for the characterization of coumarins in Radix Glehniae by liquid chromatography coupled with triple quadrupole-linear ion trap mass spectrometry. J. Chromatogr. A 2010, 1217, 4587–4600. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eum, H.-L.; Choi, M.-H.; Park, M.-H.; Lee, J.-S.; Chang, M.-S. Influence of MAP on the Postharvest Quality of Glehnia littoralis Fr. Schmidt ex Miq. Processes 2021, 9, 2052. https://doi.org/10.3390/pr9112052
Eum H-L, Choi M-H, Park M-H, Lee J-S, Chang M-S. Influence of MAP on the Postharvest Quality of Glehnia littoralis Fr. Schmidt ex Miq. Processes. 2021; 9(11):2052. https://doi.org/10.3390/pr9112052
Chicago/Turabian StyleEum, Hyang-Lan, Mi-Hee Choi, Me-Hea Park, Jung-Soo Lee, and Min-Sun Chang. 2021. "Influence of MAP on the Postharvest Quality of Glehnia littoralis Fr. Schmidt ex Miq." Processes 9, no. 11: 2052. https://doi.org/10.3390/pr9112052
APA StyleEum, H.-L., Choi, M.-H., Park, M.-H., Lee, J.-S., & Chang, M.-S. (2021). Influence of MAP on the Postharvest Quality of Glehnia littoralis Fr. Schmidt ex Miq. Processes, 9(11), 2052. https://doi.org/10.3390/pr9112052






