Investigation of the Process of Simple Distillation in Irrigated Pipe Elements
Abstract
:1. Introduction
- The method of coupled physical and mathematical modeling based on the variational formulation of conservation laws [9].
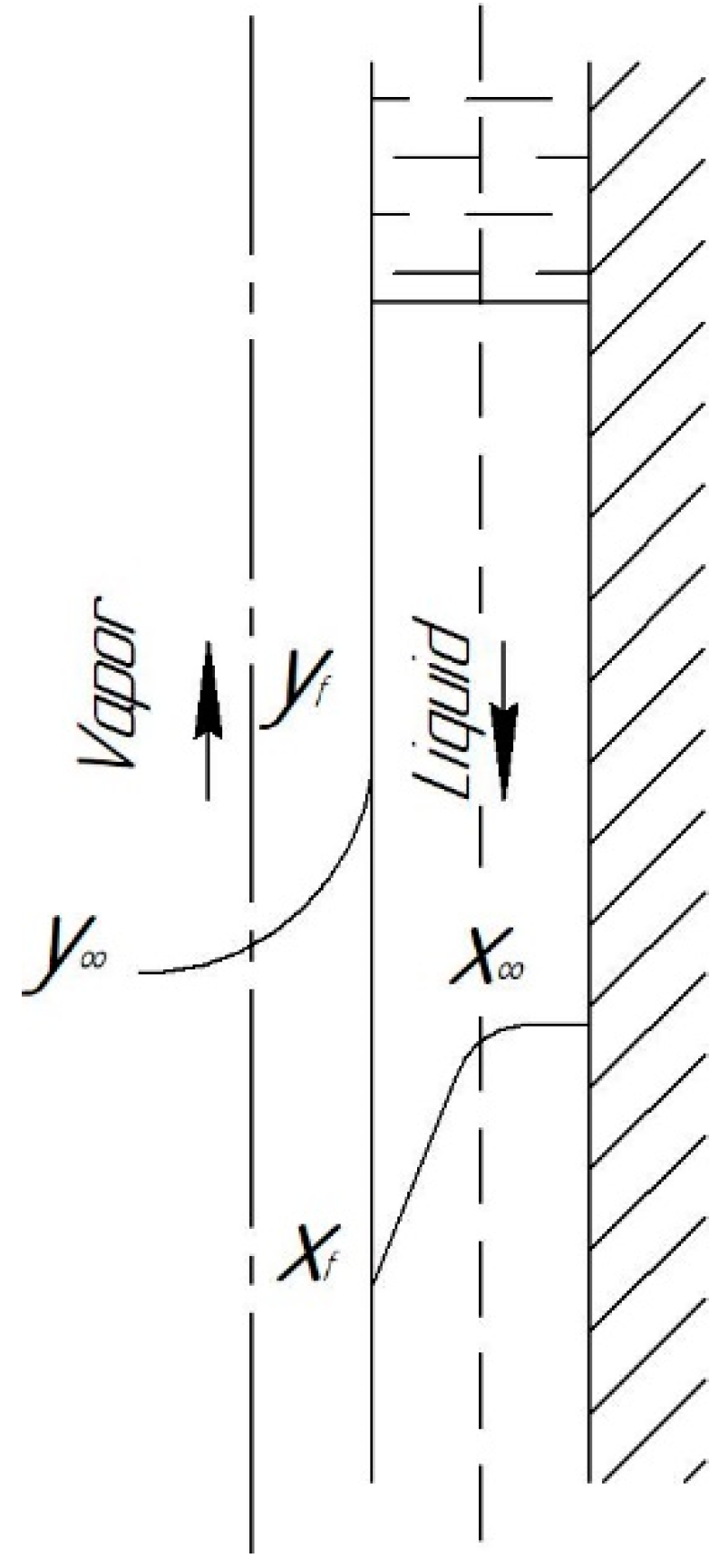
2. Mass Transfer in the Film
2.1. For the Gas (Vapor) Phase
2.2. For the Liquid Phase
- Laminar wave-free mode (). In this mode, the stability of the film flow and the very possibility of its existence is easily violated. Therefore, in this mode, the mass flow of any component was calculated without taking into account the diffusion flow (only by the convective component of the total flow).
- Laminar wave modes ()—V. A. Malyusov’s equation was used [28]:
- The turbulent mode () is represented by the equation:which was obtained by the generalization of experimental data on rectification in a film column [29].
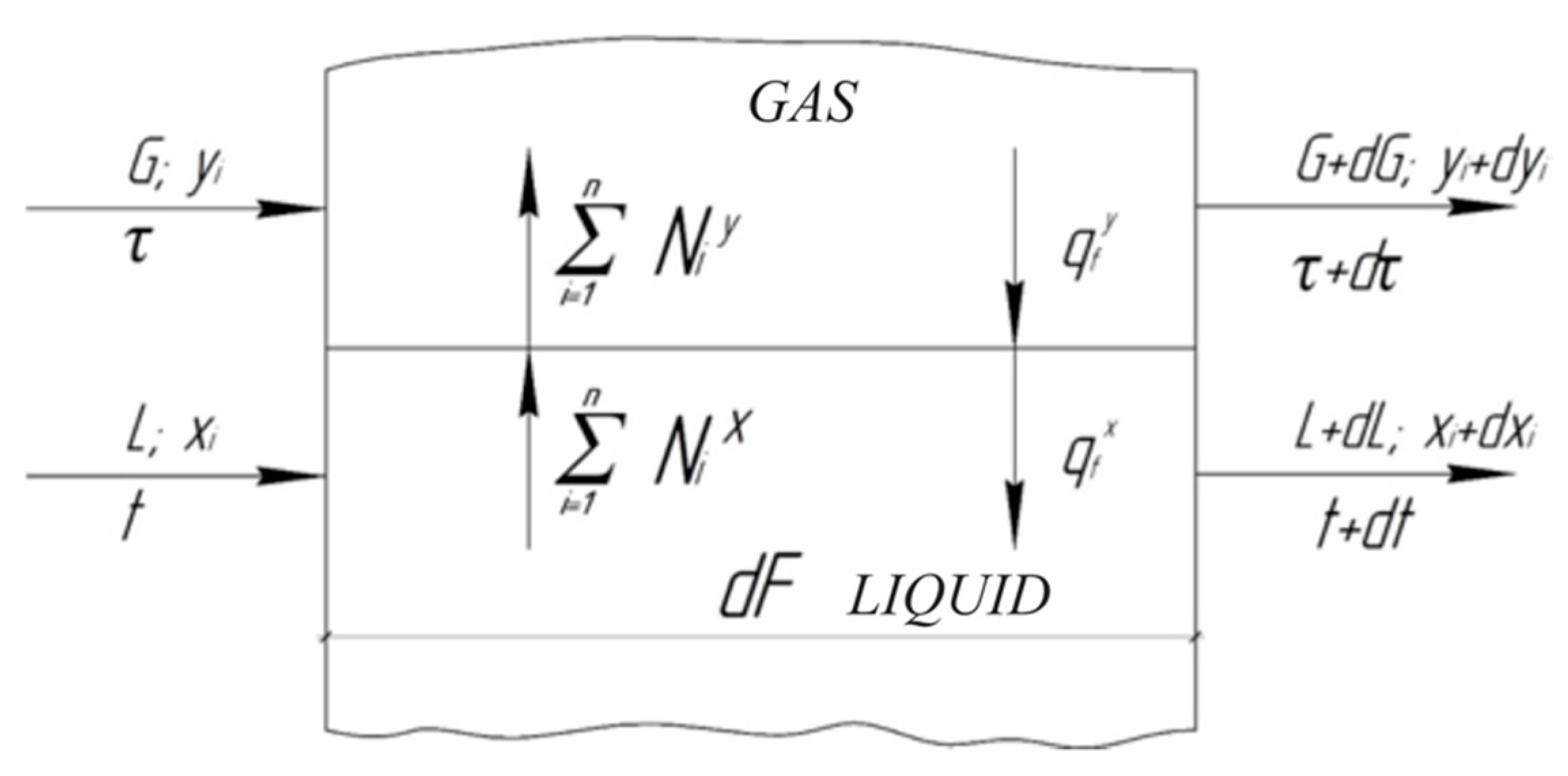
3. Mass Transfer in Irrigated Pipe Elements
3.1. Development of an Algorithm for Calculating Mass Transfer in Irrigated Pipe Elements
3.2. Experimental Study of the Simple Distillation Process
- The laminar flow regime of the gas phase is considered;
- The transverse mass flow () is constant over the entire height of the pipe;
- The equilibrium dependence is linear ;
- A linear profile of concentrations along the pipe height is adopted.
- The composition of the distillate was determined by the analytical expression (35) proposed in the work of Yu. I. Kallas [24]—model No. 1;
- The distillate composition was determined by numerical integration of the system of Equations (17)–(26) according to a specially developed iterative algorithm (Figure 4)—model No. 2;
- The distillate composition was determined by the analytical expression (39)—model No. 3.
4. Results and Discussion
5. Conclusions
5.1. Conventional Designations
- H—the height of the device, or the pipe section, m;
- B*—elements of the square matrix of coefficients of multicomponent equimolar mass transfer, m3/(m2 s); kg-mol/(m2 s);
- D—binary diffusion coefficients, m2/s;
- d—the diameter of the device, or the pipe section, m;
- F, G, L—power consumption, steam, liquid phases, respectively, m3/s; kg-mol/s;
- g—acceleration due to gravity, m/s2;
- idem—the property of an object or operation, when reapplying the operation to the object, give the same result as when the first
- k—the number of components in a multicomponent mixture;
- K—mass transfer coefficient, m3/(m2 s), kg-mol/(m2 s);
- L—length of the device (pipe), m;
- m and b—coefficients of the linearized equilibrium curve;
- —substance stream, m3/(m2 s), kg-mol/(m2 s);
- P—pressure, Pa;
- p—concentration parameter [24];
- q—substance stream, m3/(m2 s), kg-mol/(m2 s);
- R—pipe radius, m;
- S—phase separation surface, m2;
- t—temperature, K;
- τ, ˦—time, s;
- Ψ—pseudo-concentrations of the linearized equation of multicomponent mass transfer, respectively;
- y and x—molar concentrations of gas and liquid, mol. fraction;
- —average volume molar concentrations of gas and liquid, mol. fraction;
- r and z—the transverse and longitudinal coordinates, respectively;
- δ—the thickness of the liquid film, m;
- —coefficient of turbulent diffusion, m2/s;
- —the standard deviation between the calculated and experimental values;
- [] and 〈 〉—square and column (vector—column) matrices, respectively;
- —the Reynolds criterion;
- —the Sherwood criterion;
- —the Schmidt criterion.
5.2. Indexes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stage, H.; Schultze, G.R. The fundamental work on theory, apparatus, as well as procedure of distillation and rectification. Chem. Ztg 1970, 94, 271–286. [Google Scholar]
- Olevsky, V.M. Film heat and mass transfer equipment. Chemistry 1988, 195. [Google Scholar]
- Kallas, Y.I. Investigation of the Process of Continuous Distillation in a Film-Type Apparatus; Candidate of Technical Science Tallinn Polytechnic Institute: Tallin, Estonia, 1972. [Google Scholar]
- Kallas, Y.I. Theory and Methods of Calculation of Distillation with Water Vapor: Doctor of Technical Sciences; Tallinn Polytechnic Institute: Tallinn, Estonia, 1985. [Google Scholar]
- Ham, P.; Bun, S.; Painmanakul, P.; Wongwailikhit, K. Effective Analysis of Different Gas Diffusers on Bubble Hydrodynamics in Bubble Column and Airlift Reactors towards Mass Transfer Enhancement. Processes 2021, 9, 1765. [Google Scholar] [CrossRef]
- Nikeshin, V.V.; Burmistrov, D.A.; Klinov, A.V. Determination of mass transfer coefficients in steam and liquid phases during rectification in packing columns. Bull. Kaz. Nat. Res. Technol. Univ. 2011, 24, 34–38. [Google Scholar]
- Nikeshin, V.V.; Burmistrov, D.A.; Klinov, A.V. Extraction of mass transfer coefficients from experimental data on profiles of component concentration by column height. Bull. Kaz. Nat. Res. Technol. Univ. 2012, 15, 93–95. [Google Scholar]
- Nikeshin, V.V.; Klinov, A.V.; Razinov, A.I.; Nikeshina, Y.M. Description of the diffusion of liquid mixtures ethanol water, methanol water. Bull. Kaz. Nat. Res. Technol. Univ. 2014, 17, 160–161. [Google Scholar]
- Diakonov, S.G.; Elizarov, V.I.; Laptev, A.G. Theoretical Foundations and Modeling of Processes of Separation of Substances, 1st ed.; Kazan University Publishing House: Kazan, Russian, 1993; p. 437. [Google Scholar]
- Minkin, V.I.; Simkin, B.Y.; Minyaev, R.M. Theory of the structure of molecules. High. Sch. 1979, 1, 407. [Google Scholar]
- Linek, V.; Moucha, T.; Prokopova, E.; Rejl, J.F. Simultaneous determination of vapor and liquid-side volumetric mass transfer coefficients in a distillation column. Chem. Eng. Res. Des. 2005, 83, 979–986. [Google Scholar] [CrossRef]
- Dyakonov, G.S.; Dinmukhametova, R.A.; Klinov, A.V.; Dyakonov, S.G. Algorithm of prototyping devices for separation of substances. Bull. Kaz. Nat. Res. Technol. Univ. 2013, 16, 186–189. [Google Scholar]
- Serafimov, L.A.; Timoshenko, A.V. Equation of mass transfer in multicomponent mixtures Timoshenko. Theor. Fund. Chem. Technol. 2005, 39, 337–343. [Google Scholar]
- Nasr, A.; Debbissi, C.; Nasrallah, B.S. Numerical study of evaporation by mixed convection of binary liquid film. Energy 2011, 36, 2316–2327. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Moulijn, J.A. Process intensification: Transforming chemical engineering. Chem. Eng. Prog. 2000, 96, 22–34. [Google Scholar]
- Costa, V.A.F. Transient natural convection in enclosures filled with humid air, including wall evaporation and condensation. Heat Mass Transfer. 2012, 55, 5479–5494. [Google Scholar] [CrossRef]
- Sadeghifar, H.A.; Kordi, A.A. new and applicable method to calculate mass and heat transfer coefficients abd efficiency of industrial distillation columns containing structured packings. Energy 2011, 36, 1415. [Google Scholar] [CrossRef]
- Huang, J.; Guo, R.; Tao, L.; Wang, Q.; Liu, Z.; Zhang, S.; Sun, J. Mass transfer coefficient and effective internal diffusion coefficient for coke solution loss reaction with non-equimolar diffusion. Fuel 2020, 278, 118225. [Google Scholar] [CrossRef]
- Huang, J.; Guo, R.; Wang, Q.; Liu, Z.; Zhang, S.; Sun, J. Coke solution-loss degradation model with non-equimolar diffusion and changing local pore structure. Fuel 2020, 263, 116694. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhao, L.; Liu, S.; Gao, J.; Xu, C.; Ma, M.; Meng, Q. Modeling, simulation, and optimization for producing ultra-low sulfur and high-octane number gasoline by separation and conversion of fluid catalytic cracking naphtha. Fuel 2021, 299, 1–12. [Google Scholar] [CrossRef]
- Ponikarov, A.S. Influence of mass flow transfer on velocity profile formation. J. Phys. Conf. Ser. 2020, 971, 052023. [Google Scholar] [CrossRef]
- Telyakov, E.S.; Osipova, L.E.; Ponikarov, A.S. Kinetics of Nonequimolar Mass Transfer in Multicomponent Gas (Vapor)—Liquid Systems. Theor. Found. Chem. Eng. 2018, 52, 11–23. [Google Scholar] [CrossRef]
- Telyakov, E.S.; Osipova, L.E.; Ponikarov, A.S. Nonequimolar mass transfer in gas (vapor)-liquid systems. Theor. Found. Chem. Eng. Nauka, Moskow. 2015, 49, 261–270. [Google Scholar] [CrossRef]
- Alexandrov, I.A. Rectification and Absorption Devices, 3rd ed.; Chemistry; 1978; p. 280. [Google Scholar]
- Kallas, Y.I.; Siirde, E.K. The simple distillation process in a film apparatus. Theor. Found. Chem. Eng. 1974, 8, 264–268. [Google Scholar]
- Konstantinov, E.N.; Kuznechikov, V.A. Mathematical model of turbulent mass transfer in a multicomponent mixture. Theor. Found. Chem. Technol. 1975, 9, 149–154. [Google Scholar]
- Ramm, V.M. Absorption of gases. Chemistry 1971, 767. [Google Scholar]
- Kvashnin, S.Y.; Baklachyan, R.A.; Lotkhov, V.A.; Kulov, V.A.; Malyusov, V.A. Kinetics of rectification of binary systems in film-type columns. Theor. Found. Chem. Technol. 2003, 37, 265–270. [Google Scholar]
- Telyakov, E.S.; Nikolaev, A.M. A method for determining the partial heights of transfer units in the process of rectification. Theor. Found. Chem. Technol. 1969, 3, 826–830. [Google Scholar]
- Osipov, E.; Telyakov, E.; Ponikarov, S. Coupled Simulation of a Vacuum Creation System and a Rectification Column Block. Processes 2020, 8, 1333. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Z.; Lu, H.; Yang, L. Experimental Study of the Convective Heat Transfer and Local Thermal Equilibrium in Ceramic Foam. Processes 2020, 8, 1490. [Google Scholar] [CrossRef]


| Series No. | Separated Mixture | Composition of the Initial Mixture, Mol. Fraction | Relative Distillate Selection, (D/F) | Root-Mean-Square Error | ||
|---|---|---|---|---|---|---|
| Calculation by Models | ||||||
| 1 | 2 | 3 | ||||
| 1 | Benzene—Toluene | 0.807 | 0.023–0.497 | 0.0008 | 0.0005 | 0.0027 |
| 2 | 0.615 | 0.0094 | 0.0048 | 0.0070 | ||
| 3 | 0.435 | 0.0092 | 0.0059 | 0.0119 | ||
| 4 | 0.131 | 0.0081 | 0.0023 | 0.0097 | ||
| 5 | N. hexane—N. heptane | 0.725 | 0.047–0.754 | 0.0270 | 0.0164 | 0.0173 |
| 6 | 0.804 | 0.0233 | 0.0160 | 0.0356 | ||
| 7 | Ethanol—water | 0.350 | 0.012–0.144 | 0.0210 | 0.0168 | 0.0178 |
| 8 | H. Hexane-benzene | 0.164 | 0.041–0.620 | 0.0101 | 0.0068 | 0.0133 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponikarov, A.S.; Ponikarov, S.I.; Osipov, E.V. Investigation of the Process of Simple Distillation in Irrigated Pipe Elements. Processes 2021, 9, 2047. https://doi.org/10.3390/pr9112047
Ponikarov AS, Ponikarov SI, Osipov EV. Investigation of the Process of Simple Distillation in Irrigated Pipe Elements. Processes. 2021; 9(11):2047. https://doi.org/10.3390/pr9112047
Chicago/Turabian StylePonikarov, Artem Sergeevich, Sergey Ivanovich Ponikarov, and Eduard Vladislavovich Osipov. 2021. "Investigation of the Process of Simple Distillation in Irrigated Pipe Elements" Processes 9, no. 11: 2047. https://doi.org/10.3390/pr9112047
APA StylePonikarov, A. S., Ponikarov, S. I., & Osipov, E. V. (2021). Investigation of the Process of Simple Distillation in Irrigated Pipe Elements. Processes, 9(11), 2047. https://doi.org/10.3390/pr9112047






