Process Intensification in Photocatalytic Decomposition of Formic Acid over a TiO2 Catalyst by Forced Periodic Modulation of Concentration, Temperature, Flowrate and Light Intensity
Abstract
1. Introduction
2. Kinetic Model of Formic Acid Decomposition over a Titania Photocatalyst
3. Non-Stationary CSTR Model
4. The NFR Method for Evaluating Forced Periodic Operations
5. Application of the NFR Method for Evaluating Potential Forced Periodic Operations of a Photocatalytic Reactor for Formic Acid Decomposition
5.1. Modulated Inputs and Outputs of Interest and Performance Criteria
5.2. Possible Periodic Operations with One or Two Modulated Inputs
- Case 1: Periodic operation of the volumetric flowrate of the feed stream;
- Case 2: Periodic operation of the feed molar fraction of formic acid;
- Case 3: Periodic operation of the reactor temperature;
- Case 4: Periodic operation of the light intensity.
- Case 5: Modulation of the acid flowrate and the acid molar fraction;
- Case 6: Modulation of the acid molar fraction and temperature;
- Case 7: Modulation of the temperature and the acid flowrate;
- Case 8: Modulation of the acid molar fraction and the light intensity;
- Case 9: Modulation of the temperature and the light intensity;
- Case 10: Modulation of the light intensity and the acid flowrate.
5.3. Frequency Response Functions for Evaluating Periodic Operations
- Sixteen first order FRFs relating each output to each input;
- Sixteen asymmetrical second order FRFs relating each output to each input;
- Twenty-four cross asymmetrical second order FRFs relating each output to each combination of two inputs defined in Case 5 to Case 10.
5.4. Performance Indicators
- For co-sinusoidal modulation of one input (x) with frequency ω and amplitude Ax (Cases 1–4)
- For simultaneous co-sinusoidal modulations of two inputs (x and z) with frequency ω, amplitudes Ax and Az and phase difference φ (Cases 5–10)
6. NFR Analysis for Different Cases
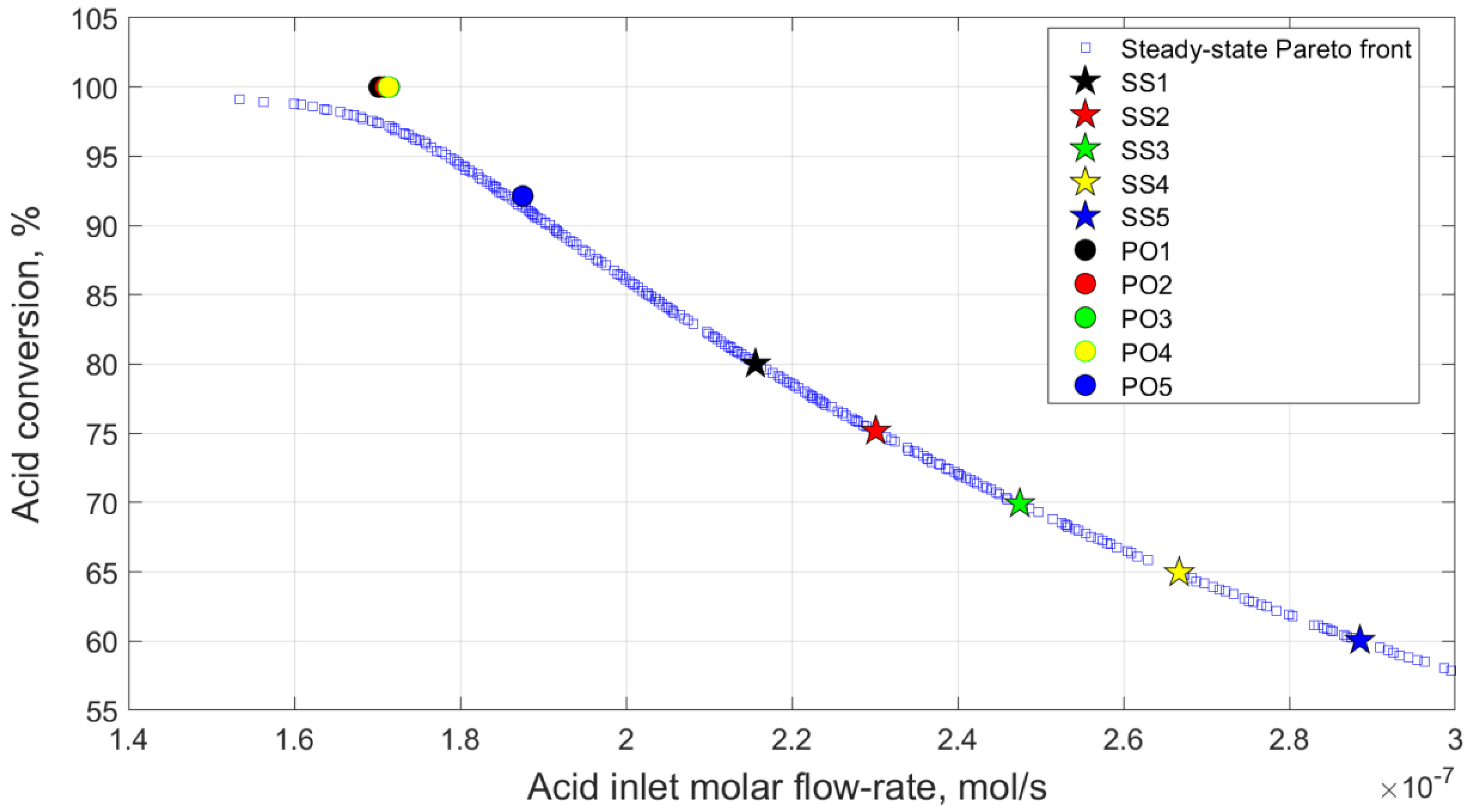
6.1. Selection of the Steady-State Points for Analysis
6.2. Evaluation of Periodic Modulations with a Single Input
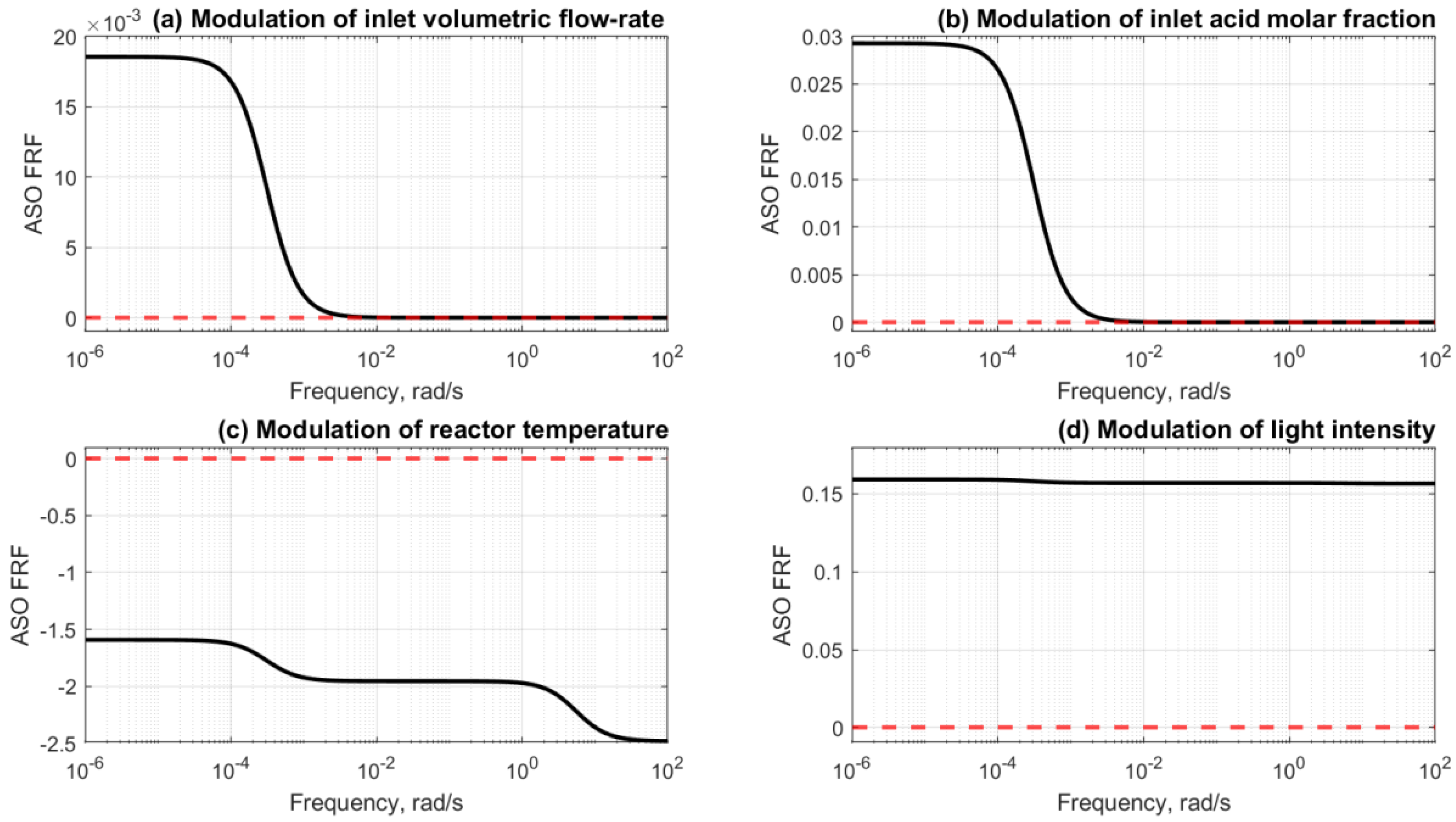
6.3. Periodic Modulation of Two Input Parameters
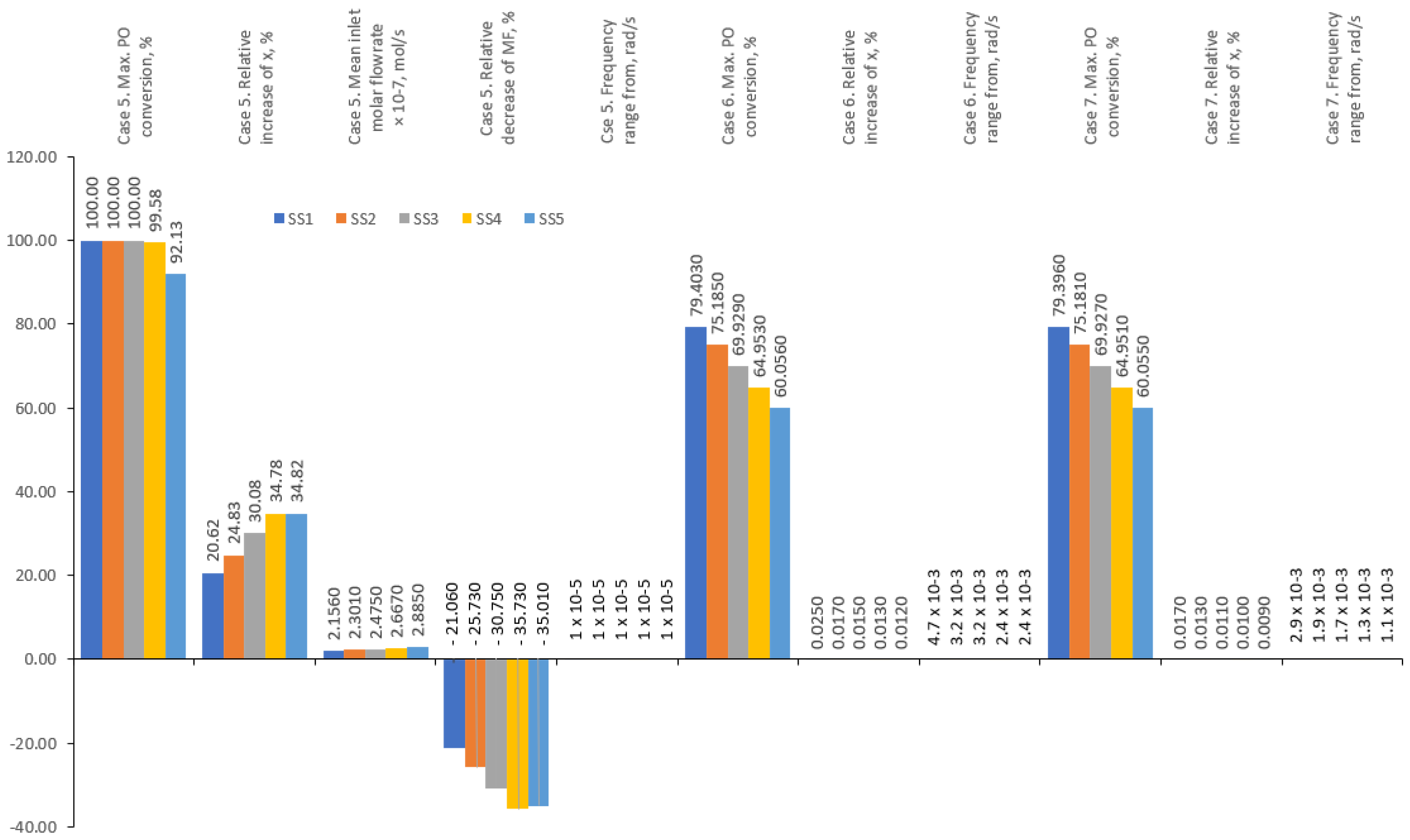
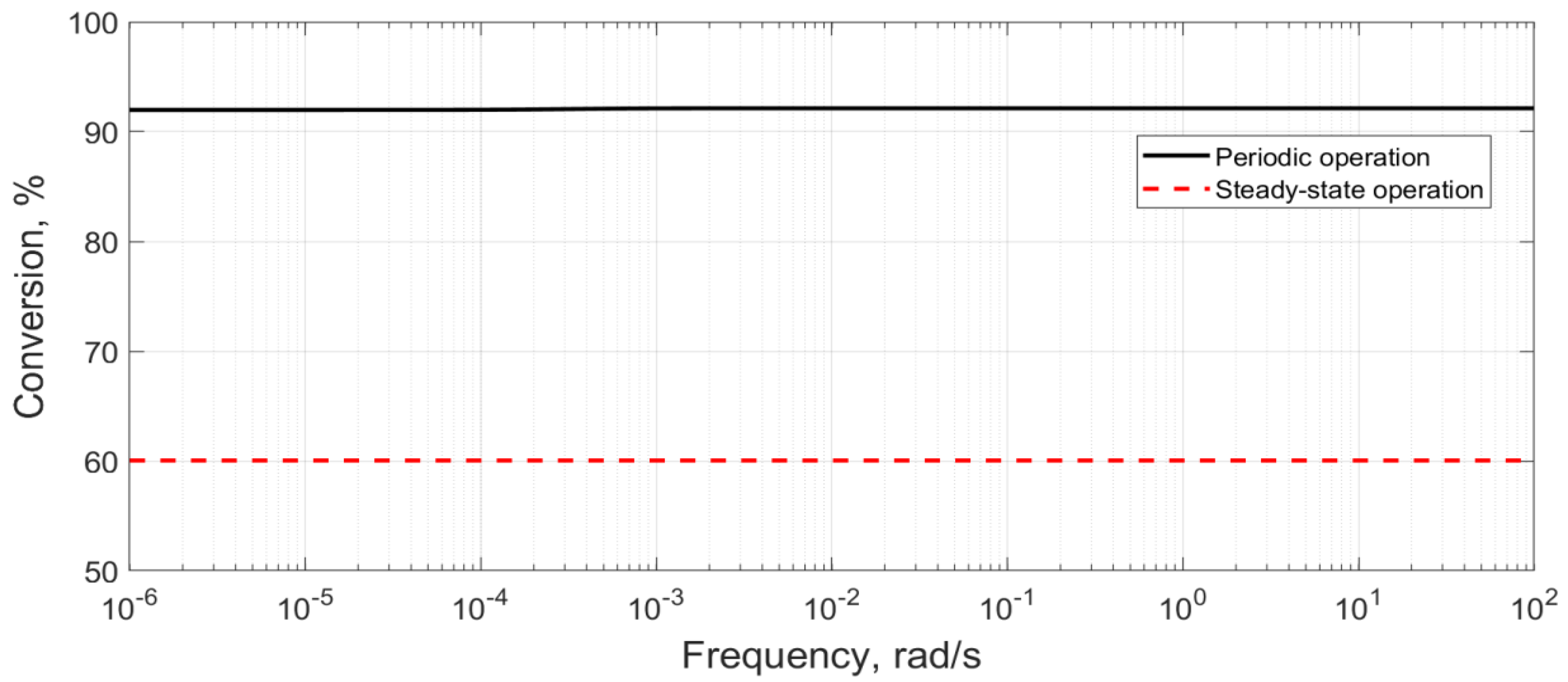
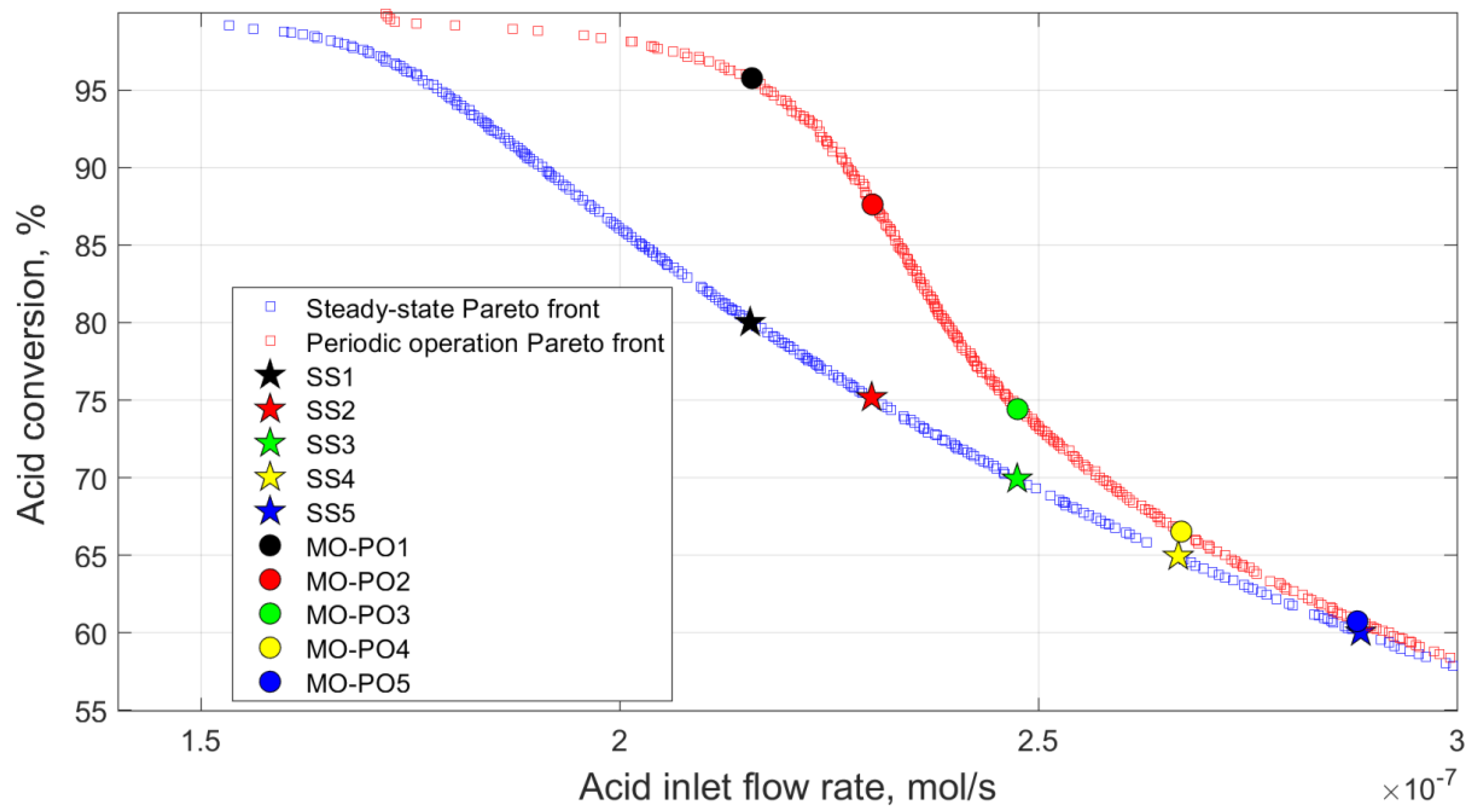
6.4. Multi-Objective Optimisation for Case 5
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| ABET | Specific surface area | Greek Letters | |
| Ax | Amplitude of periodic modulation | Reciprocal absorption length | |
| Concentration | Heating rate | ||
| Volumetric flowrate | Surface coverage | ||
| G | Frequency response function | Density | |
| ΔH | Enthalpy | Phase difference | |
| I | Intensity of light | Frequency of periodic modulation | |
| Kinetic constant | Subscripts | ||
| Reaction rate | |||
| Mass | A | Formic acid | |
| Mass flowrate | C, H | CO2, H2 | |
| Molar mass | N | Nitrogen | |
| Avogadro number | ads | Adsorption | |
| Concentration of catalyst sites | des | Desorption | |
| Pressure | in | Inlet | |
| Ideal gas constant | max | Maximum | |
| Temperature | opt | Optimal phase difference | |
| Time | out | Outlet | |
| Reactor volume | R | Reactor | |
| Input function | 2 | Step 2. Photochemical reaction | |
| Output function | tot | Total | |
| DC component of the output function | x | First input parameter | |
| Molar fraction | z | Second input parameter | |
Appendix A
References
- Lanzafame, P.; Abate, S.; Ampelli, C.; Genovese, C.; Passalacqua, R.; Centi, G.; Perathoner, S. Beyond Solar Fuels: Renewable Energy-Driven Chemistry. ChemSusChem 2017, 10, 4409–4419. [Google Scholar] [CrossRef] [PubMed]
- Abiev, R.S.; Sladkovskiy, D.A.; Semikin, K.V.; Murzin, D.Y.; Rebrov, E.V. Non-Thermal Plasma for Process and Energy Intensification in Dry Reforming of Methane. Catalysts 2020, 10, 1358. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology-a novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Probing paramagnetic species in titania-based heterogeneous photocatalysis by electron spin resonance (ESR) spectroscopy—A mini review. Chem. Eng. J. 2010, 170, 353–362. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Ismagilov, Z.; Matus, E.; Yakutova, A.; Protasova, L.; Ismagilov, I.; Kerzhentsev, M.; Rebrov, E.; Schouten, J. Design of Pt–Sn catalysts on mesoporous titania films for microreactor application. Catal. Today 2009, 147, S81–S86. [Google Scholar] [CrossRef]
- Protasova, L.N.; Rebrov, E.V.; Glazneva, T.S.; Berenguer-Murcia, A.; Ismagilov, Z.R.; Schouten, J.C. Control of the thickness of mesoporous titania films for application in multiphase catalytic microreactors. J. Catal. 2010, 271, 161–169. [Google Scholar] [CrossRef]
- Protasova, L.N.; Rebrov, E.V.; Skelton, H.E.; Wheatley, A.E.H.; Schouten, J.C. A kinetic study of the liquid-phase hydrogenation of citral on Au/TiO2 and Pt-Sn/TiO2 thin films in capillary microreactors. Appl. Catal. A Gen. 2011, 399, 12–21. [Google Scholar] [CrossRef]
- Liu, Y.; Cherkasov, N.; Gao, P.; Fernández, J.; Lees, M.R.; Rebrov, E.V. The enhancement of direct amide synthesis reaction rate over TiO2@SiO2@NiFe2O4 magnetic catalysts in the continuous flow under radiofrequency heating. J. Catal. 2017, 355, 120–130. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, P.; Cherkasov, N.; Rebrov, E.V. Direct amide synthesis over core-shell TiO2@NiFe2O4 catalysts in a continuous flow radiofrequency-heated reactor. RSC Adv. 2016, 6, 100997–101007. [Google Scholar] [CrossRef]
- Tang, J.; Liu, T.; Miao, S.; Cho, Y. Emerging energy harvesting technology for electro/photo-catalytic water splitting application. Catalysts 2021, 11, 142. [Google Scholar] [CrossRef]
- Chatterjee, S.; Houlding, T.K.; Doluda, V.; Molchanov, V.P.; Matveeva, V.G.; Rebrov, E.V. Thermal Behavior of a Catalytic Packed-Bed Milli-reactor Operated under Radio Frequency Heating. Ind. Eng. Chem. Res. 2017, 56, 13273–13280. [Google Scholar] [CrossRef]
- Zuyev, A.; Seidel-Morgenstern, A.; Benner, P. An isoperimetric optimal control problem for a non-isothermal chemical reactor with periodic inputs. Chem. Eng. Sci. 2017, 161, 206–214. [Google Scholar] [CrossRef][Green Version]
- Brzić, D.; Petkovska, M. A study of applicability of nonlinear frequency response method for investigation of gas adsorption based on numerical experiments. Ind. Eng. Chem. Res. 2013, 52, 16341–16351. [Google Scholar] [CrossRef]
- Petkovska, M.; Nikolić, D.; Seidel-Morgenstern, A. Nonlinear Frequency Response Method for Evaluating Forced Periodic Operations of Chemical Reactors. Isr. J. Chem. 2018, 58, 663–681. [Google Scholar] [CrossRef]
- Nikolić, D.; Seidel, C.; Felischak, M.; Miličić, T.; Kienle, A.; Seidel-Morgenstern, A.; Petkovska, M. Forced periodic operations of a chemical reactor for methanol synthesis—The search for the best scenario based on the nonlinear frequency response method. Part I Single input modulations. Chem. Eng. Sci. 2021, 248, 117134. [Google Scholar] [CrossRef]
- Eze, V.C.; Fisher, J.C.; Phan, A.N.; Harvey, A.P. Intensification of carboxylic acid esterification using a solid catalyst in a mesoscale oscillatory baffled reactor platform. Chem. Eng. J. 2017, 322, 205–214. [Google Scholar] [CrossRef]
- Abiev, R. Process Intensification by Pulsations in Chemical Engineering: Some General Principles and Implementation. Ind. Eng. Chem. Res. 2017, 56, 13497–13507. [Google Scholar] [CrossRef]
- Petkovska, M.; Seidel-Morgenstern, A.; Silveston, R. Evaluation of Periodic Processes. In Periodic Operation of Reactors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 387–413. [Google Scholar]
- Weiner, D.; Spina, J. Sinusoidal Analysis and Modeling of Weakly Nonlinear Circuits; Van Nostrand Reinhold Company: New York, NY, USA, 1980. [Google Scholar]
- Nikolic, D. Forced Periodically Operated Chemical Reactors-Evaluation and Analysis by the Nonlinear Frequency Response Method; University of Belgrade: Belgrade, Serbia, 2016. [Google Scholar]
- Marković, A.; Morgenstern, A.-S.; Petkovska, M. Evaluation of the potential of periodically operated reactors based on the second order frequency response function. Chem. Eng. Res. Des. 2008, 86, 682–691. [Google Scholar] [CrossRef]
- Currie, R.; Nikolic, D.; Petkovska, M.; Simakov, D.S.A. CO2 Conversion Enhancement in a Periodically Operated Sabatier Reactor: Nonlinear Frequency Response Analysis and Simulation-based Study. Isr. J. Chem. 2018, 58, 762–775. [Google Scholar] [CrossRef]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M.; Felischak, M.; Seidel-Morgenstern, A.; Petkovska, M. Periodic Operation with Modulation of Inlet Concentration and Flow Rate. Part I: Nonisothermal Continuous Stirred-Tank Reactor. Chem. Eng. Technol. 2016, 39, 2020–2028. [Google Scholar] [CrossRef]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Periodic Operation with Modulation of Inlet Concentration and Flow Rate. Part II: Adiabatic Continuous Stirred-Tank Reactor. Chem. Eng. Technol. 2016, 39, 2126–2134. [Google Scholar] [CrossRef]
- Felischak, M.; Kaps, L.; Hamel, C.; Nikolic, D.; Petkovska, M.; Seidel-Morgenstern, A. Analysis and experimental demonstration of forced periodic operation of an adiabatic stirred tank reactor: Simultaneous modulation of inlet concentration and total flow-rate. Chem. Eng. J. 2021, 410, 128197. [Google Scholar] [CrossRef]
- Živković, L.A.; Kandaswamy, S.; Petkovska, M.; Vidaković-Koch, T. Evaluation of Electrochemical Process Improvement Using the Computer-Aided Nonlinear Frequency Response Method: Oxygen Reduction Reaction in Alkaline Media. Front. Chem. 2020, 8, 579869. [Google Scholar] [CrossRef]
- Živković, L.A.; Vidakovic-Koch, T.; Petkovska, M. Computer-Aided Nonlinear Frequency Response Method for Investigating the Dynamics of Chemical Engineering Systems. Processes 2020, 8, 1354. [Google Scholar] [CrossRef]
- Nikolić, D.; Seidel, C.; Felischak, M.; Miličić, T.; Kienle, A.; Seidel-Morgenstern, A.; Petkovska, M. Forced periodic operations of a chemical reactor for methanol synthesis—The search for the best scenario based on the nonlinear frequency response method. Part II Simultaneous modulation of two inputs. Chem. Eng. Sci. 2022, 248, 117133. [Google Scholar]
- Paunic, D.N.; Petkovska, M. Evaluation of periodic processes with two modulated inputs based on nonlinear frequency response analysis. Case study: CSTR with modulation of the inlet concentration and flow-rate. Chem. Eng. Sci. 2013, 104, 208–219. [Google Scholar] [CrossRef]
- Nikolić, D.; Petkovska, M. Evaluation of Performance of Periodically Operated Reactors for Single Input Modulations of General Waveforms. Chem. Ing. Tech. 2016, 88, 1715–1722. [Google Scholar] [CrossRef]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operation of non-isothermal CSTR using single input modulations. Part II: Modulation of inlet temperature or temperature of the cooling/heating fluid. Chem. Eng. Sci. 2014, 117, 31–44. [Google Scholar] [CrossRef]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operation of non-isothermal CSTR using single input modulations. Part I: Modulation of inlet concentration or flow-rate. Chem. Eng. Sci. 2014, 117, 71–84. [Google Scholar] [CrossRef][Green Version]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operation of non-isothermal CSTR with simultaneous modulation of inlet concentration and inlet temperature. Chem. Eng. Sci. 2015, 137, 40–58. [Google Scholar] [CrossRef][Green Version]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operations with simultaneous modulation of two general waveform inputs with applications on adiabatic CSTR with square-wave modulations. Chem. Eng. Sci. 2020, 226, 115842. [Google Scholar] [CrossRef]
- Živković, L.A.; Milić, V.; Vidaković-koch, T.; Petkovska, M. Rapid multi-objective optimization of periodically operated processes based on the computer-aided nonlinear frequency response method. Processes 2020, 8, 1357. [Google Scholar] [CrossRef]
- Tang, Y.; Roberts, C.; Perkins, R.T.; Wachs, I.E. Revisiting formic acid decomposition on metallic powder catalysts: Exploding the HCOOH decomposition volcano curve. Surf. Sci. 2016, 650, 103–110. [Google Scholar] [CrossRef]
- Ai, M. Activities for the decomposition of formic acid and the acid-base properties of metal oxide catalysts. J. Catal. 1977, 50, 291–300. [Google Scholar] [CrossRef]
- Vittadini, A.; Selloni, A.; Rotzinger, F.P.; Grätzel, M. Formic Acid Adsorption on Dry and Hydrated TiO2 Anatase (101) Surfaces by DFT Calculations. J. Phys. Chem. B 2000, 104, 1300–1306. [Google Scholar] [CrossRef]
- Gong, X.Q.; Selloni, A.; Vittadini, A. Density functional theory study of formic acid adsorption on anatase TiO2(001): Geometries, energetics, and effects of coverage, hydration, and reconstruction. J. Phys. Chem. B 2006, 110, 2804–2811. [Google Scholar] [CrossRef]
- Dijkstra, M.; Panneman, H.; Winkelman, J.; Kelly, J.; Beenackers, A. Modeling the photocatalytic degradation of formic acid in a reactor with immobilized catalyst. Chem. Eng. Sci. 2002, 57, 4895–4907. [Google Scholar] [CrossRef]
- Uslu, H. Adsorption equilibria of formic acid by weakly basic adsorbent Amberlite IRA-67: Equilibrium, kinetics, thermodynamic. Chem. Eng. J. 2009, 155, 320–325. [Google Scholar] [CrossRef]
- Sadovskaya, E.; Chesalov, Y.; Goncharov, V.; Sobolev, V.; Andrushkevich, T. Formic acid decomposition over V-Ti oxide catalyst: Mechanism and kinetics. Mol. Catal. 2017, 430, 54–62. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Dillon, J.K.; Grassian, V.H. Surface adsorption and photochemistry of gas-phase formic acid on TiO2 nanoparticles: The role of adsorbed water in surface coordination, adsorption kinetics, and rate of photoproduct formation. J. Phys. Chem. C 2014, 118, 25487–25495. [Google Scholar] [CrossRef]
- Dong, H.; Zhuang, Z.; Gu, Y.; Gao, J. The adsorption and activation of formic acid on different anatase TiO2 surfaces. J. Energy Chem. 2017, 26, 738–742. [Google Scholar] [CrossRef][Green Version]
- Popova, G.Y.; Andrushkevich, T.V.; Chesalov, Y.A.; Stoyanov, E.S. In situ FTIR study of the adsorption of formaldehyde, formic acid, and methyl formiate at the surface of TiO2 (anatase). Kinet. Catal. 2020, 41, 885–891. [Google Scholar]
- Kim, K.S.; Barteau, M.A. Pathways for carboxylic acid decomposition on TiO2. Langmuir 1988, 4, 945–953. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Simakov, A.V.; Sazonova, N.N.; Rogov, V.A.; Barannik, G.B. Propane and oxygen action on NOx adspecies on low-exchanged Cu-ZSM-5. Catal. Lett. 1988, 51, 3–4. [Google Scholar]
- Delgado, J.A.; Sotelo, J.L.; Gómez, J.M.; Gómez, P. Estimation of adsorption parameters from temperature-programmed desorption thermograms: Application to the adsorption of carbon dioxide onto alumina. Adsorpt. Sci. Technol. 2007, 25, 113–127. [Google Scholar] [CrossRef]
- Ivanov, E.A.; Popova, G.Y.; Chesalov, Y.A.; Andrushkevich, T.V. In situ FTIR study of the kinetics of formic acid decomposition on V-Ti oxide catalyst under stationary and non-stationary conditions. Determination of kinetic constants. J. Mol. Catal. A Chem. 2009, 312, 92–96. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical Identification of Surface Groups. Adv. Catal. 1966, 16, 179–274. [Google Scholar]
- Liu, B.; Cheng, K.; Nie, S.; Zhao, X.; Yu, H.; Yu, J.; Nakata, K. Ice-Water Quenching Induced Ti3+ Self-doped TiO2 with Surface Lattice Distortion and the Increased Photocatalytic Activity. J. Phys. Chem. C 2017, 121, 19836–19848. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the Photoelectronic and Photocatalytic Activities of Various Anatase and Rutile Forms of Titania in Pure Liquid Organic Phases and in Aqueous Solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Liu, B.; Wu, H.; Parkin, I.P. Gaseous Photocatalytic Oxidation of Formic Acid over TiO2: A Comparison between the Charge Carrier Transfer and Light-Assisted Mars-van Krevelen Pathways. J. Phys. Chem. C 2019, 123, 22261–22272. [Google Scholar] [CrossRef]
- Salvador, P. Semiconductors’ Photoelectrochemistry: A Kinetic and Thermodynamic Analysis in the Light of Equilibrium and Nonequilibrium Models. J. Phys. Chem. B 2001, 105, 6128–6141. [Google Scholar] [CrossRef]
- Lim, T.H.; Jeong, S.M.; Kim, S.D.; Gyenis, J. Photocatalytic decomposition of NO by TiO2 particles. J. Photochem. Photobiol. A Chem. 2000, 134, 209–217. [Google Scholar] [CrossRef]
- Zhang, L.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and mechanisms of charge transfer processes in photocatalytic systems: A review. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 263–276. [Google Scholar] [CrossRef]
- Bloh, J.Z. A Holistic Approach to Model the Kinetics of Photocatalytic Reactions. Front. Chem. 2019, 7, 128. [Google Scholar] [CrossRef]
- Sta, I.; Jlassi, M.; Hajji, M.; Boujmil, M.F.; Jerbi, R.; Kandyla, M.; Kompitsas, M.; Ezzaouia, H. Structural and optical properties of TiO2 thin films prepared by spin coating. J. Sol-Gel Sci. Technol. 2014, 72, 421–427. [Google Scholar] [CrossRef]
- Douglas, J. Process Dynamics and Control; Prentice-Hall: Englewood Cliffs, NJ, USA, 1972. [Google Scholar]
- Petkovska, M.; Nikolić, D.; Marković, A.; Seidel-Morgenstern, A. Fast evaluation of periodic operation of a heterogeneous reactor based on nonlinear frequency response analysis. Chem. Eng. Sci. 2010, 65, 3632–3637. [Google Scholar] [CrossRef]
- Ghiasi, H.; Pasini, D.; Lessard, L. A non-dominated sorting hybrid algorithm for multi-objective optimization of engineering problems. Eng. Optim. 2011, 43, 39–59. [Google Scholar] [CrossRef]
- Yaws, C. The Yaws Handbook of Vapor Pressure, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]




| Parameter | Description | Value | Units |
|---|---|---|---|
| Regression fit parameter 1 | 1.503 | ||
| Regression fit parameter 2 | 1.50 × 10−5 | ||
| Enthalpy of adsorption | 3.243 × 104 | ||
| Desorption pre-exponential factor 1 | 1.00 × 10−13 | ||
| Desorption pre-exponential factor 2 | 2.05 | ||
| Recombination rate | 0.24 | ||
| Estimation of first desorption energy | 1.131 × 105 | ||
| Second desorption energy | 2.092 × 104 | ||
| Reaction activation energy | 8.480 × 103 | ||
| Reference light intensity | 1.6 |
| Parameter | Value | Units |
|---|---|---|
| Photocatalyst loading | ||
| SS1 | SS2 | SS3 | SS4 | SS5 | |
|---|---|---|---|---|---|
| Inlet molar fraction, - | 0.121 | 0.154 | 0.139 | 0.153 | 0.142 |
| 4.966 | 4.171 | 4.983 | 4.876 | 5.682 | |
| Outlet molar fraction, - | 0.023 | 0.034 | 0.038 | 0.049 | 0.052 |
| Surface coverage, - | 99.28 | 99.52 | 99.57 | 99.66 | 99.68 |
| 5.447 | 4.654 | 5.466 | 5.359 | 6.165 | |
| SS conversion, % | 79.383 | 75.172 | 69.919 | 64.944 | 60.049 |
| 2.156 | 2.301 | 2.475 | 2.667 | 2.885 | |
| −2.3473 | −2.6324 | −4.0278 | −5.1532 | −5.5210 | |
| −0.0003 | −0.0002 | −0.0003 | −0.0003 | −0.0003 |
| Temperature Modulation | SS1 | SS2 | SS3 | SS4 | SS5 |
|---|---|---|---|---|---|
| Highest conversion at modulation, % | 79.391 | 75.179 | 69.925 | 64.950 | 60.054 |
| Relative increase in conversion over steady state, % | +0.010 | +0.010 | +0.010 | +0.010 | +0.010 |
| >2.4 × 10−4 | >0 | >0 | >0 | >0 |
| Optimisation Parameter | Lower Bound | Upper Bound |
|---|---|---|
| , - | 0.010 | 0.3189 |
| 10−9 | 10−6 | |
| , - | 0 | 0.7 |
| , - | 0 | 1 |
| 0 | ||
| 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellwood, T.; Živković, L.A.; Denissenko, P.; Abiev, R.S.; Rebrov, E.V.; Petkovska, M. Process Intensification in Photocatalytic Decomposition of Formic Acid over a TiO2 Catalyst by Forced Periodic Modulation of Concentration, Temperature, Flowrate and Light Intensity. Processes 2021, 9, 2046. https://doi.org/10.3390/pr9112046
Ellwood T, Živković LA, Denissenko P, Abiev RS, Rebrov EV, Petkovska M. Process Intensification in Photocatalytic Decomposition of Formic Acid over a TiO2 Catalyst by Forced Periodic Modulation of Concentration, Temperature, Flowrate and Light Intensity. Processes. 2021; 9(11):2046. https://doi.org/10.3390/pr9112046
Chicago/Turabian StyleEllwood, Thomas, Luka A. Živković, Petr Denissenko, Rufat Sh. Abiev, Evgeny V. Rebrov, and Menka Petkovska. 2021. "Process Intensification in Photocatalytic Decomposition of Formic Acid over a TiO2 Catalyst by Forced Periodic Modulation of Concentration, Temperature, Flowrate and Light Intensity" Processes 9, no. 11: 2046. https://doi.org/10.3390/pr9112046
APA StyleEllwood, T., Živković, L. A., Denissenko, P., Abiev, R. S., Rebrov, E. V., & Petkovska, M. (2021). Process Intensification in Photocatalytic Decomposition of Formic Acid over a TiO2 Catalyst by Forced Periodic Modulation of Concentration, Temperature, Flowrate and Light Intensity. Processes, 9(11), 2046. https://doi.org/10.3390/pr9112046









