Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
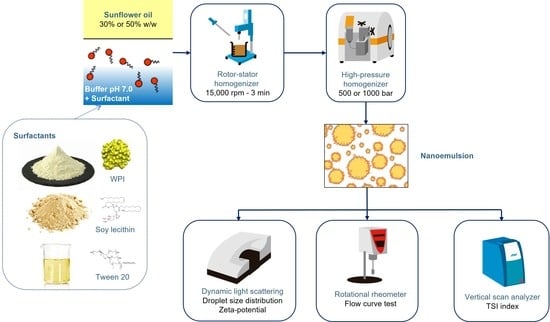
2.2. Fabrication of Emulsions
2.3. Measurements of Dynamic Interfacial Tension (DIT) between Surfactant Dispersions and Sunflower Oil
2.4. Measurement of Droplet Size Distributions of the Emulsions
2.5. Zeta-Potential Measurements
2.6. Rheological Characterization of the Emulsions
2.7. Physical Stability of Emulsions
2.8. Statistical Analysis of Data
3. Results and Discussion
3.1. Dynamic Interfacial Tension (DIT) at the Oil/Water Interface
3.2. Effect of the Formulation and Processing Conditions on Droplet Size Distribution of Emulsions
3.3. Effect of Surfactant on Z-Potential of the Emulsions
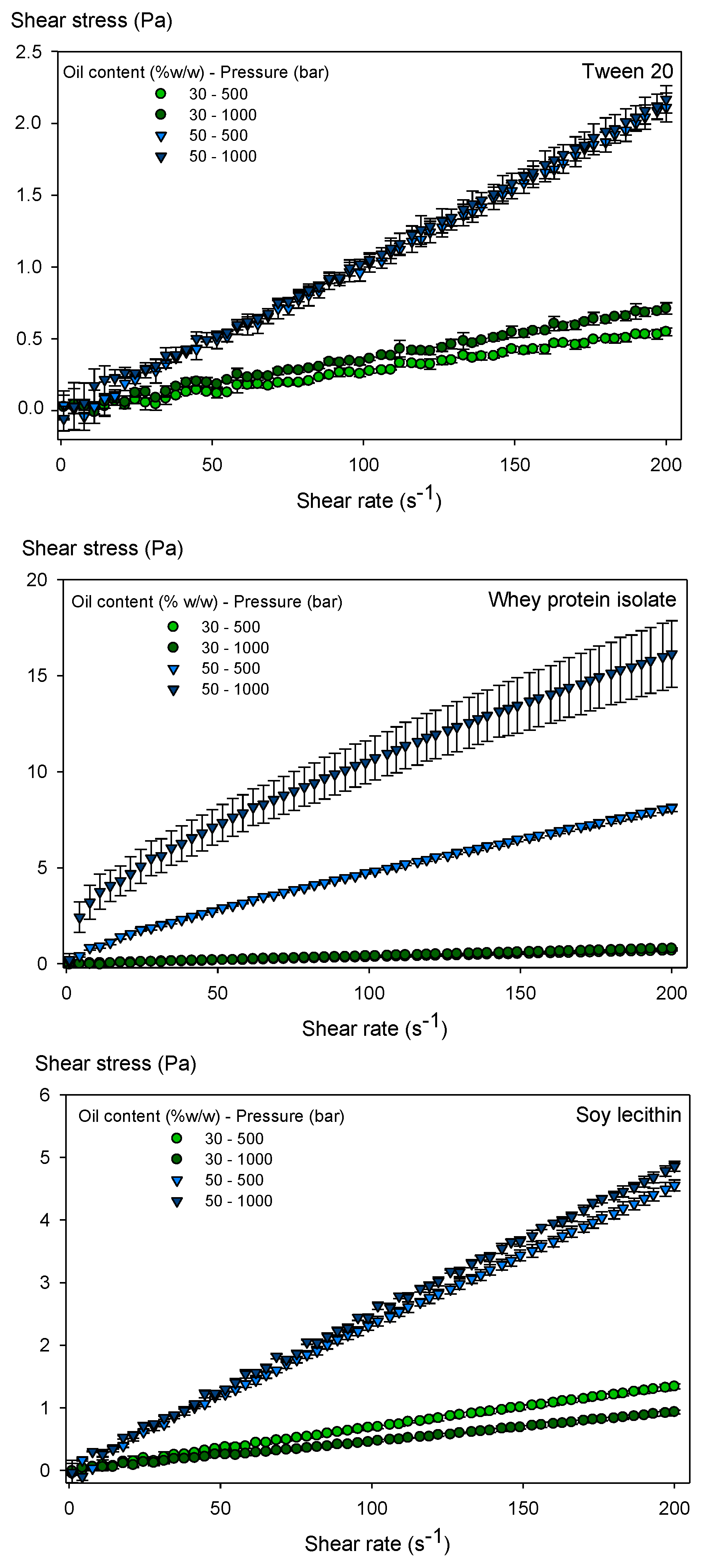
3.4. Rheological Behavior of the Emulsions
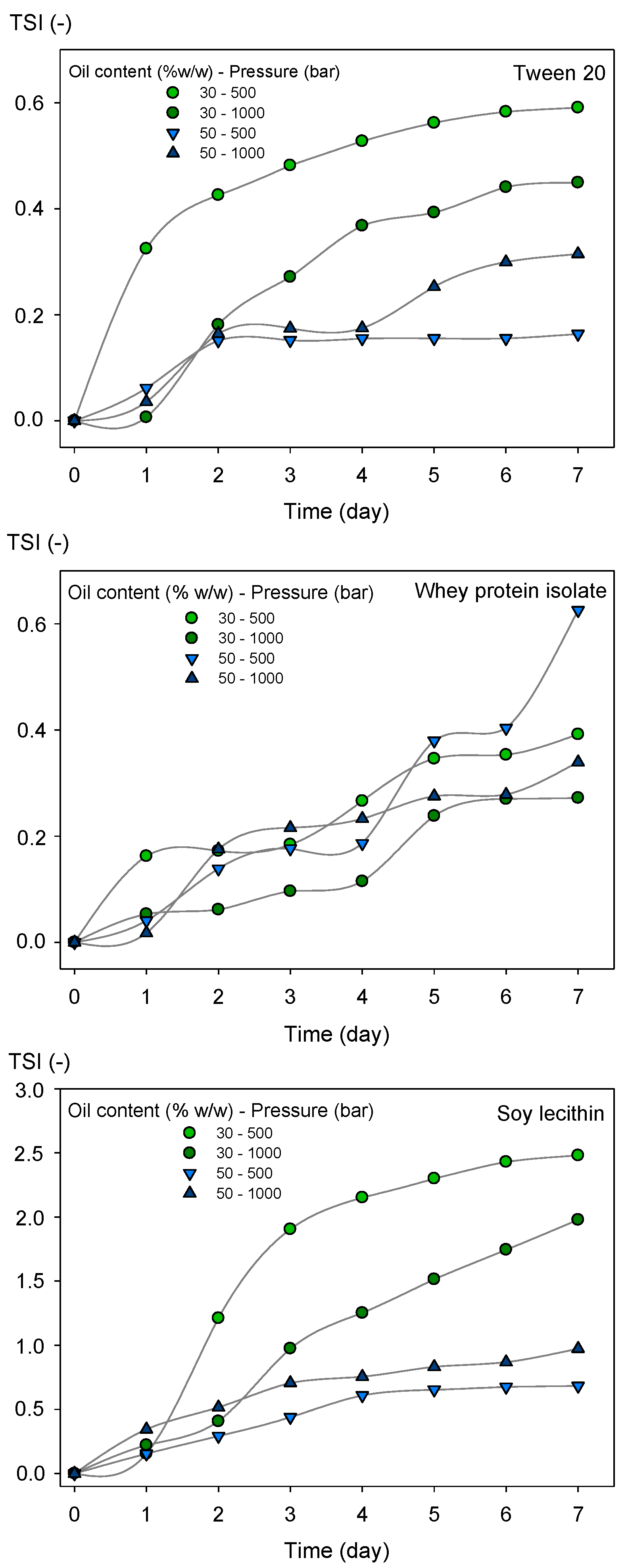
3.5. Physical Stability of the Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arancibia, C.; Miranda, M.; Matiacevich, S.; Troncoso, E. Physical properties and lipid bioavailability of nanoemulsion-based matrices with different thickening agents. Food Hydrocoll. 2017, 73, 243–254. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 6, 1719–1729. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys.-Condens. Mat. 2006, 18, R635–R666. [Google Scholar] [CrossRef] [Green Version]
- Troncoso, E.; Aguilera, J.M.; McClements, D.J. Influence of particle size on the in vitro digestibility of protein-coated lipid nanoparticles. J. Colloid Interface Sci. 2012, 382, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, E.; Aguilera, J.M.; McClements, D.J. Fabrication, characterization and lipase digestibility of food-grade nanoemulsions. Food Hydrocoll. 2012, 27, 355–363. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Hashim, A.F.; Youssef, K.; Abd-Elsalam, K.A. The role of nanoemulsions as antimicrobial agents in plant protection. In Nanobiotechnology Applications in Plant Protection, 1st ed.; Abd-Elsalam, K., Prasad, R., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2018; pp. 137–153. [Google Scholar]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Anton, N.; Gayet, P.; Benoit, J.P.; Saulnier, P. Nano-emulsions and nanocapsules by the PIT method: An investigation on the role of the temperature cycling on the emulsion phase inversion. Int. J. Pharmaceut. 2007, 344, 44–52. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Tabilo-Munizaga, G.; Villalobos-Carvajal, R.; Herrera-Lavados, C.; Moreno-Osorio, L.; Jarpa-Parra, M.; Pérez-Won, M. Physicochemical properties of high-pressure treated lentil protein-based nanoemulsions. LWT Food Sci. Technol. 2019, 101, 590–598. [Google Scholar] [CrossRef]
- Yukuyama, M.M.; Kato, E.T.M.; de Araujo, G.L.B.; Löbenberg, R.; Monteiro, L.M.; Lourenço, F.R.; Bou-Chacra, N.A. Olive oil nanoemulsion preparation using high-pressure homogenization and d-phase emulsification—A design space approach. J. Drug Deliv. Sci. Technol. 2019, 49, 622–631. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yia, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Guo, Y.; Jiang, Z.; Shi, L.; Zhang, T.; Wang, Y.; Gong, M.; Wang, T.; Lin, R.; Liu, R.; et al. Sea buckthorn pulp oil nanoemulsions fabricated by ultra-high pressure homogenization process: A promising carrier for nutraceutical. J. Food Eng. 2020, 287, 110129. [Google Scholar] [CrossRef]
- Gazolu-Rusanova, D.; Lesov, I.; Tcholakova, S.; Denkov, N.; Ahtchi, B. Food grade nanoemulsions preparation by rotor-stator homogenization. Food Hydrocoll. 2020, 102, 105579. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Xie, F.; Zhang, S.; Li, Y.; Qi, B. Homogenization pressure and soybean protein concentration impact the stability of perilla oil nanoemulsions. Food Hydrocoll. 2020, 101, 105575. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Ciacciarelli, A.; Quassinti, L.; Bramucci, M.; Miano, A.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Properties and stability of nanoemulsions: How relevant is the type of surfactant? J. Drug Deliv. Sci. Technol. 2020, 58, 101772. [Google Scholar] [CrossRef]
- Rave, M.C.; Echeverria, J.D.; Salamanca, C.H. Improvement of the physical stability of oil-in-water nanoemulsions elaborated with Sacha inchi oil employing ultra-high-pressure homogenization. J. Food Eng. 2020, 273, 109801. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Santamaria-Echart, A.; Ribeiro, A.; Peres, A.M.; Dias, M.M.; Pinho, S.P.; Barreiro, M.F. Formulation and optimization of nanoemulsions using the natural surfactant saponin from Quillaja bark. Molecules 2020, 25, 1538. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yue, W.; Luo, Y.; Luo, Q.; Liu, S.; Chen, H.; Qin, W.; Zhang, Q. Preparation and stability characterization of soybean protein isolate/sodium alginate complexes-based nanoemulsions using high-pressure homogenization. LWT Food Sci. Technol. 2021, 154, 112607. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Cossetin, L.F.; Garlet, Q.I.; Velho, M.C.; Gündel, S.; Ourique, A.F.; Heinzmann, B.M.; Gonzalez Monteiro, S. Development of nanoemulsions containing Lavandula dentata or Myristica fragrans essential oils: Influence of temperature and storage period on physical-chemical properties and chemical stability. Ind. Crop Prod. 2021, 160, 113115. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Yan, J.; Zhang, S.; Shi, C.; McClements, D.J.; Liu, X.; Liu, F. Development of antibacterial nanoemulsions incorporating thyme oil: Layer-by-layer self-assembly of whey protein isolate and chitosan hydrochloride. Food Chem. 2021, 339, 128016. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, L.; Kang, Y.; He, D.; Yang, B.; Wu, K. Cinnamon essential oil nanoemulsions by high-pressure homogenization: Formulation, stability, and antimicrobial activity. LWT Food Sci. Technol. 2021, 147, 111660. [Google Scholar] [CrossRef]
- Haritha, S.P.B.; Koteswara, R.P.; Chakravarthi, V. A brief introduction to methods of preparation, applications and characterization of nanoemulsion drug delivery systems. Indian J. Res. Pharm. Biotechnol. 2013, 1, 25–28. [Google Scholar]
- McClements, D.J.; Öztürk, B. Utilization of nanotechnology to improve the handling, storage and biocompatibility of bioactive lipids in food applications. Foods 2021, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for food applications: Development and characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, N.; Ranjan, S.; Gandhi, M. Nanoemulsions in food: Market demand. Environ. Chem. Lett. 2019, 17, 1003–1009. [Google Scholar] [CrossRef]
- Pawlik, A.; Cox, P.W.; Norton, I.T. Food grade duplex emulsions designed and stabilized with different osmotic pressures. J. Colloid Interface Sci. 2010, 352, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Rodrigues Costa, A.L.; Lopes Cunha, R. Impact of oil type and WPI/Tween 80 ratio at the oil-water interface: Adsorption, interfacial rheology and emulsion features. Colloids Surf B Biointerfaces 2018, 164, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, R.N.; Aguilera, J.M. Structure–fracture relationships in gas-filled gelatin gels. Food Hydrocoll. 2009, 23, 1351–1357. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Dapueto, N.; Troncoso, E.; Mella, C.; Zúñiga, R.N. The effect of denaturation degree of protein on the microstructure, rheology and physical stability of oil-in-water (O/W) emulsions stabilized by whey protein isolate. J. Food Eng. 2019, 263, 253–261. [Google Scholar] [CrossRef]
- Arancibia, C.; Riquelme, N.; Zúñiga, R.N.; Matiacevich, S. Comparing the effectiveness of natural and synthetic emulsifiers on oxidative and physical stability of avocado oil-based nanoemulsions. Innov. Food Sci. Emerg. Technol. 2017, 44, 159–166. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion Stability. Crit. Rev. Food Sci. 2007, 47, 611–694. [Google Scholar] [CrossRef]
- Flores-Andrade, E.; Allende-Baltazar, Z.; Sandoval-González, P.E.; Jiménez-Fernández, M.; Beristain, C.I.; Pascual-Pineda, L.A. Carotenoid nanoemulsions stabilized by natural emulsifiers: Whey protein, gum Arabic, and soy lecithin. J. Food Eng. 2021, 290, 110208–110216. [Google Scholar] [CrossRef]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108–109, 63–71. [Google Scholar] [CrossRef]
- Zhai, J.; Wooster, T.J.; Hoffmann, S.V.; Lee, T.-H.; Augustin, M.A.; Aguilar, M.-I. Structural rearrangement of β-lactoglobulin at different oil–water interfaces and its effect on emulsion stability. Langmuir 2011, 27, 9227–9236. [Google Scholar] [CrossRef]
- Gülseren, I.; Corredig, M. Interactions at the interface between hydrophobic and hydrophilic emulsifiers: Polyglycerol polyricinoleate (PGPR) and milk proteins, studied by drop shape tensiometry. Food Hydrocoll. 2012, 29, 193–198. [Google Scholar] [CrossRef]
- Van Nieuwenhuyzen, W.; Szuhaj, B.F. Effects of lecithins and proteins on the stability of emulsions. Lipid/Fett 1998, 100, 282–291. [Google Scholar] [CrossRef]
- Rodríguez Patino, J.M.; Caro, A.L.; Rodríguez Niño, R.; Mackie, A.; Gunning, P.; Morris, V. Some implications of nanoscience in food dispersion formulations containing phospholipids as emulsifiers. Food Chem. 2007, 102, 532–541. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Deng, L. Current progress in the utilization of soy-based emulsifiers in food applications—A review. Foods 2021, 10, 1354. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Sullo, A.; Winston, S.; Norton, I.T.; Norton-Welch, A.B. Influence of ethanol on emulsions stabilized by low molecular weight surfactants. J. Food Sci. 2020, 85, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Šegota, S.; Težak, D. Spontaneous formation of vesicles. Adv. Colloid Interface Sci. 2006, 121, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Valenta, C. Lecithin-based nanoemulsions. J. Drug Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Liu, W.; Zou, L.; McClements, D.J. A review of the rheological properties of dilute and concentrated food emulsions. J. Texture Stud. 2020, 51, 45–55. [Google Scholar] [CrossRef]
- Bot, F.; Cossuta, D.; O’Mahony, J.A. Inter-relationships between composition, physicochemical properties and functionality of lecithin ingredients. Trends Food Sci. Technol. 2021, 111, 261–270. [Google Scholar] [CrossRef]
- Hu, Y.-T.; Ting, Y.; Hu, J.-Y.; Hsieh, S.-C. Techniques and methods to study functional characteristics of emulsion systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Food Emulsions. Principles, Practices and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Nash, J.J.; Erk, K.A. Stability and interfacial viscoelasticity of oil-water nanoemulsions stabilized by soy lecithin and Tween 20 for the encapsulation of bioactive carvacrol. Colloids Surf. A Physicochem. Eng. Asp. 2017, 517, 1–11. [Google Scholar] [CrossRef]
- Guan, H.; Diao, X.; Liu, D.; Han, J.; Kong, B.; Liu, D.; Gao, C.; Zhang, L. Effect of high-pressure processing enzymatic hydrolysates of soy protein isolate on the emulsifying and oxidative stability of myofibrillar protein-prepared oil-in-water emulsions. J. Sci. Food Agric. 2020, 100, 3910–3919. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]

| Surfactant Concentration (% by Weight) | Tween 20 | Whey Protein Isolate | Soy Lecithin | |||
|---|---|---|---|---|---|---|
| τ0 (mN/m) | k (mN/m s) | τ0 (mN/m) | k (mN/m s) | τ0 (mN/m) | k (mN/m s) | |
| 2.86 | 6.47 ± 0.17 aB | −0.263 ± 0.011 cA | 21.20 ± 0.73 cB | −0.739 ± 0.019 bA | 13.81 ± 0.18 bB | −1.056 ± 0.007 aA |
| 6.66 | 5.49 ± 0.03 aA | −0.195 ± 0.002 cA | 18.08 ± 0.24 cA | −0.576 ± 0.026 bA | 14.55 ± 1.01 bA | −1.204 ± 0.129 aA |
| Surfactant | Oil Content (% w/w) | Emulsification Pressure (Bar) | D10 (nm) | D50 (nm) | D90 (nm) | Dispersal Coefficient (Dimensionless) | Distribution Shape |
|---|---|---|---|---|---|---|---|
| Tween 20 | 30 | 500 | 141 ± 3 bA | 251 ± 8 aA | 399 ± 15 aA | 1.03 ± 0.02 aA | Monomodal |
| 30 | 1000 | 145 ± 0 bA | 237 ± 2 aA | 349 ± 7 aA | 0.86 ± 0.02 aA | Monomodal | |
| 50 | 500 | 173 ± 5 bB | 288 ± 4 aA | 427 ± 4 aA | 0.88 ± 0.03 aA | Monomodal | |
| 50 | 1000 | 202 ± 8 bB | 332 ± 16 aB | 503 ± 52 aA | 0.90 ± 0.09 aA | Monomodal | |
| Whey protein isolate | 30 | 500 | 136 ± 10 bA | 262 ± 24 aA | 508 ± 83 aA | 1.44 ± 0.42 aA | Bimodal |
| 30 | 1000 | 115 ± 6 bA | 194 ± 7 aA | 316 ± 20 aA | 1.04 ± 0.17 aA | Monomodal | |
| 50 | 500 | 173 ± 10 bB | 302 ± 6 aA | 537 ± 29 aA | 1.20 ± 0.12 aA | Bimodal | |
| 50 | 1000 | 131 ± 3 bB | 214 ± 6 aB | 326 ± 26 aA | 0.91 ± 0.10 aA | Monomodal | |
| Soy lecithin | 30 | 500 | 121 ± 19 aA | 344 ± 19 bA | 1016 ± 340 bA | 2.65 ± 1.23 bA | Bimodal |
| 30 | 1000 | 67 ± 3 aA | 146 ± 11 bA | 935 ± 655 bA | 5.91 ± 4.38 bA | Bimodal | |
| 50 | 500 | 120 ± 16 aB | 310 ± 1 bA | 780 ± 171 bA | 2.13 ± 0.50 bA | Bimodal | |
| 50 | 1000 | 351 ± 15 aB | 615 ± 46 bB | 1145 ± 154 bA | 1.29 ± 0.19 bA | Bimodal |
| Oil Content (% by Weight) | Emulsification Pressure (Bar) | Tween 20 | Whey Protein Isolate | Soy Lecithin |
|---|---|---|---|---|
| 30 | 500 | −41.3 ± 2.2 aA | −56.7 ± 4.0 bA | −88.3 ± 4.0 cA |
| 30 | 1000 | −43.6 ± 1.2 aAB | −54.7 ± 1.7 bAB | −77.4 ± 1.4 cAB |
| 50 | 500 | −43.6 ± 0.2 aAB | −55.7 ± 2.7 bAB | −76.1 ± 0.4 cAB |
| 50 | 1000 | −45.9 ± 1.4 aB | −50.1 ± 1.5 bB | −74.6 ± 0.2 cB |
| Oil Content(% by Weight) | Emulsification Pressure (Bar) | Tween 20 | Whey Protein Isolate | Soy Lecithin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K (mPa sn) | n (-) | μapp (mPa s) | K (mPa sn) | n (-) | μapp (mPa s) | K (mPa sn) | n (-) | μapp (mPa s) | ||
| 30 | 500 | 2.59 ± 0.7 aA | 1.02 ± 0.05 bB | 2.71 ± 0.1 aA | 3.55 ± 0.6 bA | 0.99 ± 0.02 aB | 3.43 ± 0.2 bA | 7.41 ± 1.4 aA | 0.98 ± 0.03 bB | 6.86 ± 0.4 aA |
| 30 | 1000 | 4.85 ± 0.5 aA | 0.94 ± 0.01 bB | 3.79 ± 0.2 aA | 3.47 ± 0.8 bA | 1.03 ± 0.04 aB | 3.88 ± 0.3 bA | 4.72 ± 0.8 aA | 1.00 ± 0.03 bB | 4.66 ± 0.2 aA |
| 50 | 500 | 7.23 ± 1.7 aA | 1.08 ± 0.04 bAB | 9.67 ± 0.8 aB | 143.7 ± 20 bA | 0.76 ± 0.03 aAB | 53.6 ± 0.6 bB | 27.91 ± 0.5 aA | 0.96 ± 0.01 bAB | 23.9 ± 0.4 aB |
| 50 | 1000 | 9.04 ± 0.3 aB | 1.03 ± 0.01 bA | 10.34 ± 0.3 aC | 866.3 ± 269 bB | 0.56 ± 0.06 aA | 135.8 ± 17 bC | 32.26 ± 1.5 aB | 0.95 ± 0.01 bA | 25.9 ± 0.4 aC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, K.; Matamala, C.; Martínez, N.; Zúñiga, R.N.; Troncoso, E. Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants. Processes 2021, 9, 2002. https://doi.org/10.3390/pr9112002
Fuentes K, Matamala C, Martínez N, Zúñiga RN, Troncoso E. Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants. Processes. 2021; 9(11):2002. https://doi.org/10.3390/pr9112002
Chicago/Turabian StyleFuentes, Karen, Claudia Matamala, Nayaret Martínez, Rommy N. Zúñiga, and Elizabeth Troncoso. 2021. "Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants" Processes 9, no. 11: 2002. https://doi.org/10.3390/pr9112002
APA StyleFuentes, K., Matamala, C., Martínez, N., Zúñiga, R. N., & Troncoso, E. (2021). Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants. Processes, 9(11), 2002. https://doi.org/10.3390/pr9112002