The Biological Effects of Ozone Gas on Soft and Hard Dental Tissues and the Impact on Human Gingival Fibroblasts and Gingival Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Part One of the Experiment
2.1.1. Study Design
2.1.2. Ozonation Procedure
2.2. Part Two of the Experiment
2.2.1. Cell Lines
2.2.2. Cell Culture
2.2.3. Experimental Design
2.2.4. Impact on Cell Morphology and Confluence
3. Results
3.1. Experiment Part ONE
Evolution of the Degree of Mineralization of All the Studied Teeth
3.2. Experiment Part Two
3.2.1. Gaseous Ozone Affects Primary Gingival Fibroblasts’ Morphology and Confluence
3.2.2. Effect of Ozone Gas on the Morphology and Confluence of Human Primary Gingival Keratinocytes—PGK
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| O3 | Ozone |
| SARS -CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| GSH | Glutathione |
| NICE | National Institute of Clinical Excellence |
| M1 | Permanent molar |
| ATCC | American Type Culture Collection |
| PGK | Primary gingival keratinocytes |
| HGF | Primary gingival fibroblast |
| DNA | Deoxyribonucleic acid |
| FGF | Fibroblast Growth Factor |
References
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R. Ozone therapy in dentistry: A strategic review. J. Nat. Sci. Biol. Med. 2011, 2, 151–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarpazhooh, A.; Limeback, H. The application of ozone in dentistry: A systematic review of literature. J. Dent. 2008, 36, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuadros, M.E.; Albaladejo-Florín, M.J.; Peña-Lora, D.; Álava-Rabasa, S.; Pérez-Moro, O.S. Ozone (O3) and SARS-CoV-2: Physiological Bases and Their Therapeutic Possibilities According to COVID-19 Evolutionary Stage. SN Compr. Clin. Med. 2020, 2, 1094–1102. [Google Scholar] [CrossRef]
- Dähnhardt, J.E.; Jaeggi, T.; Lussi, A. Treating open carious lesions in anxious children with ozone. A prospective controlled clinical study. Am. J. Dent. 2006, 19, 267–270. [Google Scholar] [PubMed]
- Gulafsha, M.; Anuroopa, P. Miracle of ozone in dentistry: An overview. World J. Pharm. Res. 2019, 8, 665–667. [Google Scholar]
- Alpan, A.L.; Bakar, O. Ozone in Dentistry. In Ozone in Nature and Practice; Derco, J., Koman, M., Eds.; IntechOpen: London, UK, 2018; pp. 57–76. [Google Scholar] [CrossRef] [Green Version]
- Grootveld, M.; Chang, H. Oxidative Consumption of Oral Biomolecules by Therapeutically-Relevant Doses of Ozone. Adv. Chem. Eng. Sci. 2012, 2, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Pattanaik, B.; DJetwa, D.; Pattanaik, S.; Manglekar, S.; Naitam, D.N.; Dani, A. Ozone therapy in dentistry: A literature review. J Interdiscip. Dent. 2011, 1, 87–92. [Google Scholar] [CrossRef]
- Gonzalez-Munoz, L.; Flichy-Fernandez, A.; Ata-Ali, J.; Pascual-Moscardo, A.; Penarrocha-Diago, M. Effect of ozone therapy upon clinical and bacteriological parameters of the oral cavity: An update. J. Clin. Exp. Dent. 2011, 3, e325–e327. [Google Scholar] [CrossRef]
- Tiwari, S.; Avinash, A.; Katiyar, S.; Iyer, A.A.; Jain, S. Dental applications of ozone therapy: A review of literature. Saudi J. Dent. Res. 2017, 8, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.V.; Rajeshwari, K.; Kohli, S.; Zohabhasan, S.; Bhatia, S. Ozone- A Biological Therapy in Dentistry- Reality or Myth? Open Dent. J. 2016, 10, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domb, W.C. Ozone therapy in dentistry. A brief review for physicians. Interv. Neuroradiol. 2014, 20, 632–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travagli, V.; Zanardi, I.; Valacchi, G.; Bocci, V. Ozone and Ozonated Oils in Skin Diseases: A Review. Int. J. Ozone Ther. 2010, 9, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Deepa, D.; Gupta, S. Applications of ozone therapy in dentistry. J. Oral Res. Rev. 2016, 8, 86. [Google Scholar] [CrossRef]
- Nikhade, P.; Chandak, M. Evaluation of unique property of ozone in comparison with 3% sodium hypochlorite in eradication of enterococcus faecalis. Int. J. Clin. Dent. 2011, 3, 18–20. [Google Scholar]
- Johansson, E.; Claesson, R.; van Dijken, J. Antibacterial effect of ozone on cariogenic bacterial species. J. Dent. 2009, 37, 449–453. [Google Scholar] [CrossRef]
- Huth, K.C.; Jakob, F.M.; Saugel, B.; Cappello, C.; Paschos, E.; Hollweck, R.; Hickel, R.; Brand, K. Effect of ozone on oral cells compared with established antimicrobials. Eur. J. Oral Sci. 2006, 114, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, O.; Pelz, K.; Hahn, P. Antibacterial effect of an ozone device and its comparison with two dentin-bonding systems. Eur. J. Oral Sci. 2006, 114, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Baysan, A.; Lynch, E. The use of ozone in dentistry and medicine. Part 2. Ozone and root caries. Prim. Dent. Care 2006, 13, 37–41. [Google Scholar] [CrossRef]
- Huth, K.C.; Paschos, E.; Brand, K.; Hickel, R. Effect of ozone on non-cavitated fissure carious lesions in permanent molars. A controlled prospective clinical study. Am. J. Dent. 2005, 18, 223–228. [Google Scholar] [PubMed]
- Magni, E.; Ferrari, M.; Hickel, R.; Huth, K.C.; Ilie, N. Effect of ozone gas application on the mechanical properties of dental adhesives bonded to dentin. Dent. Mater. 2008, 24, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Celiberti, P.; Pazera, P.; Lussi, A. The impact of ozone treatment on enamel physical properties. Am. J. Dent. 2006, 19, 67–72. [Google Scholar] [PubMed]
- Petrucci, M.T.; Gallucci, C.; Agrillo, A.; Mustazza, M.C.; Foà, R. Role of ozone therapy in the treatment of osteonecrosis of the jaws in multiple myeloma patients. Haematologica 2007, 92, 1289–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogales, C.G.; Ferrari, P.H.; Kantorovich, E.O.; Lage-Marques, J.L. Ozone Therapy in Medicine and Dentistry. J. Contemp. Dent. Pract. 2008, 9, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Nagayoshi, M.; Fukuizumi, T.; Okinaga, T.; Masumi, S.; Morikawa, M.; Kakinoki, Y.; Nishihara, T. Microbicidal efficacy of ozonated water against Candida albicans adhering to acrylic denture plates. Oral Microbiol. Immunol. 2005, 20, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Estrela, C.R.; Decurcio, D.d.A.; Silva, J.A.; Bammann, L.L. Antimicrobial potential of ozone in an ultrasonic cleaning system against Staphylococcus aureus. Br. Dent. J. 2006, 17, 134–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torabzadeh, H.; Jamali, M.; Razmavar, S.; Baghban, A.; Zanjani, V.; Ghasemi, A. Bleaching effect of ozone on pigmented teeth. Dent. Res. J. 2015, 12, 20–24. [Google Scholar] [CrossRef]
- NICE Report July 2005. National Institute of Clinical Excellence: HealOzone for the Treatment of Tooth Decay (Oclussal Pit and Fissure Caries and Root Caries). Available online: www.nice.org.uk/guidance/ta92 (accessed on 27 July 2005).
- Millar, B.J.; Hodson, N. Assessment of the safety of two ozone delivery devices. J. Dent. 2007, 35, 195–200. [Google Scholar] [CrossRef]
- Srinivasan, S.R.; Amaechi, B.T. Ozone: A paradigm shift in dental therapy. J. Int. Oral Health 2019, 2, 68–77. [Google Scholar] [CrossRef]
- Karawia, I.M.; Mohamed, O.S. The Effect of Ozone Gas Using Different Remineralizing Materials on Non-Cavitated Caries-Like Lesions in Permanent Teeth. Oral Health Dent. Manag. 2017, 16. [Google Scholar]
- Kapdan, A.; Oztaş, N.; Sümer, Z. Comparing the antibacterial activity of gaseous ozone and chlorhexidine solution on a tooth cavity model. J. Clin. Exp. Dent. 2013, 5, e133–e137. [Google Scholar] [CrossRef] [PubMed]
- Atabek, D.; Oztas, N. Effectiveness of Ozone with or without the Additional Use of Remineralizing Solution on Non-Cavitated Fissure Carious Lesions in Permanent Molars. Eur. J. Dent. 2011, 5, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libonati, A.; Di Taranto, V.; Mea, A.; Montemurro, E.; Gallusi, G.; Angotti, V.; Nardi, R.; Paglia, L.; Marzo, G.; Campanella, V. Clinical antibacterial effectiveness of Healozone Technology after incomplete caries removal. Eur. J. Paediatr. Dent. 2019, 20, 73–78. [Google Scholar]
- Primary Gingival Keratinocytes|ATCC. Available online: https://www.atcc.org/products/pcs-200-014 (accessed on 26 August 2021).
- Primary Gingival Fibroblast; Normal, Human, Adult (HGF)|ATCC. Available online: https://www.atcc.org/products/pcs-201-018 (accessed on 26 August 2021).
- Hodson, N.; Dunne, S.M.; Swift, E.J. Using ozone to treat dental caries. J. Esthet. Restor. Dent. 2007, 19, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Duggal, M.; Nikolopoulou, A.; Tahmassebi, J. The Additional Effect of Ozone in combination with adjunct remineralisation products on Inhibition of Demineralisation of the Dental Hard Tissues in Situ. J. Dent. 2012, 40, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.Á.; Elias, S.T.; da Silva, S.M.M.; Magalhaes, P.O.; Macedo, S.B.; Ribeiro, A.P.D.; Guerra, E.N.S. In vitro evaluation of wound healing and antimicrobial potential of ozonotherapy. J. Cranio-Maxillofac. Surg. 2017, 45, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.; Olga, L.; Sladana, M. The Influence of Healozone on Microleakage and Fissure Penetration of Different Sealing Materials. Coll. Antropol. 2009, 33, 157–162. [Google Scholar]
- Polydorou, O.; Halili, A.; Wittmer, A.; Pelz, K.; Hahn, P. The antibacterial effect of gas ozone after 2 months of in vitro evaluation. Clin. Oral Investig. 2011, 16, 545–550. [Google Scholar] [CrossRef]
- Sathyajith, N.; Sabha, S.; Shashi, B.; Subba, R. Ozone therapy in dentistry. CODS 2012, 4, 38–43. [Google Scholar]
- Nam-Kyoung, K.; Sanghwa, H.; Shin Woak, C. Poster Session: Oral Presentation; Cell Cycle and Cell Death: In vitro ozone exposure induces oxidative stress and cell death in keratinocyte). In Proceedings of the KSBMB Annual Meeting, Seoul, Korea, 19–20 May 2005. [Google Scholar]
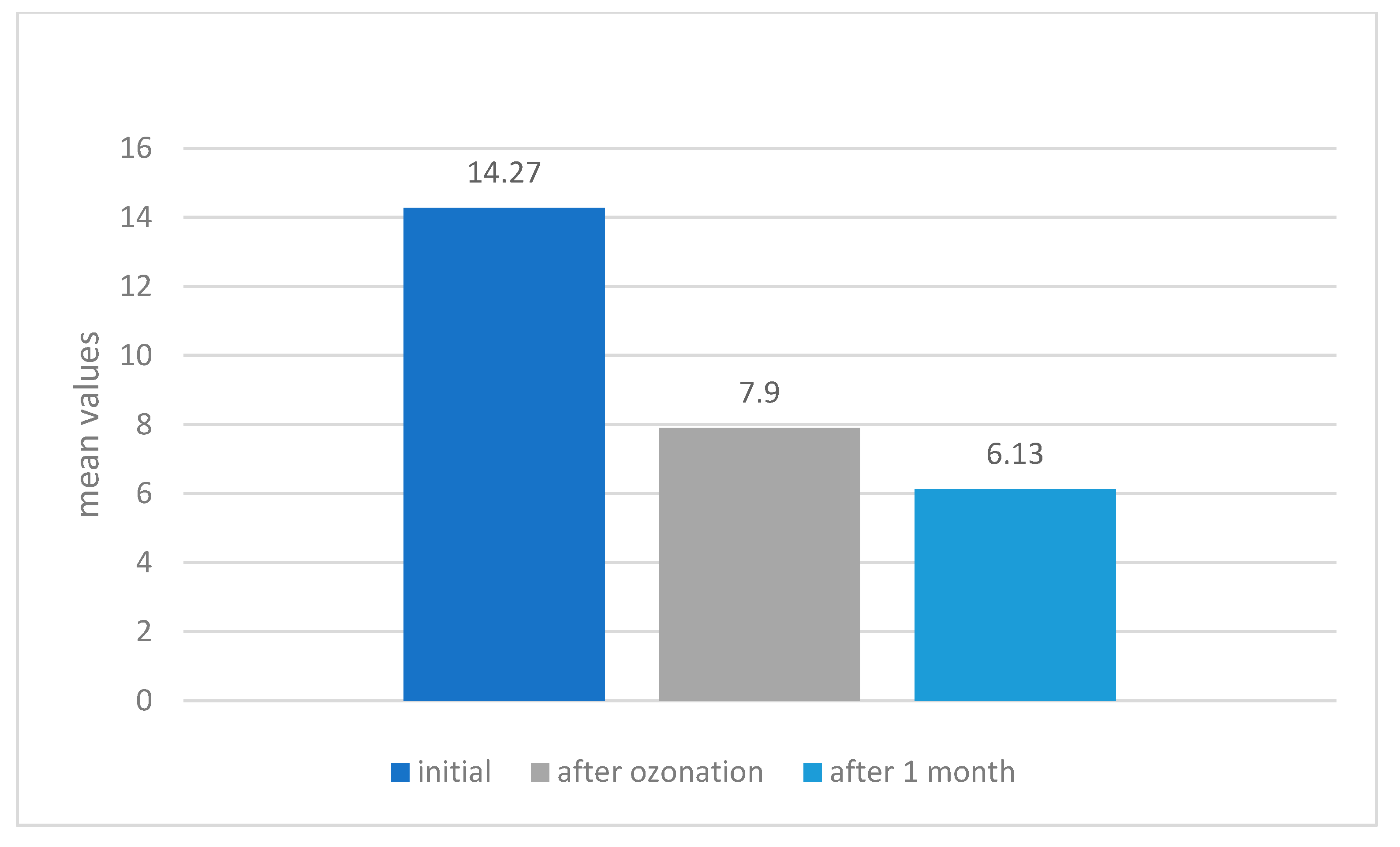
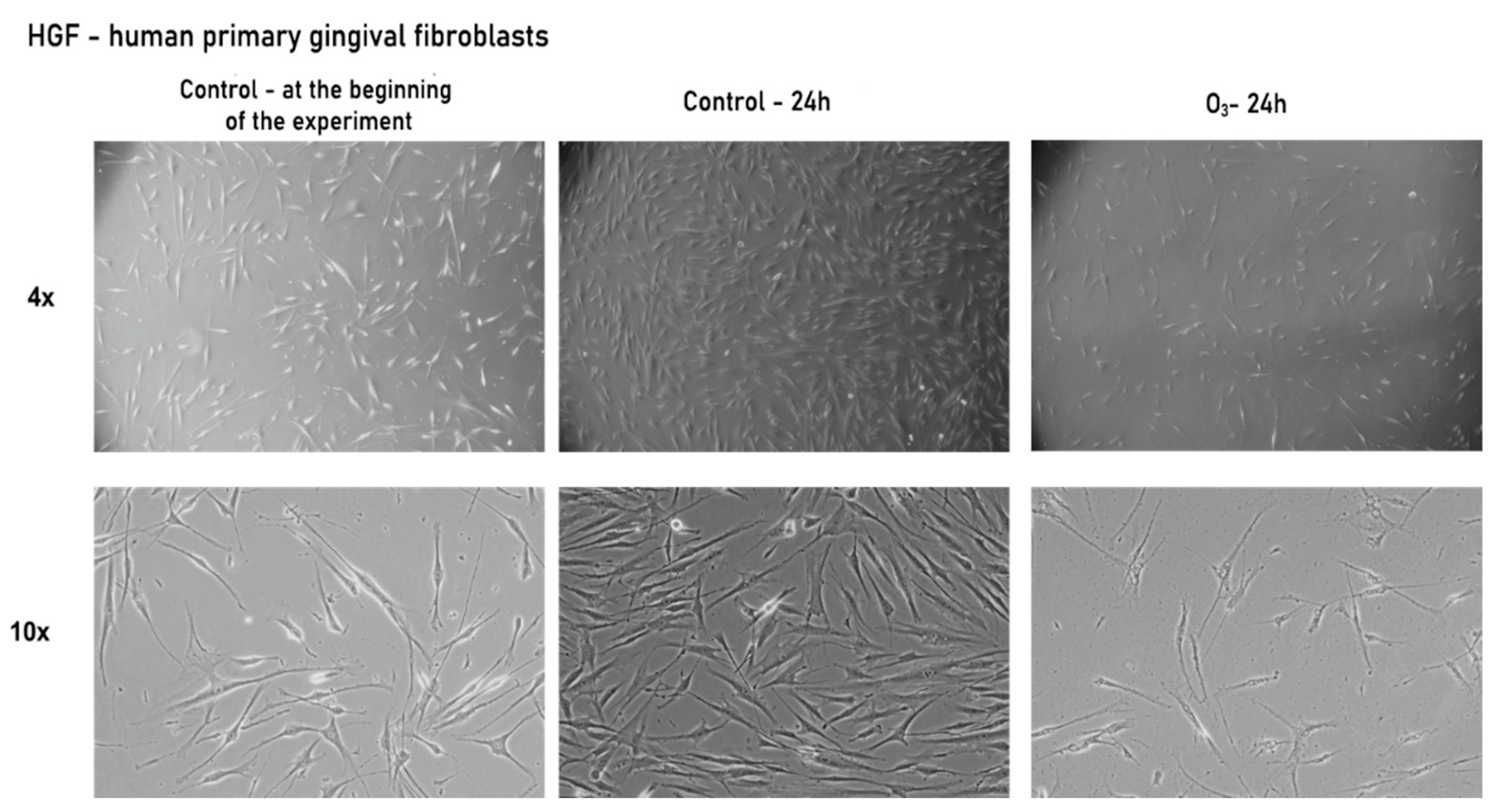
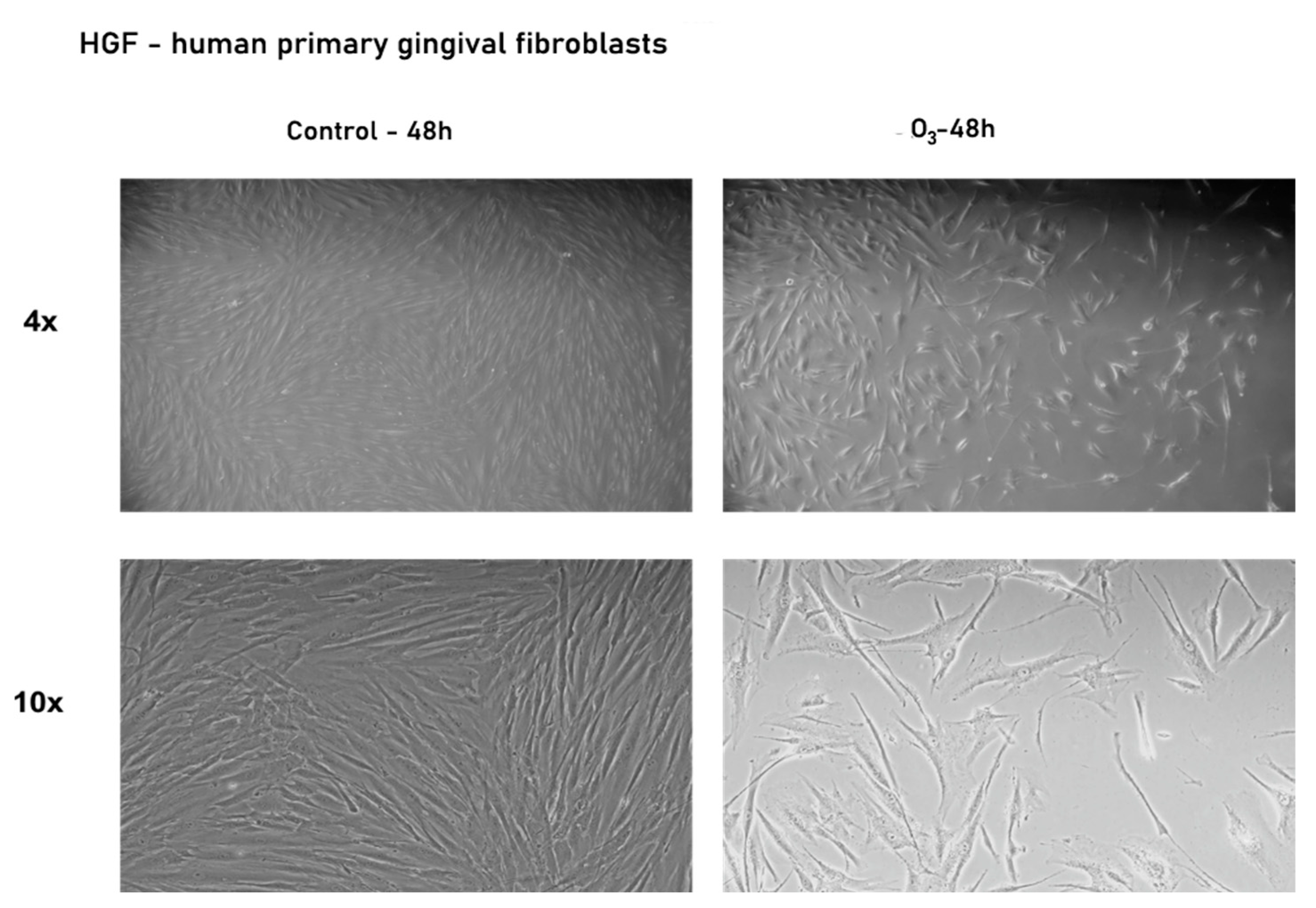


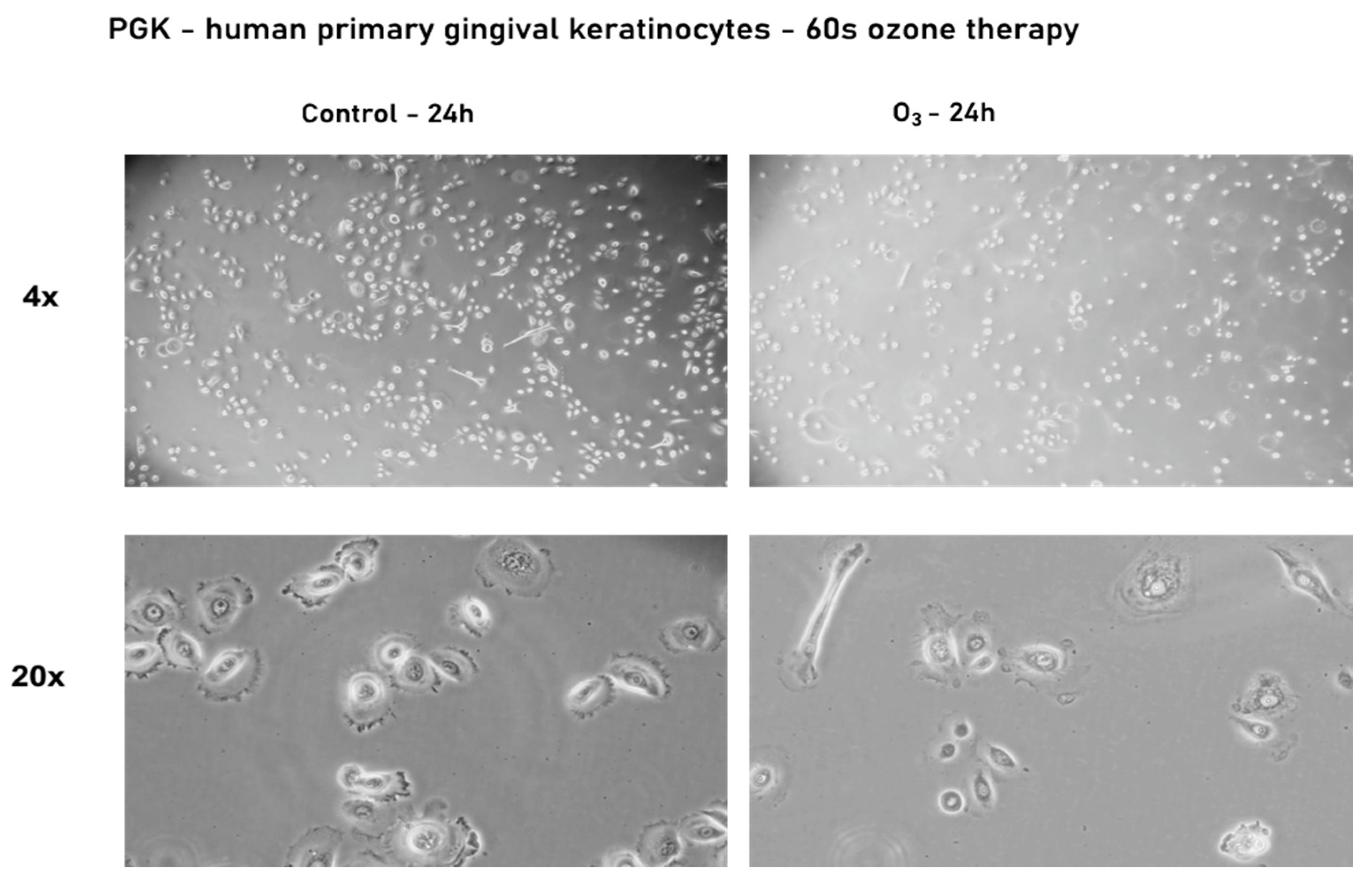
| Evaluation Stage | Mean Difference | Std. Error | 95% Confidence Interval for Difference | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Initial stage | immediate post-ozone | 6.375 * | 1.032 | 3.358 | 9.392 |
| 1 month post-ozone | 8.150 * | 1.060 | 5.051 | 11.249 | |
| Immediate post-ozone | initial | −6.375 * | 1.032 | −9.392 | −3.358 |
| 1 month post-ozone | 1.775 * | 0.251 | 1.041 | 2.509 | |
| 1 month post-ozone | initial | −8.150 * | 1.060 | −11.249 | −5.051 |
| immediate post-ozone | −1.775 * | 0.251 | −2.509 | −1.041 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floare, A.D.; Scurtu, A.D.; Balean, O.I.; Chioran, D.; Buzatu, R.; Sava Rosianu, R.; Alexa, V.T.; Jumanca, D.; Rusu, L.-C.; Racea, R.C.; et al. The Biological Effects of Ozone Gas on Soft and Hard Dental Tissues and the Impact on Human Gingival Fibroblasts and Gingival Keratinocytes. Processes 2021, 9, 1978. https://doi.org/10.3390/pr9111978
Floare AD, Scurtu AD, Balean OI, Chioran D, Buzatu R, Sava Rosianu R, Alexa VT, Jumanca D, Rusu L-C, Racea RC, et al. The Biological Effects of Ozone Gas on Soft and Hard Dental Tissues and the Impact on Human Gingival Fibroblasts and Gingival Keratinocytes. Processes. 2021; 9(11):1978. https://doi.org/10.3390/pr9111978
Chicago/Turabian StyleFloare, Alin Daniel, Alexandra Denisa Scurtu, Octavia Iulia Balean, Doina Chioran, Roxana Buzatu, Ruxandra Sava Rosianu, Vlad Tiberiu Alexa, Daniela Jumanca, Laura-Cristina Rusu, Robert Cosmin Racea, and et al. 2021. "The Biological Effects of Ozone Gas on Soft and Hard Dental Tissues and the Impact on Human Gingival Fibroblasts and Gingival Keratinocytes" Processes 9, no. 11: 1978. https://doi.org/10.3390/pr9111978
APA StyleFloare, A. D., Scurtu, A. D., Balean, O. I., Chioran, D., Buzatu, R., Sava Rosianu, R., Alexa, V. T., Jumanca, D., Rusu, L.-C., Racea, R. C., Coricovac, D., Pinzaru, I., Dehelean, C. A., & Galuscan, A. (2021). The Biological Effects of Ozone Gas on Soft and Hard Dental Tissues and the Impact on Human Gingival Fibroblasts and Gingival Keratinocytes. Processes, 9(11), 1978. https://doi.org/10.3390/pr9111978












