Purification of Textile Effluents Containing C.I. Acid Violet 1: Adsorptive Removal versus Hydrogen Peroxide and Peracetic Acid Based Advanced Oxidation
Abstract
:1. Introduction
2. Materials and Methods
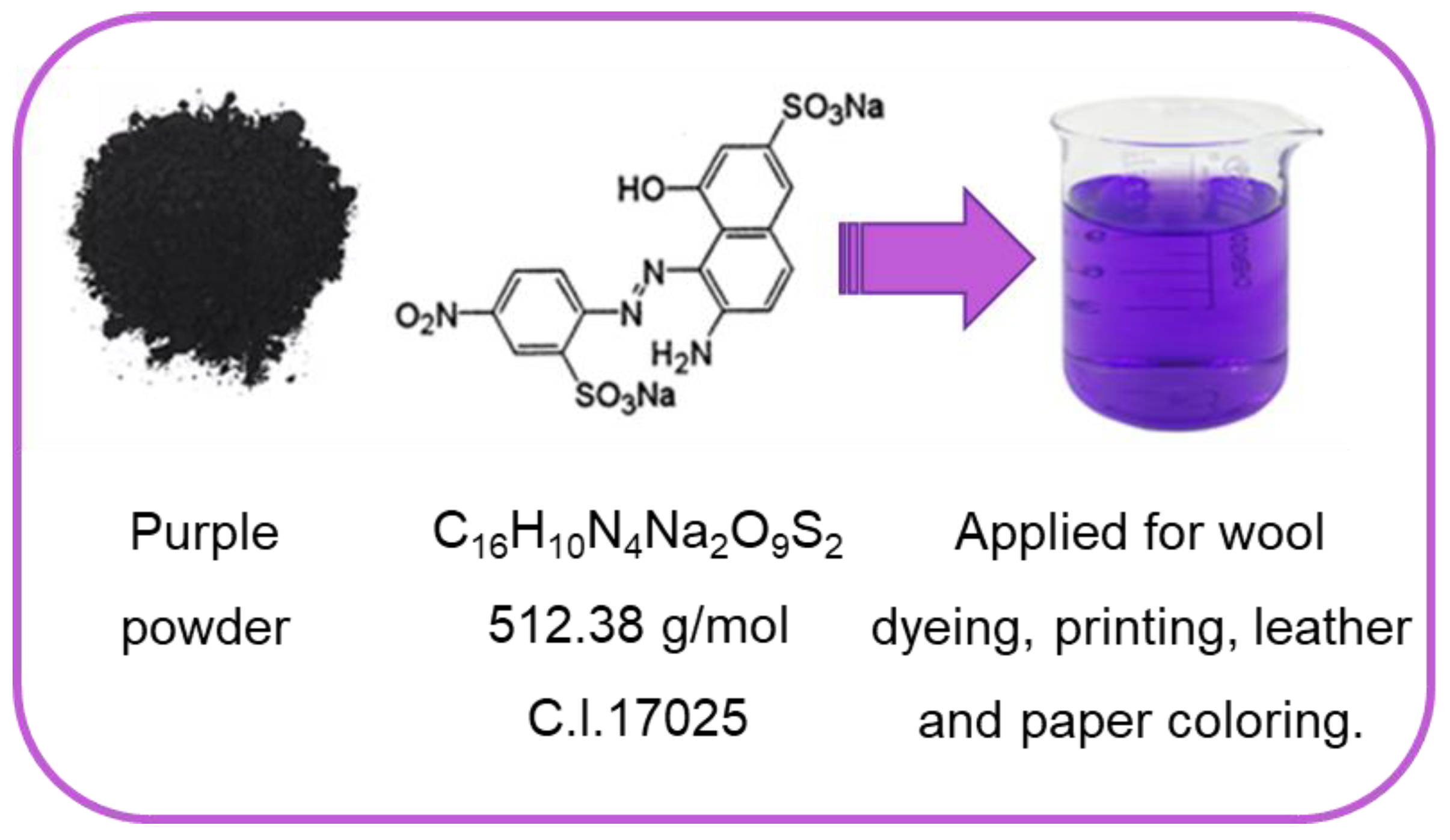
2.1. Materials
2.2. Methods
2.2.1. Adsorption and Desorption Tests
2.2.2. Advanced Oxidation Processes
3. Results
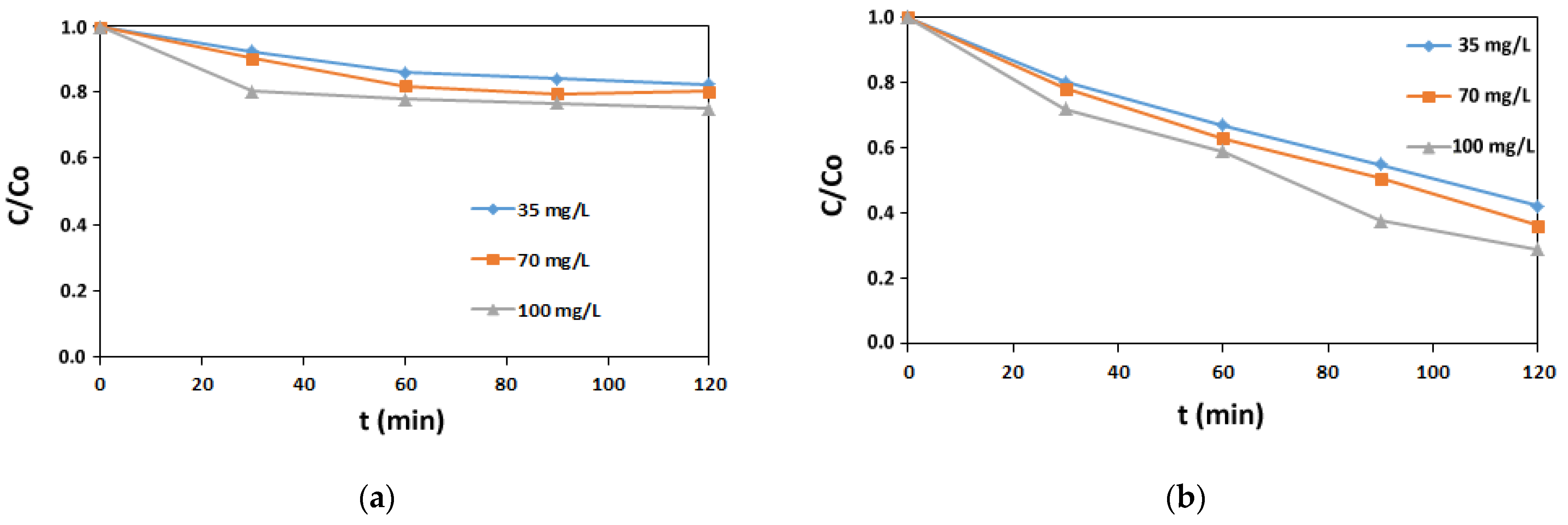
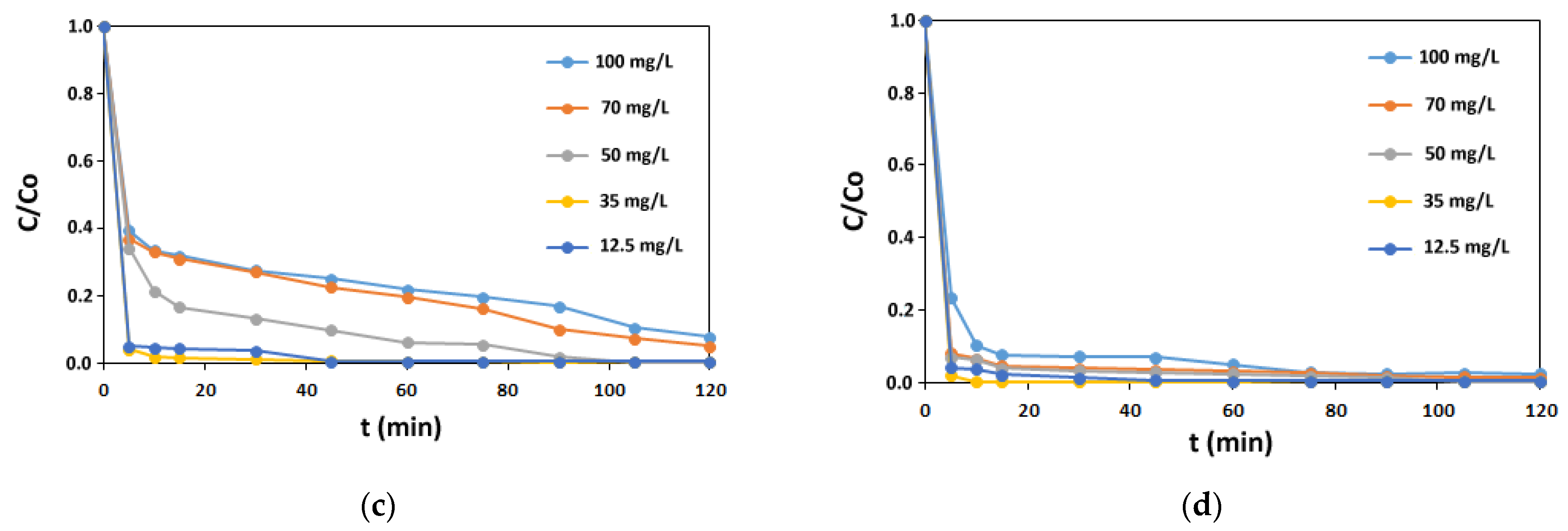
3.1. Adsorption Studies
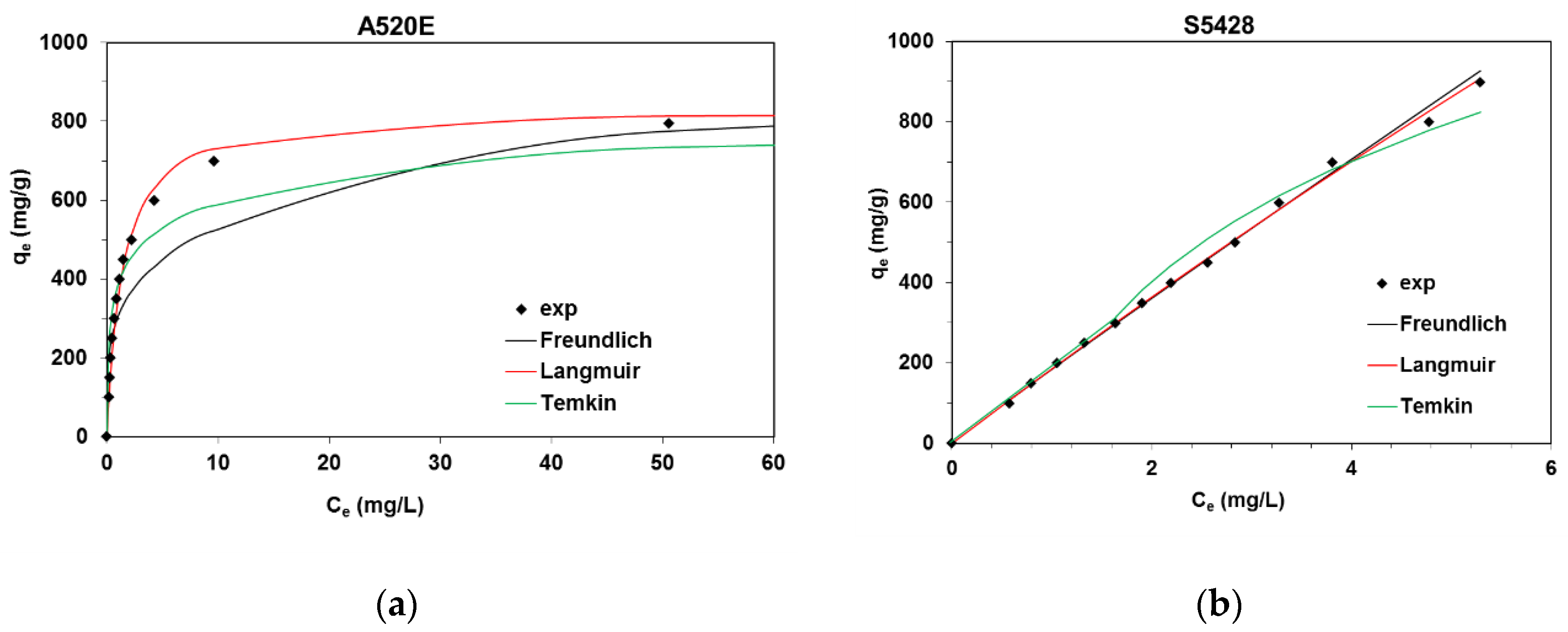
3.1.1. Isotherm Experiments
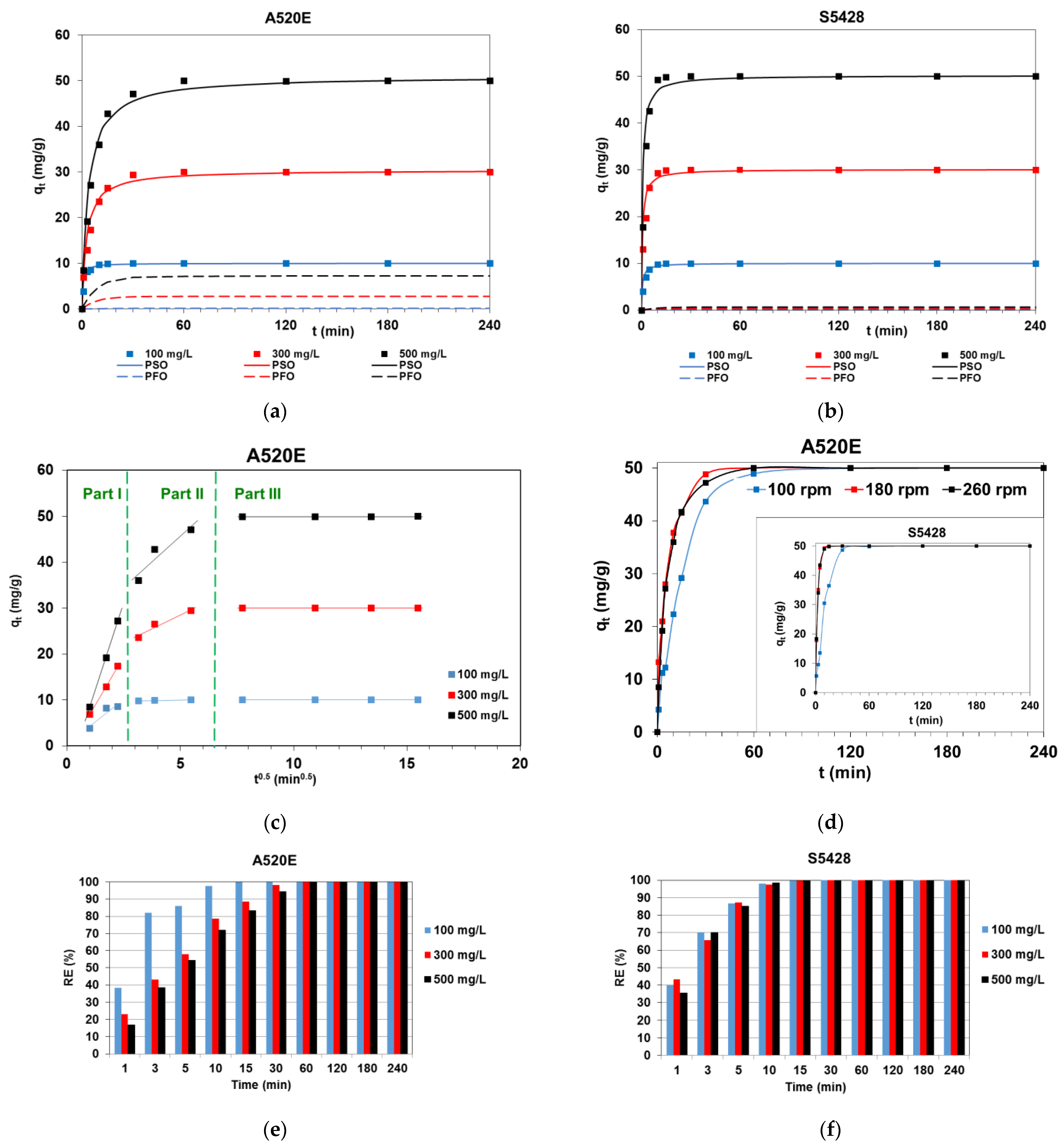
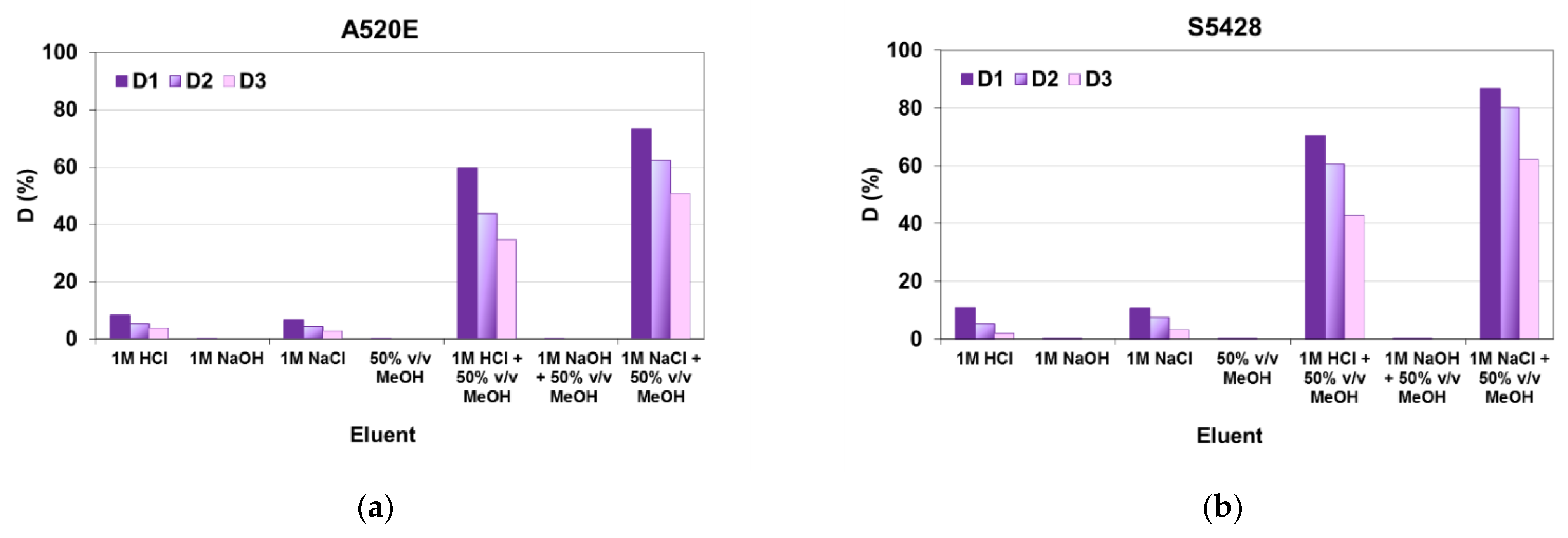
3.1.2. Kinetic Experiments
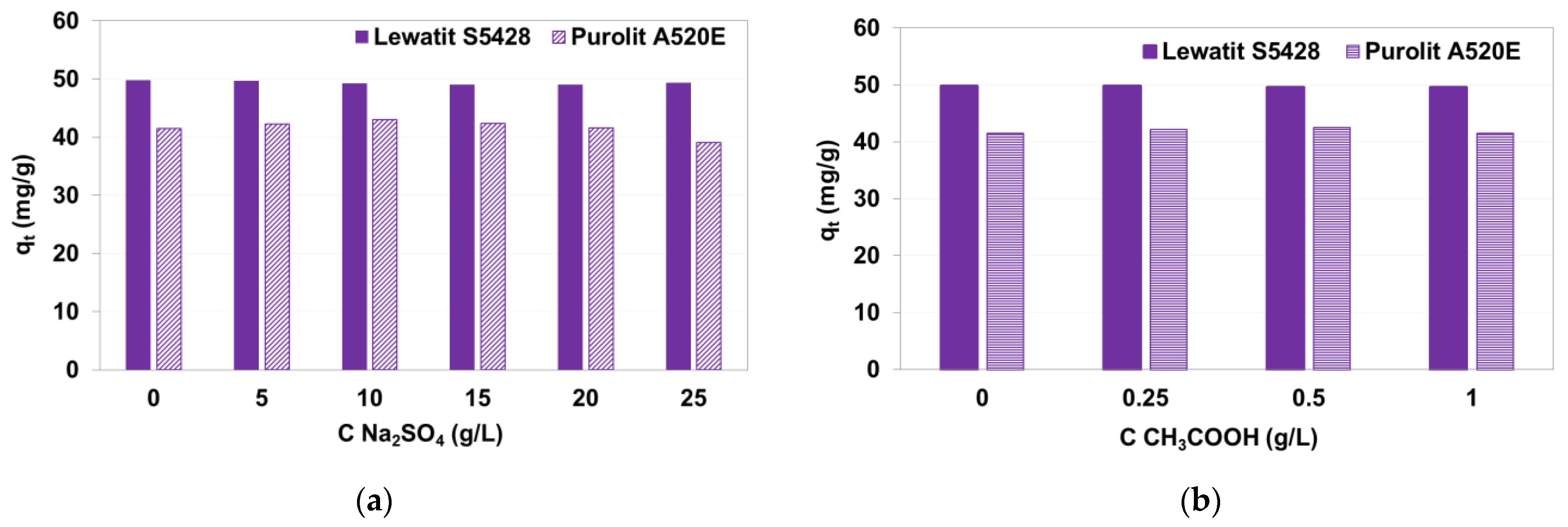
3.1.3. Impact of Auxiliaries on AV1 Sorption
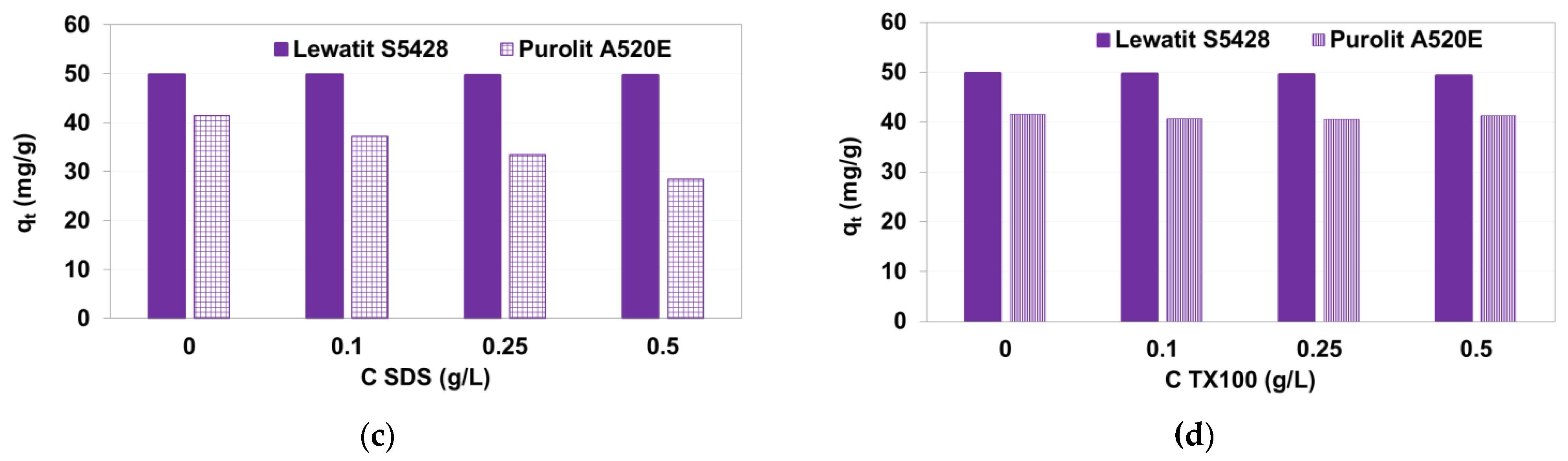
3.1.4. Desorption Experiments
3.2. Advanced Oxidation Processes
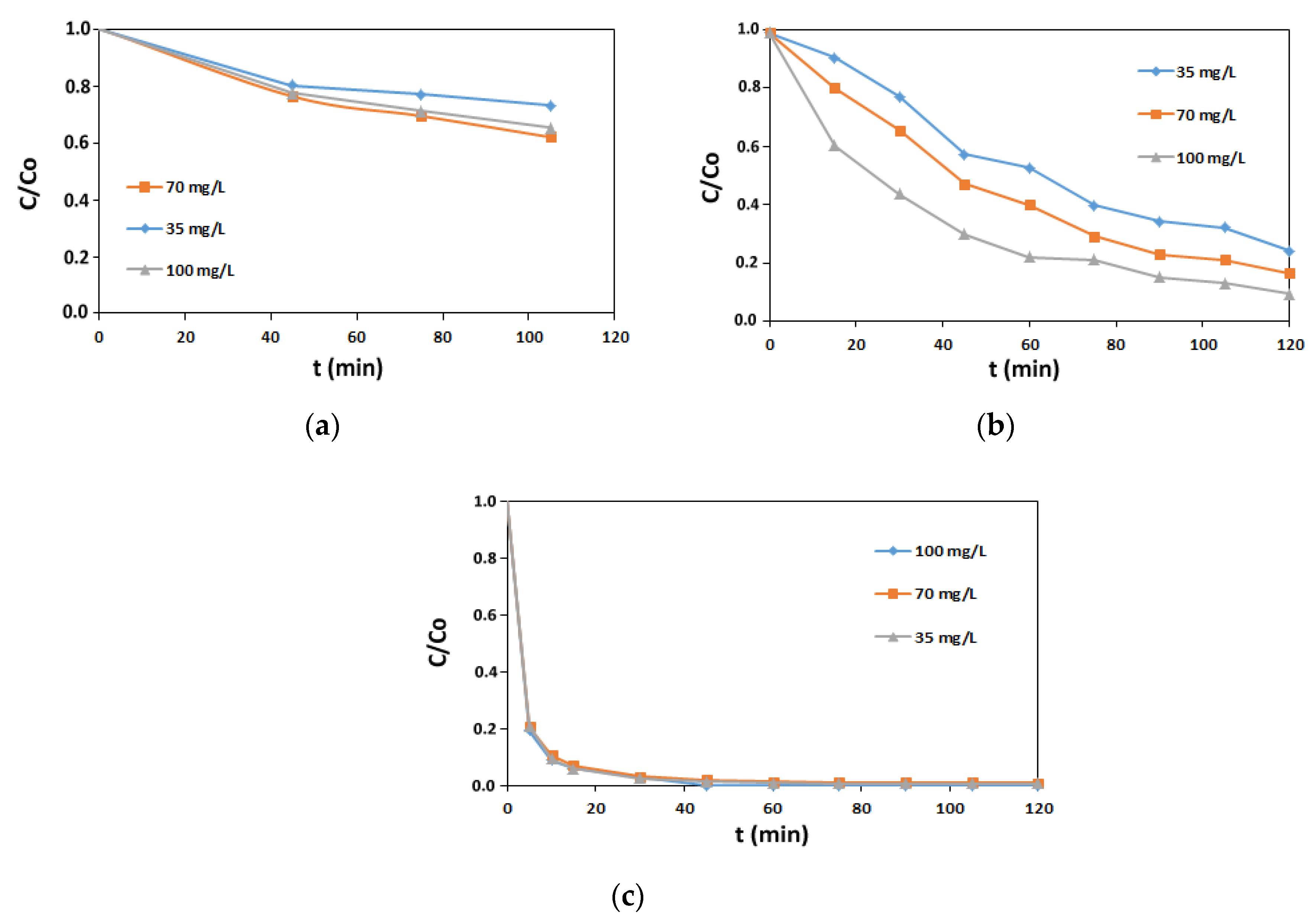
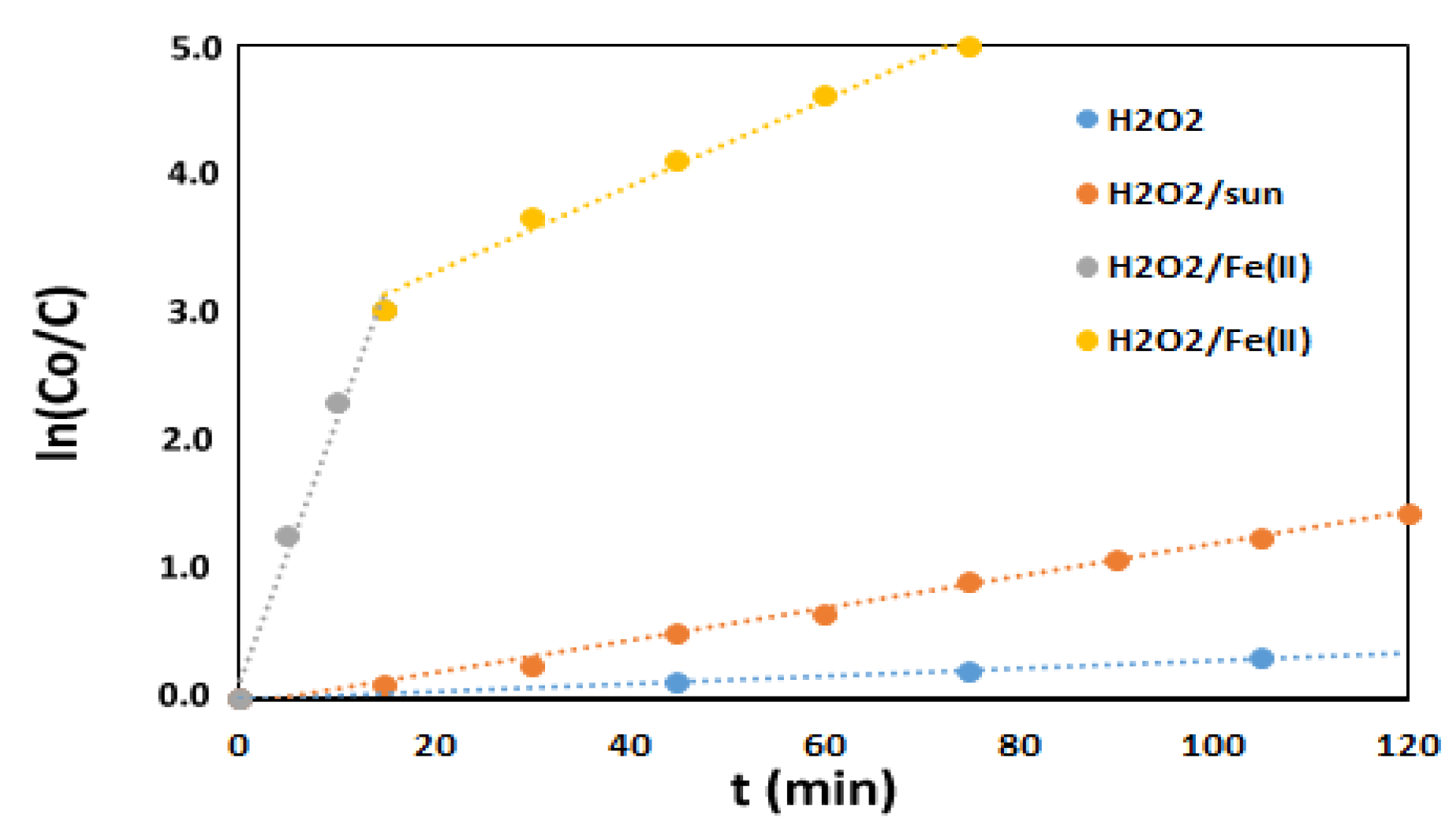
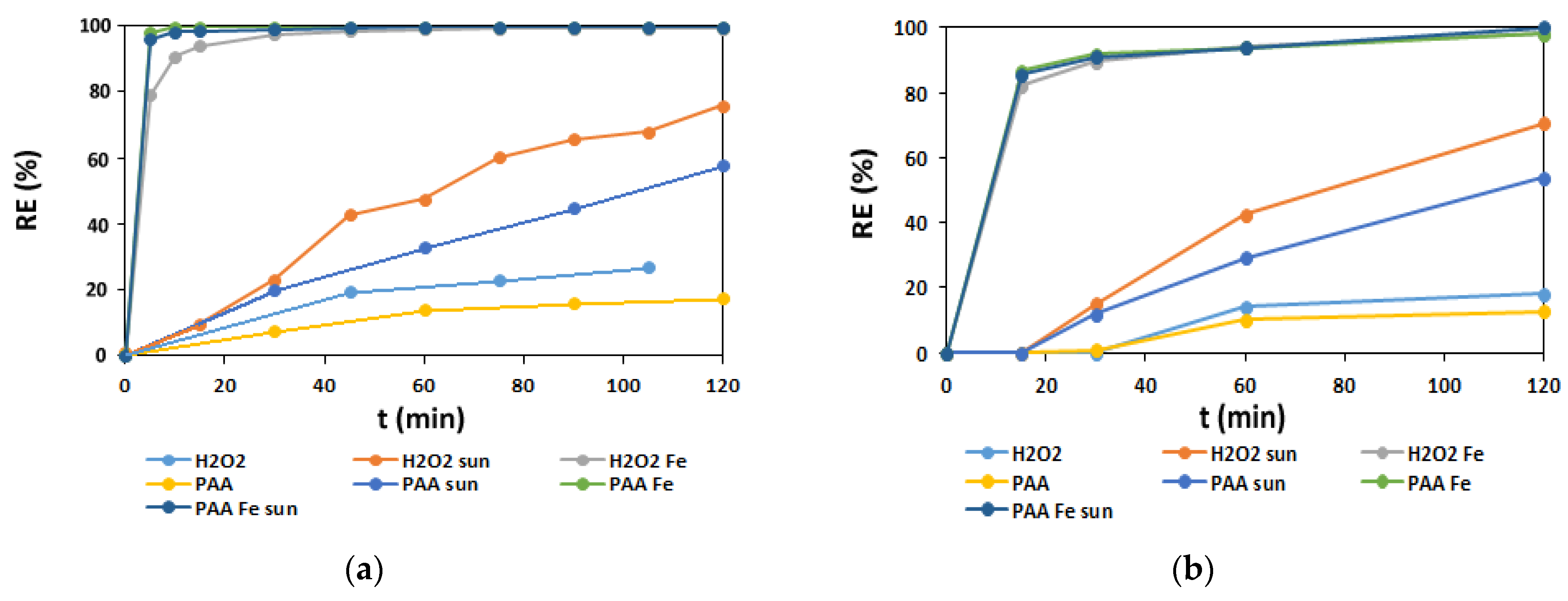
3.2.1. H2O2-Based Oxidation
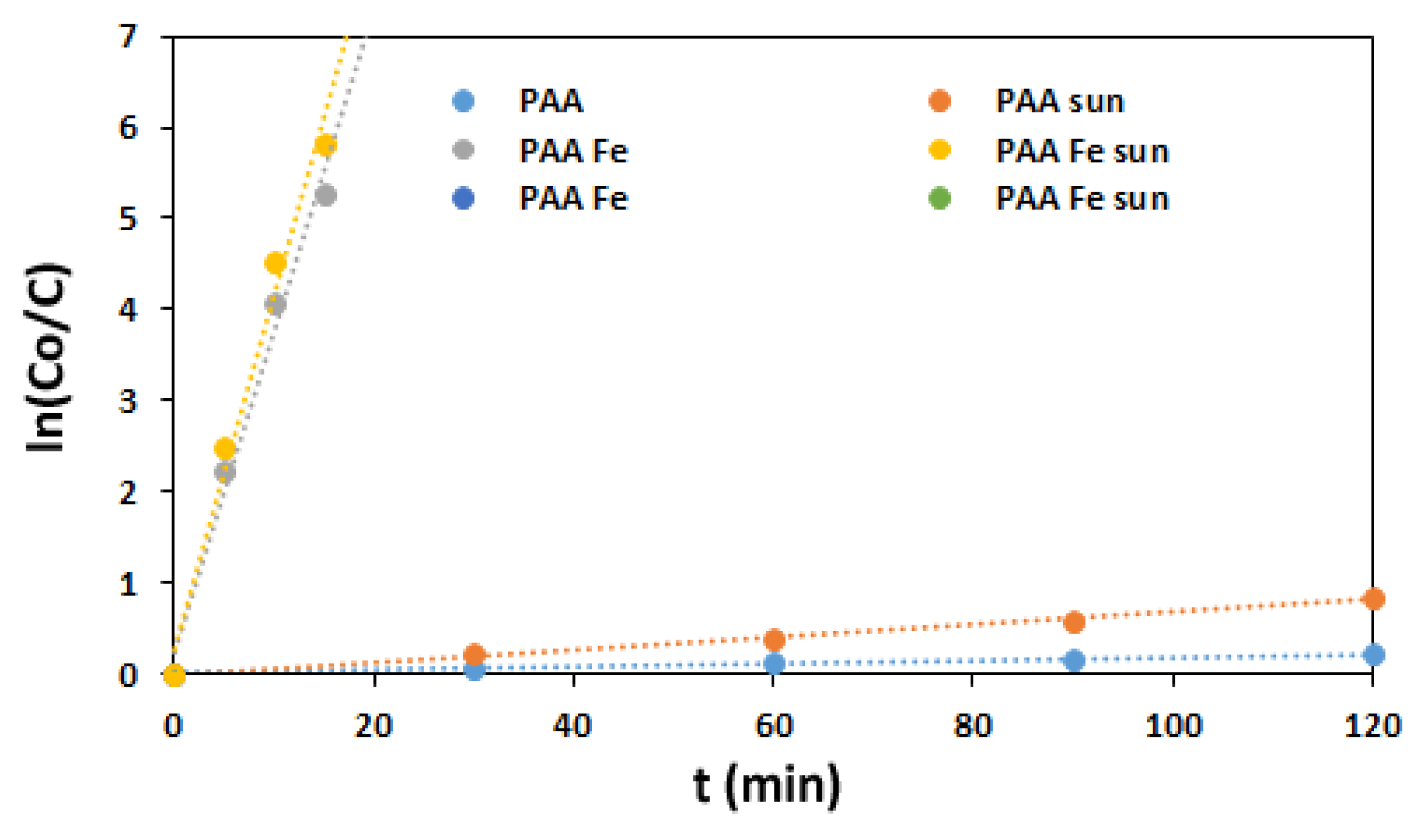
3.2.2. PAA Based Oxidation
3.2.3. AV1 Removal Efficiency in Oxidation Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sen, T.K.; Afroze, S.; Ang, H. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, H. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Mittal, A.K.; Gupta, S. Biosorption of cationic dyes by dead macro fungus Fomitopsis carnea: Batch studies. Water Sci. Technol. 1996, 34, 81–87. [Google Scholar] [CrossRef]
- Fu, Y.; Viraraghavan, T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- Mishra, G.; Tripathy, M.A. critical review of the treatments for decolourization of textile effluent. Colourage 1993, 40, 35–45. [Google Scholar]
- Lazar, T. Color chemistry: Synthesis, properties, and applications of organic dyes and pigments. Color. Res. Appl. 2005, 30, 313–314. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Ishak, S.A.; Murshed, M.F.; Md Akil, H.; Ismail, N.; Md Rasib, S.Z.; Al-Gheethi, A.A.S. The application of modified natural polymers in toxicant dye compounds wastewater: A review. Water 2020, 12, 2032. [Google Scholar] [CrossRef]
- Argumedo-Delira, R.; Gómez-Martínez, M.J.; Uribe-Kaffure, R. Trichoderma biomass as an alternative for removal of congo red and malachite green industrial dyes. Appl. Sci. 2021, 11, 448. [Google Scholar] [CrossRef]
- Duhan, M.; Kaur, R. Nano-structured polyaniline as a potential adsorbent for methylene blue dye removal from effluent. J. Compos. Sci. 2021, 5, 7. [Google Scholar] [CrossRef]
- Ma, C.M.; Hong, G.B.; Wang, Y.K. Performance evaluation and optimization of dyes removal using rice bran-based magnetic composite adsorbent. Materials 2020, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.; Haghighi, R. Chemical catalytic reaction and biological oxidation for treatment of non-biodegradable textile effluent. Chem. Eng. J. 2003, 95, 163–169. [Google Scholar] [CrossRef]
- Yagub, M.T.; Kanti Sen, T.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Kant, R. Adsorption of dye eosin from an aqueous solution on two different samples of activated carbon by static batch method. J. Water Resour. Prot. 2012, 4, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Seifikar, F.; Azizian, S.; Sillanpää, M. Microwave-assisted synthesis of carbon powder for rapid dye removal. Mater. Chem. Phys. 2020, 250, 123057. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Han, J.I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Laia, L.; Meia, P.; Lia, Y.; Lia, Y.-H.; Liu, Y. Enhanced removal efficiency of acid red 18 from aqueous solution using wheat bran modified by multiple quaternary ammonium salts. Chem. Phys. Lett. 2018, 710, 193–201. [Google Scholar] [CrossRef]
- Gupta, V. Application of low-cost adsorbents for dye removal-a review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Karcher, S.; Kornmüller, A.; Jekel, M. Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res. 2002, 36, 4717–4724. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Hubicki, Z. Remazol Black B removal from aqueous solutions and wastewater using weakly basic anion exchange resins. Cent. Eur. J. Chem. 2011, 9, 867–876. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Comparison of gel anion exchangers of various basicity in direct dye removal from aqueous solutions and wastewaters. Chem. Eng. J. 2011, 173, 773–781. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Anion exchange resins as effective sorbents for acidic dye removal from aqueous solutions and wastewaters. Solvent Extr. Ion Exch. 2012, 30, 507–523. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Comparison of the efficiency of Amberlite IRA 478RF for acid, reactive, and direct dyes removal from aqueous media and wastewaters. Ind. Eng. Chem. Res. 2012, 51, 8069–8078. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Anion-exchange resins for C.I. Direct Blue 71 removal from aqueous solutions and wastewaters: Effects of basicity and matrix composition and structure. Ind. Eng. Chem. Res. 2014, 53, 11838–11849. [Google Scholar] [CrossRef]
- Kaušpėdienė, D.; Gefenienė, A.; Kazlauskienė, E.; Ragauskas, R.; Selskienė, A. Simultaneous removal of azo and phthalocyanine dyes from aqueous solutions using weak base anion exchange resin. Water Air Soil Pollut. 2013, 224, 1769–1781. [Google Scholar] [CrossRef]
- Polska-Adach, E.; Wawrzkiewicz, M. Removal of acid, direct and reactive dyes on the polyacrylic anion exchanger. Physicochem. Probl. Miner. Process. 2019, 55, 1496–1508. [Google Scholar] [CrossRef] [Green Version]
- Wawrzkiewicz, M.; Polska-Adach, E. Physicochemical interactions in systems C.I. Direct Yellow 50—weakly basic resins: Kinetic, equilibrium, and auxiliaries addition aspects. Water 2021, 13, 385. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Podkościelna, B.; Podkościelny, P. Application of functionalized DVB-co-GMA polymeric microspheres in the enhanced sorption process of hazardous dyes from dyeing baths. Molecules 2020, 25, 5247. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Ikehata, K.; Gamal El-Din, M. Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: A review. J. Environ. Eng. Sci. 2006, 5, 81–135. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Corona-Bautista, M.; Picos-Benítez, A.; Villaseñor-Basulto, D.; Bandala, E.; Peralta-Hernández, J.M. Discoloration of azo dye Brown HT using different advanced oxidation processes. Chemosphere 2021, 267, 129234. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Evaluation of UV/H2O2 advanced oxidation process (AOP) for the degradation of diazo dye Reactive Green 19 in aqueous solution. Desalin. Water Treat. 2014, 52, 1571–1577. [Google Scholar] [CrossRef]
- Tarkwa, J.B.; Oturan, N.; Acayanka, E.; Laminsi, S.; Oturan, M.A. Photo-Fenton oxidation of Orange G azo dye: Process optimization and mineralization mechanism. Environ. Chem. Lett. 2019, 17, 473–479. [Google Scholar] [CrossRef]
- Oliveira, M.; Neves, N.; Santana, R.; Lucena, A.; Zaidan, L.; Cavalcanti, V.; Silva, G.; Napoleão, D. Employment of advanced oxidation processes in the degradation of a textile dye mixture: Evaluation of reaction parameters, kinetic study, toxicity and modeling by artificial neural networks. REGET 2021, 25, 12. [Google Scholar] [CrossRef]
- Çiner, F.; Gökkuş, Ö. Treatability of dye solutions containing disperse dyes by Fenton and Fenton-solar light oxidation processes. Clean-Soil Air Water 2013, 41, 80–85. [Google Scholar] [CrossRef]
- Clematis, D.; Panizza, M. Electro-Fenton, solar photoelectro-Fenton and UVA photoelectro-Fenton: Degradation of Erythrosine B dye solution. Chemosphere 2021, 270, 129480. [Google Scholar] [CrossRef]
- Kiejza, D.; Kotowska, U.; Polińska, W.; Karpińska, J. Peracids—New oxidants in advanced oxidation processes: The use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern—A review. Sci. Total Environ. 2021, 790, 148195. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.-W.; Eloranta, J.; Huang, C.-H.; Santoro, D.; Sun, W.-J.; Lu, Z.-D.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Available online: https://www.purolite.com/product-pdf/A520E.pdf (accessed on 23 February 2021).
- Available online: https://www.lenntech.com/Data-sheets/Lewatit-S-5428-L.pdf (accessed on 23 February 2021).
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physicochim. USSR 1940, 12, 327–356. [Google Scholar]
- Bulut, E.; Özacar, M.; Şengil, İ.A. Equilibrium and kinetic data and process design for adsorption of Congo Red onto bentonite. J. Hazard. Mater. 2008, 154, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Webber, T.W.; Chakkravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AlChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Namasivayam, C.; Yamuna, R.; Arasi, D.J.S.E. Removal of acid violet from wastewater by adsorption on waste red mud. Environ. Geol. 2001, 41, 269–273. [Google Scholar] [CrossRef]
- Krysztafkiewicz, A.; Binkowski, S.; Jesionowski, T. Adsorption of dyes on a silica surface. Appl. Surf. Sci. 2002, 199, 31–39. [Google Scholar] [CrossRef]
- Wrzesińska, K.; Wawrzkiewicz, M.; Szymczyk, K. Physicochemical interactions in C.I. Acid Green 16–Lewatit S 6368A systems–kinetic, equilibrium, auxiliaries addition and thermodynamic aspects. J. Mol. Liq. 2021, 331, 115748. [Google Scholar] [CrossRef]
- Kotowska, U.; Wawrzkiewicz, M.; Polska-Adach, E. Mechanism of interactions in C.I. Acid Red 18–Floating plants and polymeric resins systems: Kinetic, equilibrium, auxiliaries impact and column studies. J. Mol. Liq. 2021, 333, 115903. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Teixeira, F.A.; Bokach, N.A.; Pombeiro, A.J.L.; Shul’pin, G.B. Radical decomposition of hydrogen peroxide catalyzed by aqua complexes [M(H2O)n]2+ (M=Be, Zn, Cd). J. Catal. 2014, 313, 135–148. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, T.; Liu, W.; Du, P.; Dobson, J.T.; Huang, C.-H. Advanced oxidation process with peracetic acid and Fe(II) for contaminant degradation. Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef]
- Tosik, R. Dyes color removal by ozone and hydrogen peroxide: Some aspects and problems. Ozone Sci. Eng. 2005, 27, 265–271. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Khan, H.M.; Khattak, R. Degradation of Crystal Violet dye by Fenton and photo-Fenton oxidation processes. Z. Für Phys. Chem. 2018, 232, 1771–1786. [Google Scholar] [CrossRef]
- Rokhina, E.; Makarova, K.; Lahtinen, M.; Golovina, E.; As, H.; Virkutyte, J. Ultrasound-assisted MnO2 catalyzed homolysis of peracetic acid for phenol degradation: The assessment of process chemistry and kinetics. Chem. Eng. J. 2013, 221, 476–486. [Google Scholar] [CrossRef]
| Properties | Lewatit S5428 | Purolite A520E |
|---|---|---|
| Functional groups | Quaternary ammonium, type 1 | |
| Ionic form as shipped | Cl− | |
| Matrix | Crosslinked polyacrylamide | Crosslinked polystyrene |
| Structure | macroporous | |
| Total capacity (eq/L) | 0.85 | 0.9 |
| Water retention (%) | 63–68 | 50–56 |
| Mean bead size (mm) | 0.4–1.6 | 0.3–1.2 |
| Max. operating temperature (°C) | 80 | 100 |
| Operating pH range | 0–12 | 0–12 |
| Appearance | White, opaque | Cream, opaque |
| Manufacturer | Lenntech, The Neterhlands | Purolite, United States |
| Isotherm | Equation No. | Linear Forms | Plot |
|---|---|---|---|
| Langmuir | (4) | Ce/qe vs. Ce | |
| Freundlich | (5) | log qe vs. log Ce | |
| Temkin | (6) | qe vs. ln Ce | |
| Pseudo-first order (PFO) | (7) | vs. t | |
| Pseudo-second order (PSO) | (8) | t/qt vs. t | |
| Intraparticle diffusion (IPD) | (9) | qt vs. t0.5 |
| Isotherm | Parameters | |
|---|---|---|
| Purolite A520E | Lewatit S5428 | |
| Langmuir | ||
| Q0 (mg/g) | 835.8 | 9867.9 |
| kL (L/mg) | 0.718 | 0.019 |
| R2 | 0.999 | 0.435 |
| Freundlich | ||
| kF (mg1−1/n L1/n/g) | 305.3 | 184.5 |
| 1/n | 0.238 | 0.967 |
| R2 | 0.834 | 0.997 |
| Temkin | ||
| A (L/g) | 75.68 | 1.69 |
| bT (J/mol) | 27.82 | 6.98 |
| R2 | 0.881 | 0.927 |
| Model | Parameter | Initial Dye Concentration C0 (mg/L) | ||
|---|---|---|---|---|
| 100 | 300 | 500 | ||
| Purolite A520E | ||||
| PFO | qe (mg/g) | 0.380 | 6.40 | 16.60 |
| k1 (1/min) | 0.028 | 0.0502 | 0.0494 | |
| R2 | 0.449 | 0.733 | 0.835 | |
| PSO | qe (mg/g) | 10.02 | 30.40 | 50.90 |
| k2 (g/mg min) | 0.181 | 0.012 | 0.005 | |
| R2 | 0.999 | 0.999 | 0.999 | |
| IPD | ki1 (mg/g min0.5) | 3.99 | 8.44 | 15.09 |
| R21 | 0.887 | 0.999 | 0.999 | |
| ki2 (mg/g min0.5) | 0.078 | 2.42 | 4.45 | |
| R22 | 0.572 | 0.948 | 0.885 | |
| ki3 (mg/g min0.5) | 0.00001 | 0.00009 | 0.004 | |
| R23 | 0.691 | 0.822 | 0.791 | |
| qe,exp (mg/g) | 9.99 | 29.99 | 49.99 | |
| Lewatit S5428 | ||||
| PFO | qe (mg/g) | 1.38 | 2.94 | 1.6 |
| k1 (1/min) | 0.028 | 0.035 | 0.039 | |
| R2 | 0.421 | 0.463 | 0.504 | |
| PSO | qe (mg/g) | 10.02 | 30.10 | 50.19 |
| k2 (g/mg min) | 0.161 | 0.052 | 0.029 | |
| R2 | 0.999 | 0.999 | 0.999 | |
| IPD | ki1 (mg/g min0.5) | 4.33 | 7.21 | 14.32 |
| R21 | 0.972 | 0.965 | 0.946 | |
| ki2 (mg/g min0.5) | 1.21 | 3.59 | 6.43 | |
| R22 | 0.679 | 0.703 | 0.671 | |
| ki3 (mg/g min0.5) | 0.00002 | 0.00005 | 0.00013 | |
| R23 | 0.721 | 0.935 | 0.896 | |
| qe,exp (mg/g) | 9.99 | 29.99 | 49.99 | |
| Degradation Process | R2 | k1 (min−1) | t1/2 (min) | t99%(min) |
|---|---|---|---|---|
| H2O2 alone | 0.999 | 0.0030 | 231 | 1535 |
| H2O2/sunlight | 0.999 | 0.0123 | 56 | 374 |
| Fenton (0–15 min) | 0.993 | 0.2001 | 3.5 | 23 |
| Fenton (15–75 min) | 0.994 | 0.0330 | 21 | 140 |
| Degradation Proces | R2 | k1 (min−1) | t1/2 (min) | t99%(min) |
|---|---|---|---|---|
| PAA alone | 0.990 | 0.0017 | 408 | 2709 |
| PAA/sunlight | 0.999 | 0.0070 | 99 | 658 |
| PAA/Fe2+ (0–15 min) | 0.991 | 0.3527 | 2 | 13 |
| PAA/Fe2+/sun (0–15 min) | 0.991 | 0.3897 | 1.8 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzkiewicz, M.; Kotowska, U.; Sokół, A. Purification of Textile Effluents Containing C.I. Acid Violet 1: Adsorptive Removal versus Hydrogen Peroxide and Peracetic Acid Based Advanced Oxidation. Processes 2021, 9, 1911. https://doi.org/10.3390/pr9111911
Wawrzkiewicz M, Kotowska U, Sokół A. Purification of Textile Effluents Containing C.I. Acid Violet 1: Adsorptive Removal versus Hydrogen Peroxide and Peracetic Acid Based Advanced Oxidation. Processes. 2021; 9(11):1911. https://doi.org/10.3390/pr9111911
Chicago/Turabian StyleWawrzkiewicz, Monika, Urszula Kotowska, and Aneta Sokół. 2021. "Purification of Textile Effluents Containing C.I. Acid Violet 1: Adsorptive Removal versus Hydrogen Peroxide and Peracetic Acid Based Advanced Oxidation" Processes 9, no. 11: 1911. https://doi.org/10.3390/pr9111911
APA StyleWawrzkiewicz, M., Kotowska, U., & Sokół, A. (2021). Purification of Textile Effluents Containing C.I. Acid Violet 1: Adsorptive Removal versus Hydrogen Peroxide and Peracetic Acid Based Advanced Oxidation. Processes, 9(11), 1911. https://doi.org/10.3390/pr9111911