Investigating the Solubility and Activity of a Novel Class of Ice Recrystallization Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Ice Recrystallization Inhibition (IRI) Assay
2.2. HepG2 Cell Culture
2.3. AlamarBlueTM Assay for Cytotoxicity
3. Results
3.1. Solubility of Sodium Phosphonate Compounds
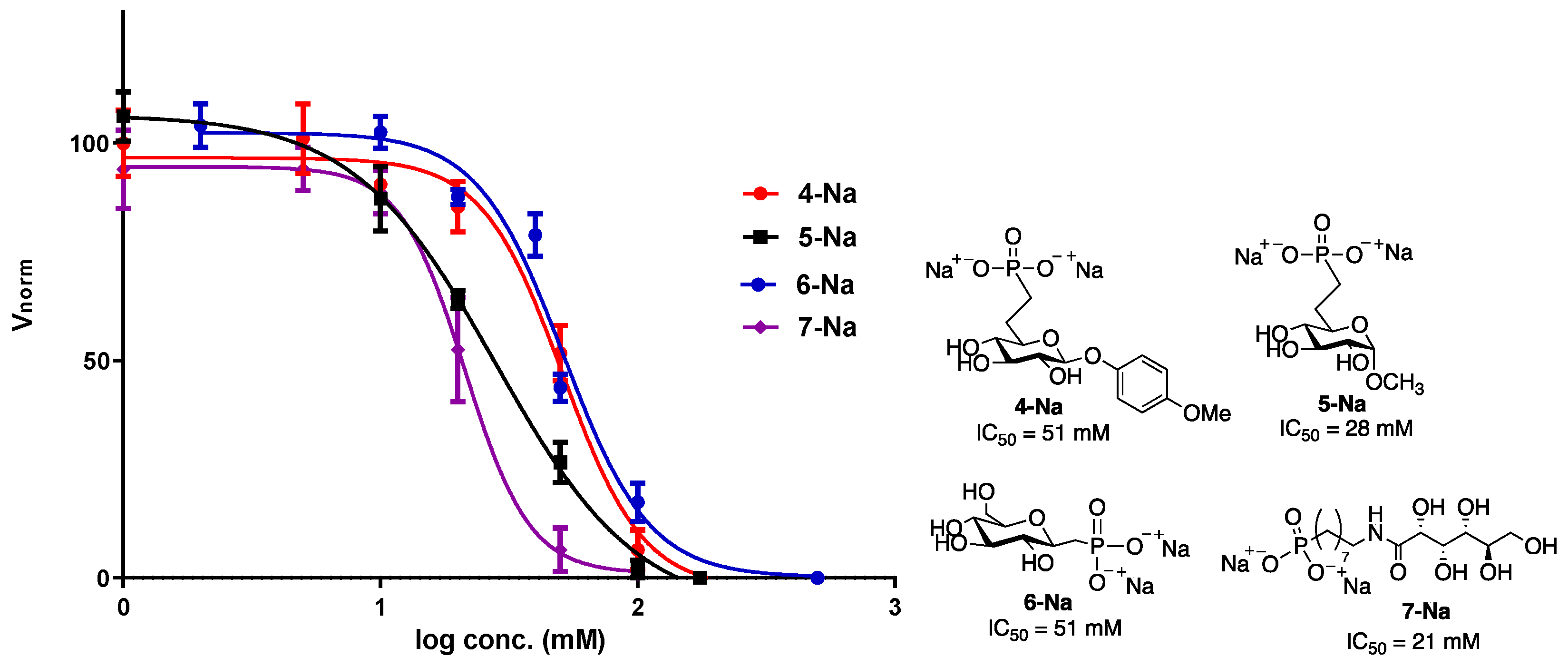
3.2. IRI Analysis of Sodium Phosphonate Derivatives
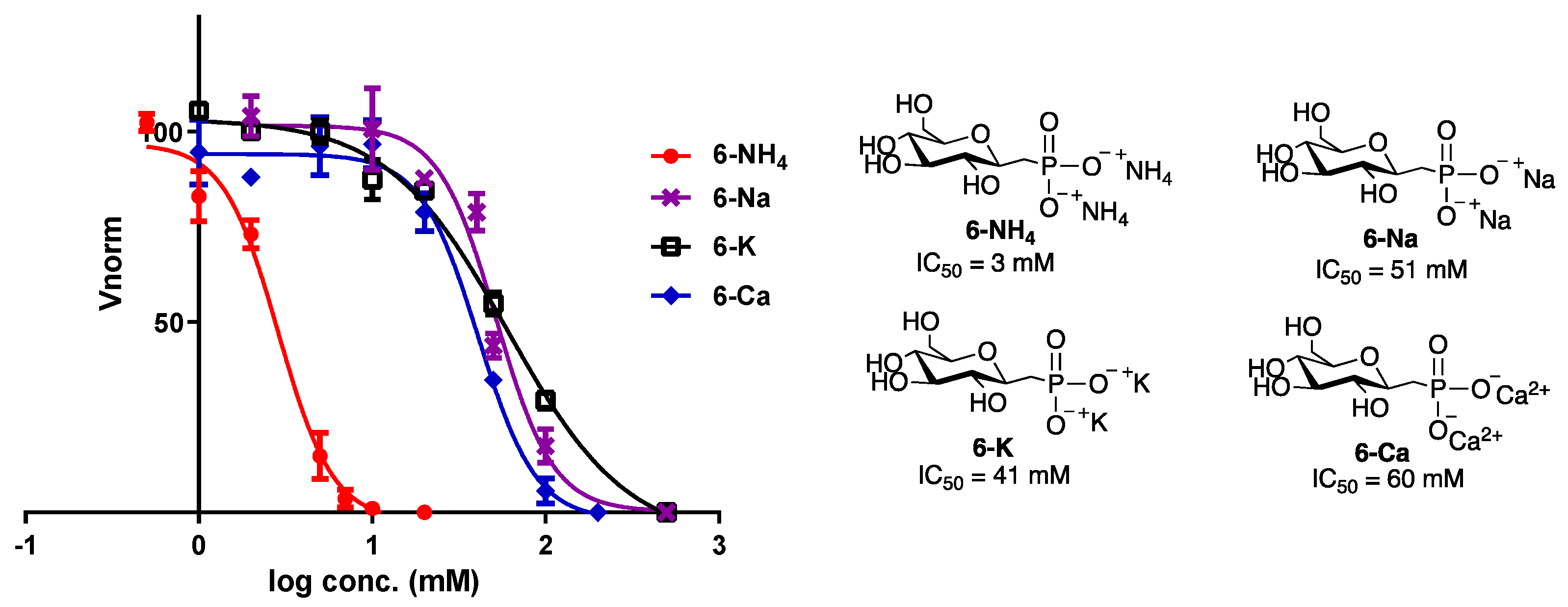
3.3. IRI Activity of Phosphonate Compounds with Varying Counterions
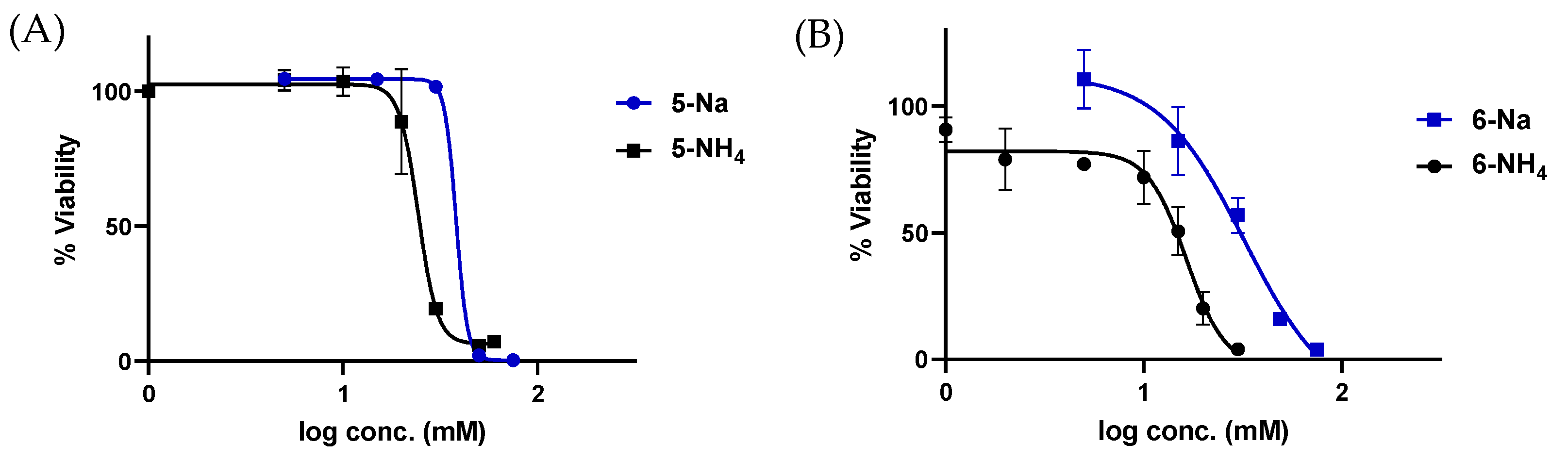
3.4. Toxicity of Phosphonates 5 and 6 in HepG2 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and Its Clinical Applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Jahan, S.; Kaushal, R.; Pasha, R.; Pineault, N. Current and Future Perspectives for the Cryopreservation of Cord Blood Stem Cells. Transfus. Med. Rev. 2021, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meneghel, J.; Kilbride, P.; Morris, G.J. Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review. Front. Med. 2020, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.; et al. The Promise of Organ and Tissue Preservation to Transform Medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Lahmann, J.M.; Sanchez, C.C.; Benson, J.D.; Acker, J.P.; Higgins, A.Z. Implications of Variability in Cell Membrane Permeability for Design of Methods to Remove Glycerol from Frozen-Thawed Erythrocytes. Cryobiology 2020, 92, 168–179. [Google Scholar] [CrossRef]
- Awan, M.; Buriak, I.; Fleck, R.; Fuller, B.; Goltsev, A.; Kerby, J.; Lowdell, M.; Mericka, P.; Petrenko, A.; Petrenko, Y.; et al. Dimethyl Sulfoxide: A Central Player since the Dawn of Cryobiology, Is Efficacy Balanced by Toxicity? Regen. Med. 2020, 15, 1463–1491. [Google Scholar] [CrossRef]
- Baboo, J.; Kilbride, P.; Delahaye, M.; Milne, S.; Fonseca, F.; Blanco, M.; Meneghel, J.; Nancekievill, A.; Gaddum, N.; Morris, G.J. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Rall, W.F.; Mazur, P.; Souzu, H. Physical-Chemical Basis of the Protection of Slowly Frozen Human Erythrocytes by Glycerol. Biophys. J. 1978, 23, 101–120. [Google Scholar] [CrossRef]
- Honadel, T.E.; Killian, G.J. Cryopreservation of Murine Embryos with Trehalose and Glycerol. Cryobiology 1988, 25, 331–337. [Google Scholar] [CrossRef]
- Krijnen, H.W.; de Wit, J.J.; Kuivenhoven, A.C.; Loos, J.A.; Prins, H. Glycerol Treated Human Red Cells Frozen with Liquid Nitrogen. Vox Sang 1964, 9, 559–572. [Google Scholar] [CrossRef]
- Meira, C.; Alvarenga, M.A.; Papa, F.O.; Oba, E.; Alvarenga, F.C.L. Cryopreservation of Equine Embryos Using Glycerol and 1,2-Propanediol as Cryoprotectants. Equine Vet. J. 2010, 25, 64–66. [Google Scholar] [CrossRef]
- Lovelock, J.E. Het Mechanism of the Protective Action of Glycerol against Haemolysis by Freezing and Thawing. Biochim. Biophys. Acta 1953, 11, 28–36. [Google Scholar] [CrossRef]
- Meryman, H.T.; Hornblower, M. A Method for Freezing and Washing Red Blood Cells Using a High Glycerol Concentration. Transfusion 1972, 12, 145–156. [Google Scholar] [CrossRef]
- Fahy, G.M. Analysis of “Solution Effects” Injury: Rabbit Renal Cortex Frozen in the Presence of Dimethyl Sulfoxide. Cryobiology 1980, 17, 371–388. [Google Scholar] [CrossRef]
- Lovelock, J.E. The Protective Action of Neutral Solutes against Haemolysis by Freezing and Thawing. Biochem. J. 1954, 56, 265–270. [Google Scholar] [CrossRef]
- Lovelock, J.E.; Bishop, M.W.H. Prevention of Freezing Damage to Living Cells by Dimethyl Sulphoxide. Nature 1959, 183, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P. Cryobiology: The Freezing of Biological Systems. Science 1970, 168, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P. Freezing of Cells: Mechanisms and Implications. Am. J. Physiol. 1984, 247, C125–C142. [Google Scholar] [CrossRef]
- Poisson, J.S.; Briard, J.G.; Turner, T.R.; Acker, J.P.; Ben, R.N. Hydroxyethyl Starch Supplemented with Ice Recrystallization Inhibitors Greatly Improves Cryopreservation of Human Red Blood Cells. Bioprocess. J. 2017, 15, 16–21. [Google Scholar] [CrossRef]
- Capicciotti, C.J.; Kurach, J.D.; Turner, T.R.; Mancini, R.S.; Acker, J.P.; Ben, R.N. Small Molecule Ice Recrystallization Inhibitors Enable Freezing of Human Red Blood Cells with Reduced Glycerol Concentrations. Nat. Sci. Rep. 2015, 5, 9692. [Google Scholar] [CrossRef]
- Briard, J.G.; Poisson, J.S.; Turner, T.R.; Capiccioti, C.J.; Acker, J.P.; Ben, R.N. Small Molecule Ice Recrystallization Inhibitors Mitigate Red Blood Cell Lysis during Freezing, Transient Warming and Thawing. Sci. Rep. 2016, 6, 23619. [Google Scholar] [CrossRef] [PubMed]
- Briard, J.G.; Jahan, S.; Chandran, P.; Allan, D.; Pineault, N.; Ben, R.N. Small-Molecule Ice Recrystallization Inhibitors Improve the Post-Thaw Function of Hematopoietic Stem and Progenitor Cells. ACS Omega 2016, 1, 1010–1018. [Google Scholar] [CrossRef]
- Jahan, S.; Adam, M.K.; Manesia, J.K.; Doxtator, E.; Ben, R.N.; Pineault, N. Inhibition of Ice Recrystallization during Cryopreservation of Cord Blood Grafts Improves Platelet Engraftment. Transfusion 2020, 60, 769–778. [Google Scholar] [CrossRef]
- Poisson, J.S.; Acker, J.P.; Briard, J.G.; Meyer, J.E.; Ben, R.N. Modulating Intracellular Ice Growth with Cell-Permeating Small-Molecule Ice Recrystallization Inhibitors. Langmuir 2019, 35, 7452–7458. [Google Scholar] [CrossRef]
- Suris-Valls, R.; Voets, I.K. The Impact of Salts on the Ice Recrystallization Inhibition Activity of Antifreeze (Glyco)Proteins. Biomolecules 2019, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Sevrain, C.M.; Berchel, M.; Couthon, H.; Jaffrès, P.-A. Phosphonic Acid: Preparation and Applications. Beilstein J. Org. Chem. 2017, 13, 2186–2213. [Google Scholar] [CrossRef] [PubMed]
- Déjugnat, C.; Etemad-Moghadam, G.; Rico-Lattes, I. Asymmetric Synthesis of (α-Amino) Phosphonic Acid Amphiphiles Using Chiral P–H Spirophosphoranes. Chem. Commun. 2003, 1858–1859. [Google Scholar] [CrossRef]
- Capicciotti, C.J.; Mancini, R.S.; Turner, T.R.; Koyama, T.; Alteen, M.G.; Doshi, M.; Inada, T.; Acker, J.P.; Ben, R.N. O-Aryl-Glycoside Ice Recrystallization Inhibitors as Novel Cryoprotectants: A Structure-Function Study. ACS Omega 2016, 1, 656–662. [Google Scholar] [CrossRef]
- Knight, C.A.; Hallett, J.; Devries, A.L. Solute Effects on Ice Recrystallization: An Assessment Technique. Cryobiology 1988, 25, 55–60. [Google Scholar] [CrossRef]
- Abraham, S.; Keillor, K.; Capiccioti, C.J.; Perley-Robertson, E.; Keillor, J.W.; Ben, R.N. Quantitative Analysis of the Efficacy and Potency of Novel Small Molecule Ice Recrystallization Inhibitors. Cryst. Growth Des. 2015, 15, 5034–5039. [Google Scholar] [CrossRef]
- Glöckler, J.; Klützke, S.; Meyer-Zaika, W.; Reller, A.; García-García, F.J.; Strehblow, H.H.; Keller, P.; Rentschler, E.; Kláui, W. With Phosphinophosphonic Acids to Nanostructured, Water-Soluble, and Catalytically Active Rhodium Clusters. Angew. Chem. Int. Ed. 2007, 46, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Clerget, M.; Gager, O.; Monteil, M.; Pirat, J.L.; Migianu-Griffoni, E.; Deschamp, J.; Lecouvey, M. Novel Easily Recyclable Bifunctional Phosphonic Acid Carrying Tripeptides for the Stereoselective Michael Addition of Aldehydes with Nitroalkenes. Adv. Synth. Catal. 2016, 358, 34–40. [Google Scholar] [CrossRef]
- Bayrakc, M.; Ertul, Ş.; Yilmaz, M. Transportation of Poorly Soluble Drug Molecules from the Organic Phase to the Aqueous Phase by Using Phosphorylated Calixarenes. J. Chem. Eng. Data 2011, 56, 4473–4479. [Google Scholar] [CrossRef]
- Martin, A.D.; Boulos, R.A.; Stubbs, K.A.; Raston, C.L. Phosphonated Calix [4] Arene-Based Amphiphiles as Scaffolds for Fluorescent Nano-Fibres. Chem. Comm. 2011, 47, 7329–7331. [Google Scholar] [CrossRef]
- Capicciotti, C.J.; Leclere, M.; Perras, F.A.; Bryce, D.L.; Paulin, H.; Harden, J.; Liu, Y.; Ben, R.N. Potent Inhibition of Ice Recrystallization by Low Molecular Weight Carbohydrate-Based Surfactants and Hydrogelators. Chem. Sci. 2012, 3, 1408–1419. [Google Scholar] [CrossRef]
- Ampaw, A.; Charlton, T.A.; Briard, J.G.; Ben, R.N. Designing the next Generation of Cryoprotectants–from Proteins to Small Molecules. Peptide Sci. 2019, 111, 1–12. [Google Scholar] [CrossRef]
- Balcerzak, A.K.; Febbraro, M.; Ben, R.N. The Importance of Hydrophobic Moieties in Ice Recrystallization Inhibitors. RSC Adv. 2013, 3, 3232–3236. [Google Scholar] [CrossRef]
- Larson, E.M.; Doughman, D.J.; Gregerson, D.S.; Obritsch, W.F. A New, Simple, Nonradioactive, Nontoxic in Vitro Assay to Monitor Corneal Endothelial Cell Viability. Invest. Ophthalmol. Vis. Sci. 1997, 38, 1929–1933. [Google Scholar]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.; Wang, Q.; Li, D.; Jia, B.; Zhou, Y.; Ye, Y.; Shen, S.; Wang, Y.; Li, S.; et al. Hyperammonia Induces Specific Liver Injury through an Intrinsic Ca2+-Independent Apoptosis Pathway. BMC Gastroenterol. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hostetler, K.Y. Alkoxyalkyl Prodrugs of Acyclic Nucleoside Phosphonates Enhance Oral Antiviral Activity and Reduce Toxicity: Current State of the Art. Antivir. Res. 2009, 82, A84–A98. [Google Scholar] [CrossRef]
- Trant, J.F.; Biggs, R.A.; Capicciotti, C.J.; Ben, R.N. Developing Highly Active Small Molecule Ice Recrystallization Inhibitors Based upon C-Linked Antifreeze Glycoprotein Analogues. RSC Adv. 2013, 3, 26005–26009. [Google Scholar] [CrossRef]
- Tam, R.Y.; Ferreira, S.S.; Czechura, P.; Chaytor, J.L.; Ben, R.N. Hydration Indexes-A Better Parameter for Explaining Small Molecule Hydration in Inhibition of Ice Recrystallization. J. Am. Chem. Soc. 2008, 130, 17494–17501. [Google Scholar] [CrossRef] [PubMed]
- Noto, R.; Martorana, V.; Migliore, M.; Fornili, S.L. Hydration of the Ammonium Ion: Monte Carlo Simulation. Z. Nat. A 1991, 46, 107–110. [Google Scholar] [CrossRef]
- Brugé, F.; Bernasconi, M.; Parrinello, M. Ab Initio Simulation of Rotational Dynamics of Solvated Ammonium Ion in Water. J. Am. Chem. Soc. 1999, 121, 10883–10888. [Google Scholar] [CrossRef]
- Brugé, F.; Bernasconi, M.; Parrinello, M. Density-Functional Study of Hydration of Ammonium in Water Clusters. J. Chem. Phys. 1999, 110, 4734. [Google Scholar] [CrossRef]
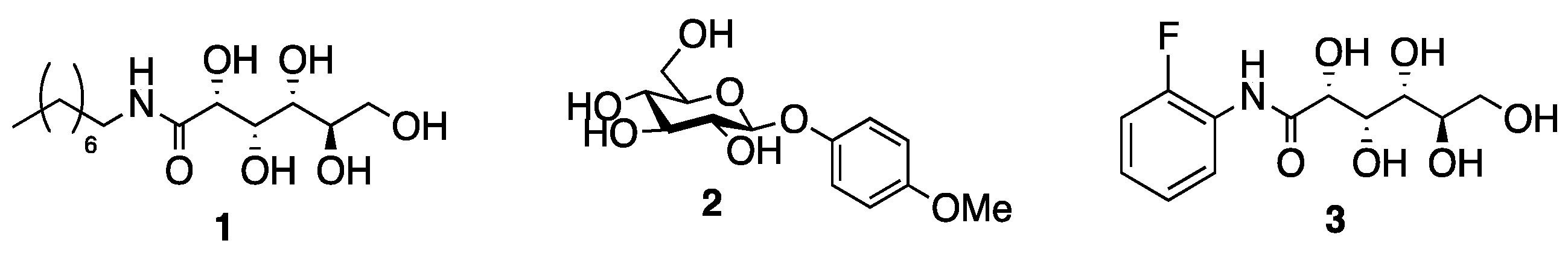

| Compound | Maximum Solubility in PBS (mM) |
|---|---|
| 1 | 1 |
| 2 | 110 |
| 4-Na | 300 |
| 5-Na | >500 |
| 6-Na | >500 |
| 7-Na | 250 |
| Compound | IC50 Values 1 (mM) |
|---|---|
| 6-NH4 | 3 |
| (NH4)2HPO4 | 2 |
| (NH4)2CO3 | 2 |
| NH4Cl | 3 |
| (NH4)2SO4 | 3 |
| NaCl | N/A 2 |
| KCl | N/A 2 |
| CaCl2 | N/A 2 |
| 5-NH4 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampaw, A.A.; Newell, K.; Ben, R.N. Investigating the Solubility and Activity of a Novel Class of Ice Recrystallization Inhibitors. Processes 2021, 9, 1781. https://doi.org/10.3390/pr9101781
Ampaw AA, Newell K, Ben RN. Investigating the Solubility and Activity of a Novel Class of Ice Recrystallization Inhibitors. Processes. 2021; 9(10):1781. https://doi.org/10.3390/pr9101781
Chicago/Turabian StyleAmpaw, Anna A., Kayla Newell, and Robert N. Ben. 2021. "Investigating the Solubility and Activity of a Novel Class of Ice Recrystallization Inhibitors" Processes 9, no. 10: 1781. https://doi.org/10.3390/pr9101781
APA StyleAmpaw, A. A., Newell, K., & Ben, R. N. (2021). Investigating the Solubility and Activity of a Novel Class of Ice Recrystallization Inhibitors. Processes, 9(10), 1781. https://doi.org/10.3390/pr9101781






