Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax senticosus and Their Enrichment and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Chemicals, and Reagents
2.2. Methods
2.2.1. Extraction Process
2.2.2. Determination of the total flavonoid yield
2.2.3. Optimization of the Extraction Rate of A. senticosus Flavonoids by Single-Factor Experiments
- (1)
- Optimization of the Amount of Cellulase
- (2)
- Optimization of the Amount of Pectinase
- (3)
- Optimization of the Ratio of the Enzymes in Mixture
- (4)
- Optimization of Amount of the Enzymes in Mixture
- (5)
- Optimization of Other Factors
2.2.4. Optimization by the Response Surface Method
2.2.5. Enrichment of Flavonoids
- (1)
- Enrichment of Flavonoids by Solvents of Different Polarities
- (2)
- Determination of the Active Ingredient Content
- (3)
- Radical Scavenging Activity Assays
- (4)
- Pearson Correlation Studies
2.3. Statistical Analysis
3. Results
3.1. Single-Factor Experiments
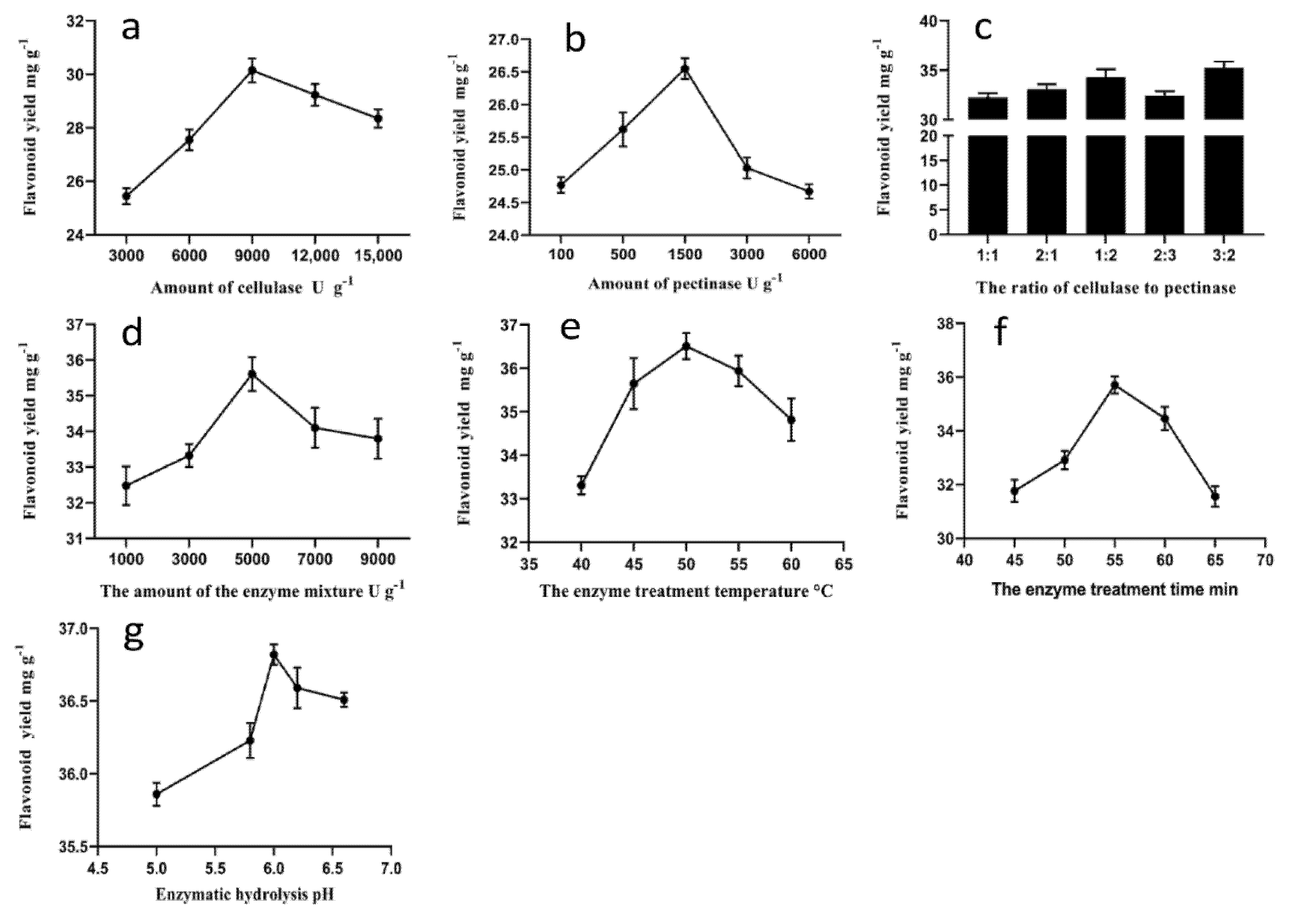
3.1.1. Effect of the Amount of Cellulase on the Flavonoid Yield
3.1.2. Effect of the Amount of Pectinase on the Flavonoid Yield
3.1.3. Effect of the Ratio of the Enzymes in Mixture on the Flavonoid Yield
3.1.4. Effect of the Amount of the Enzymes on Flavonoid Yield
3.1.5. Effect of the Enzyme Treatment Time on the Flavonoid Yield
3.1.6. Effect of the Enzyme Treatment Temperature on the Flavonoid Yield
3.1.7. Effect of pH on the Flavonoid Yield
3.2. Analysis of the Response Surfaces
0.16BC + 0.045BD + 0.065CD − 0.51A2 − 0.50B2 − 0.32C2,
3.3. Enrichment and Antioxidant Properties Test of A. senticosus Flavonoids
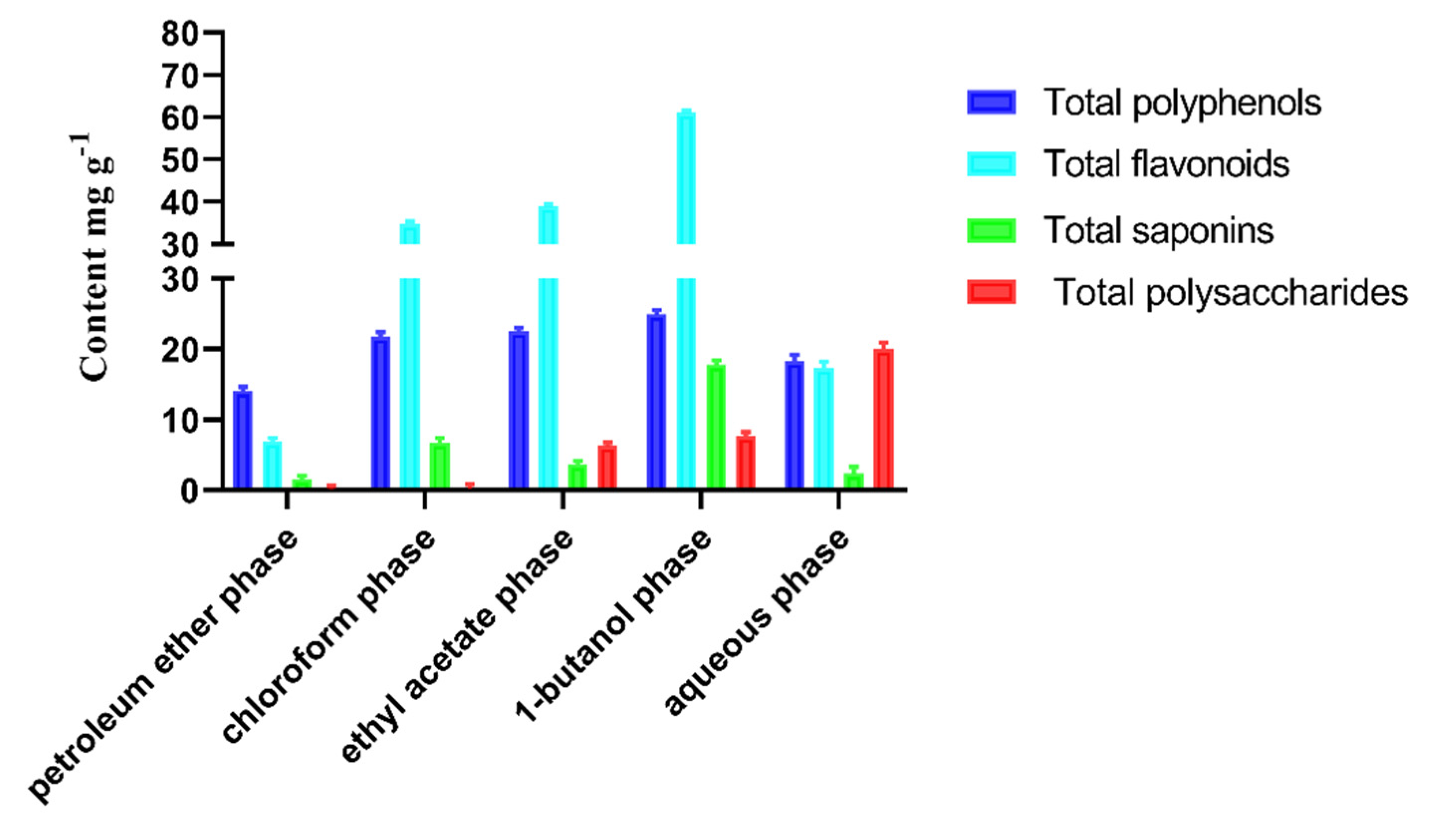
3.3.1. Determination of the Active Ingredients in the Different Extraction Phases
3.3.2. Radical Scavenging Activity in the Various Extraction Fractions
3.3.3. Pearson Correlations between the Antioxidant Activity and the Active Compounds content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Ferns, K.; Yan, Z.Q.; Yin, S.Y.; Kou, J.J.; Li, D.S.; Zeng, Z.; Yin, L.; Wang, X.Y.; Bao, H.X.; et al. Acanthopanax senticosus: Photochemistry and Anticancer Potential. Am. J. Chin. Med. 2016, 44, 1543–1558. [Google Scholar] [CrossRef] [PubMed]
- Bleakney, T.L. Deconstructing an adaptogen: Eleutherococcus senticosus. Holist. Nurs. Pract. 2008, 22, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Li, Z.; Liu, G.Z.; Wang, X.; Kwon, O.K.; Lee, H.K.; Whang, W.K.; Liu, X.Q. Quantitative determination of 15 bioactive triterpenoid saponins in different parts of Acanthopanax henryi by HPLC with charged aerosol detection and confirmation by LC-ESI-TOF-MS. J. Sep. Sci. 2016, 39, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.L.; Zhang, Y.H.; Gao, H.; Zhang, Z.; Zhang, Y.; Wang, Z.; Zhang, J.; Deng, B.; Qiu, Z.; Fu, C. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J. Ethnopharmacol. 2021, 268, 113586. [Google Scholar] [CrossRef]
- Li, F.; Li, W.; Fu, H.W.; Koike, K. Pancreatic lipase-inhibiting triterpenoid saponins from fruits of Acanthopanax senticosus. Chem. Pharm. Bull. 2007, 55, 1087–1089. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Meng, Y.H.; Zhai, C.M.; Wang, M.; Avula, B.; Yuk, J.; Smith, K.M.; Isaac, G.; Khan, I.A. The Chemical Characterization of Eleutherococcus senticosus and Ci-wu-jia Tea Using UHPLC-UV-QTOF/MS. Int. J. Mol. Sci. 2019, 20, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Zhang, A.H.; Sun, H.; Zhang, Y.Z.; Meng, X.C.; Yan, G.L.; Liu, L.; Wang, X.J. High-throughput ultra high performance liquid chromatography combined with mass spectrometry approach for the rapid analysis and characterization of multiple constituents of the fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms. J. Sep. Sci. 2017, 40, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Ren, F.; Huang, W.; Ding, R.T.; Zhou, Q.S.; Liu, X.W. Anti-Fatigue Activity of Extracts of Stem Bark from Acanthopanax senticosus. Molecules 2011, 16, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Nishida, M.; Saito, M.; Tanabe, A.; Eitsuka, T.; Yuan, S.H.; Ikekawa, N.; Nishida, H. The fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms improves insulin resistance and hepatic lipid accumulation by modulation of liver adenosine monophosphate-activated protein kinase activity and lipogenic gene expression in high-fat diet-fed obese mice. Nutr. Res. 2016, 36, 1090–1097. [Google Scholar] [CrossRef]
- Lau, K.M.; Yue, G.G.L.; Chan, Y.Y.; Kwok, H.F.; Gao, S.; Wong, C.W.; Lau, C.B.S. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin. Med. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Zaluski, D.; Olech, M.; Kuzniewski, R.; Verpoorte, R.; Nowak, R.; Smolarz, H.D. LC-ESI-MS/MS profiling of phenolics from Eleutherococcus spp. inflorescences, structure-activity relationship as antioxidants, inhibitors of hyaluronidase and acetylcholinesterase. Saudi Pharm. J. 2017, 25, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Peluso, I.; Miglio, C.; Morabito, G.; Ioannone, F.; Serafini, M. Flavonoids and Immune Function in Human: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 383–395. [Google Scholar] [CrossRef]
- Huang, L.Z.; Zhao, H.F.; Huang, B.K.; Zheng, C.J.; Peng, W.; Qin, L.P. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Pharmazie 2011, 66, 83–97. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Rodriguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339. [Google Scholar] [CrossRef]
- Ahmad, I.; Sabah, A.; Anwar, Z.; Arif, A.; Arsalan, A.; Qadeer, K. Effect of solvent polarity on the extraction of components of pharmaceutical plastic containers. Pak. J. Pharm. Sci. 2017, 30, 247–252. [Google Scholar] [PubMed]
- Chang, C.; Yang, M.; Wen, H.-M.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2020, 10, 178–182. [Google Scholar]
- Chen, H.G.; Zhou, X.; Zhang, J.Z. Optimization of enzyme assisted extraction of polysaccharides from Astragalus membranaceus. Carbohydr. Polym. 2014, 111, 567–575. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Nguyen, V.T.; Van Vuong, Q.; Bowyer, M.C.; Scarlett, C.J. Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves. Technologies 2015, 3, 285–301. [Google Scholar] [CrossRef] [Green Version]
- Hadidi, M.; Ibarz, A.; Pagan, J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. 2020, 309. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Food Compos. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Purushothaman, A.; Sheeja, A.A.; Janardanan, D. Hydroxyl radical scavenging activity of melatonin and its related indolamines. Free Radic. Res. 2020, 54, 373–383. [Google Scholar] [CrossRef]
- Kladna, A.; Berczynski, P.; Kruk, I.; Michalska, T.; Aboul-Enein, H.Y. Superoxide anion radical scavenging property of catecholamines. Luminescence 2013, 28, 450–455. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Chen, S.; Xing, X.H.; Huang, J.J.; Xu, M.S. Enzyme-assisted extraction of flavonoids from Ginkgo biloba leaves: Improvement effect of flavonol transglycosylation catalyzed by Penicillium decumbens cellulase. Enzym. Microb. Technol. 2011, 48, 100–105. [Google Scholar] [CrossRef]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The dependence of enzyme activity on temperature: Determination and validation of parameters. Biochem. J. 2007, 402, 331–337. [Google Scholar] [CrossRef]
- Li, F.J.; Ning, S.L.; Li, Y.; Yu, Y.J.; Duan, J.L. Enhanced extraction of osthole and imperatorin from Cnidium monnieri by infrared-accelerated enzyme pretreatmenta. Anal. Methods 2013, 5, 5669–5676. [Google Scholar] [CrossRef]
- Yin, X.L.; You, Q.H.; Jiang, Z.H. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2011, 86, 1358–1364. [Google Scholar] [CrossRef]
- Cai, C.Y.; Li, F.F.; Liu, L.L.; Tan, Z.J. Deep eutectic solvents used as the green media for the efficient extraction of caffeine from Chinese dark tea. Sep. Purif. Technol. 2019, 227. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-Assisted Extraction of Flavorings and Colorants from Plant Materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Ultrasound assisted intensification of enzyme activity and its properties: A mini-review. World J. Microbiol. Biotechnol. 2017, 33. [Google Scholar] [CrossRef]
- Kļaviņa, L. Composition of Mosses, Their Metabolites and Environmental Stress Impacts. Ph.D. Thesis, University of Latvia, Riga, Latvia, 2018. Available online: https://dspace.lu.lv/dspace/handle/7/38353 (accessed on 27 April 2018).
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Publ. Health Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Ajayi, A.M.; Martins, D.T.D.; Balogun, S.O.; de Oliveira, R.G.; Ascencio, S.D.; Soares, I.M.; Barbosa, R.D.; Ademowo, O.G. Ocimum gratissimum L. leaf flavonoid-rich fraction suppress LPS-induced inflammatory response in RAW 264.7 macrophages and peritonitis in mice. J. Ethnopharmacol. 2017, 204, 169–178. [Google Scholar] [CrossRef]


| Factors | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Enzyme amount (U g−1) (A) | 1000 | 5000 | 9000 |
| Enzyme treatment time (min) (B) | 45 | 55 | 65 |
| Enzyme treatment temperature (°C) (C) | 40 | 50 | 60 |
| Solvent pH value (D) | 5.4 | 6 | 6.6 |
| No. | A | B | C | D | Flavonoid Yield (mg g−1) |
|---|---|---|---|---|---|
| 1 | 0 | −1 | −1 | 0 | 35.51 |
| 2 | −1 | 1 | 0 | 0 | 35.61 |
| 3 | 1 | −1 | 0 | 0 | 35.65 |
| 4 | 0 | −1 | 0 | −1 | 35.41 |
| 5 | −1 | 0 | 0 | 1 | 35.67 |
| 6 | 1 | 0 | 0 | −1 | 36.20 |
| 7 | 0 | 0 | −1 | −1 | 36.21 |
| 8 | 0 | 0 | 1 | 1 | 36.15 |
| 9 | 0 | 1 | 0 | 1 | 36.51 |
| 10 | 1 | 0 | −1 | 0 | 36.10 |
| 11 | 1 | 1 | 0 | 0 | 36.53 |
| 12 | 0 | 0 | 0 | 0 | 36.60 |
| 13 | 1 | 0 | 1 | 0 | 36.49 |
| 14 | 0 | −1 | 0 | 1 | 35.55 |
| 15 | 0 | 1 | 1 | 0 | 36.43 |
| 16 | 1 | 0 | 0 | 1 | 36.31 |
| 17 | 0 | 0 | 0 | 0 | 36.65 |
| 18 | 0 | −1 | 1 | 0 | 35.45 |
| 19 | −1 | 0 | −1 | 0 | 35.61 |
| 20 | 0 | 0 | 0 | 0 | 36.73 |
| 21 | 0 | 0 | 0 | 0 | 36.78 |
| 22 | 0 | 1 | 0 | −1 | 36.19 |
| 23 | 0 | 1 | −1 | 0 | 35.85 |
| 24 | 0 | 0 | 0 | 0 | 36.70 |
| 25 | −1 | 0 | 0 | −1 | 35.09 |
| 26 | 0 | 0 | 1 | −1 | 36.20 |
| 27 | 0 | 0 | −1 | 1 | 35.90 |
| 28 | −1 | 0 | 1 | 0 | 35.32 |
| 29 | −1 | −1 | 0 | 0 | 35.11 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 7.1200 | 14 | 0.5087 | 25.2900 | <0.0001 | ** |
| A | 1.9800 | 1 | 1.9800 | 98.2500 | <0.0001 | ** |
| B | 1.6400 | 1 | 1.6400 | 81.6600 | <0.0001 | ** |
| C | 0.0616 | 1 | 0.0616 | 3.0600 | 0.1019 | |
| D | 0.0520 | 1 | 0.0520 | 2.5900 | 0.1302 | |
| AB | 0.0361 | 1 | 0.0361 | 1.7900 | 0.2017 | |
| AC | 0.1156 | 1 | 0.1156 | 5.7500 | 0.0310 | |
| AD | 0.0552 | 1 | 0.0552 | 2.7500 | 0.1198 | |
| BC | 0.1024 | 1 | 0.1024 | 5.0900 | 0.0406 | |
| BD | 0.0081 | 1 | 0.0081 | 0.4026 | 0.5360 | |
| CD | 0.0169 | 1 | 0.0169 | 0.8401 | 0.3749 | |
| A² | 1.700 | 1 | 1.700 | 84.4700 | <0.0001 | ** |
| B² | 1.6100 | 1 | 1.6100 | 79.9900 | <0.0001 | ** |
| C² | 0.6666 | 1 | 0.6666 | 33.1400 | <0.0001 | ** |
| D² | 0.5812 | 1 | 0.5812 | 28.8900 | <0.0001 | ** |
| Residual | 0.2816 | 14 | 0.0201 | |||
| Lack of fit | 0.2622 | 10 | 0.0262 | 5.3800 | 0.0595 | |
| Pure error | 0.0195 | 4 | 0.0049 | |||
| Cor total | 7.4 | 28 |
| Total Flavonoids | Total Polyphenols | Total Saponins | Total Polysaccharides | DPPH Radicals | ABTS Radicals | Hydroxyl Radicals | Superoxide Anion Radicals | |
|---|---|---|---|---|---|---|---|---|
| Total flavonoids | 1 | |||||||
| Total polyphenols | 0.964 ** | 1 | ||||||
| Total saponins | 0.888 * | 0.763 | 1 | |||||
| Total polysaccharides | 0.033 | 0.059 | 0.053 | 1 | ||||
| DPPH radicals | 0.701 | 0.832 * | 0.452 | 0.556 | 1 | |||
| ABTS radicals | 0.929* | 0.883 * | 0.736 | 0.109 | 0.988 ** | 1 | ||
| Hydroxyl radicals | 0.589 | 0.745 | 0.314 | 0.63 | 0.603 | 0.509 | 1 | |
| Superoxide anion radicals | 0.785 | 0.884 * | 0.646 | 0.242 | 0.868 | 0.563 | 0.805 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Chu, X.; Su, J.; Fu, X.; Kan, Q.; Wang, X.; Zhang, X. Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax senticosus and Their Enrichment and Antioxidant Properties. Processes 2021, 9, 1708. https://doi.org/10.3390/pr9101708
Liu R, Chu X, Su J, Fu X, Kan Q, Wang X, Zhang X. Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax senticosus and Their Enrichment and Antioxidant Properties. Processes. 2021; 9(10):1708. https://doi.org/10.3390/pr9101708
Chicago/Turabian StyleLiu, Ruixue, Xiuling Chu, Jianqing Su, Xiang Fu, Qibin Kan, Xiaoya Wang, and Xinyu Zhang. 2021. "Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax senticosus and Their Enrichment and Antioxidant Properties" Processes 9, no. 10: 1708. https://doi.org/10.3390/pr9101708
APA StyleLiu, R., Chu, X., Su, J., Fu, X., Kan, Q., Wang, X., & Zhang, X. (2021). Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax senticosus and Their Enrichment and Antioxidant Properties. Processes, 9(10), 1708. https://doi.org/10.3390/pr9101708






