Kanchan Arsenic Filters and the Future of Fe0-Based Filtration Systems for Single Household Drinking Water Supply
Abstract
1. Introduction
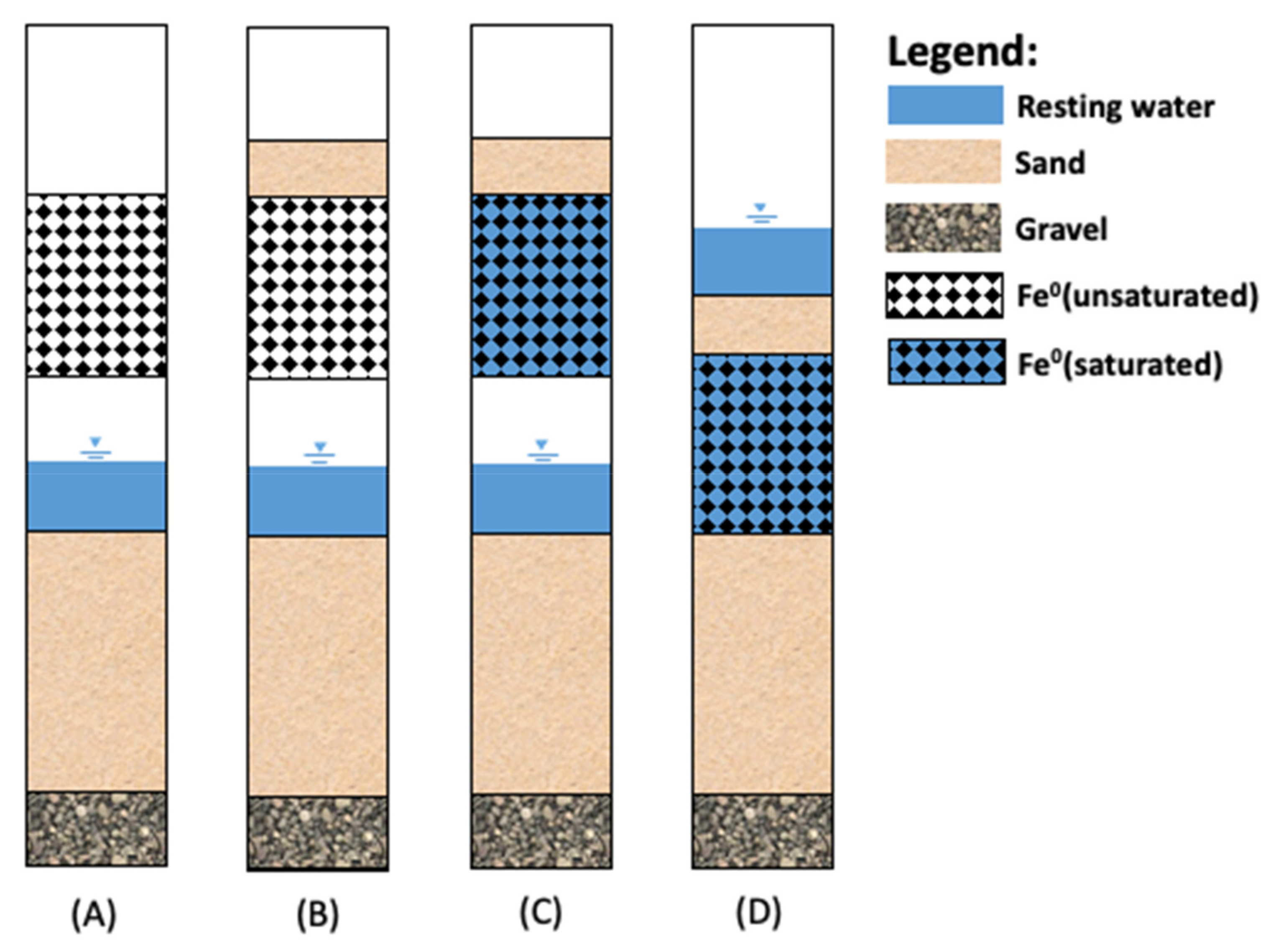
2. The Kanchan Arsenic Filter
3. The Design Limitations of KAF
4. Rationalizing the Highly Variable as Removal Efficiency of KAFs
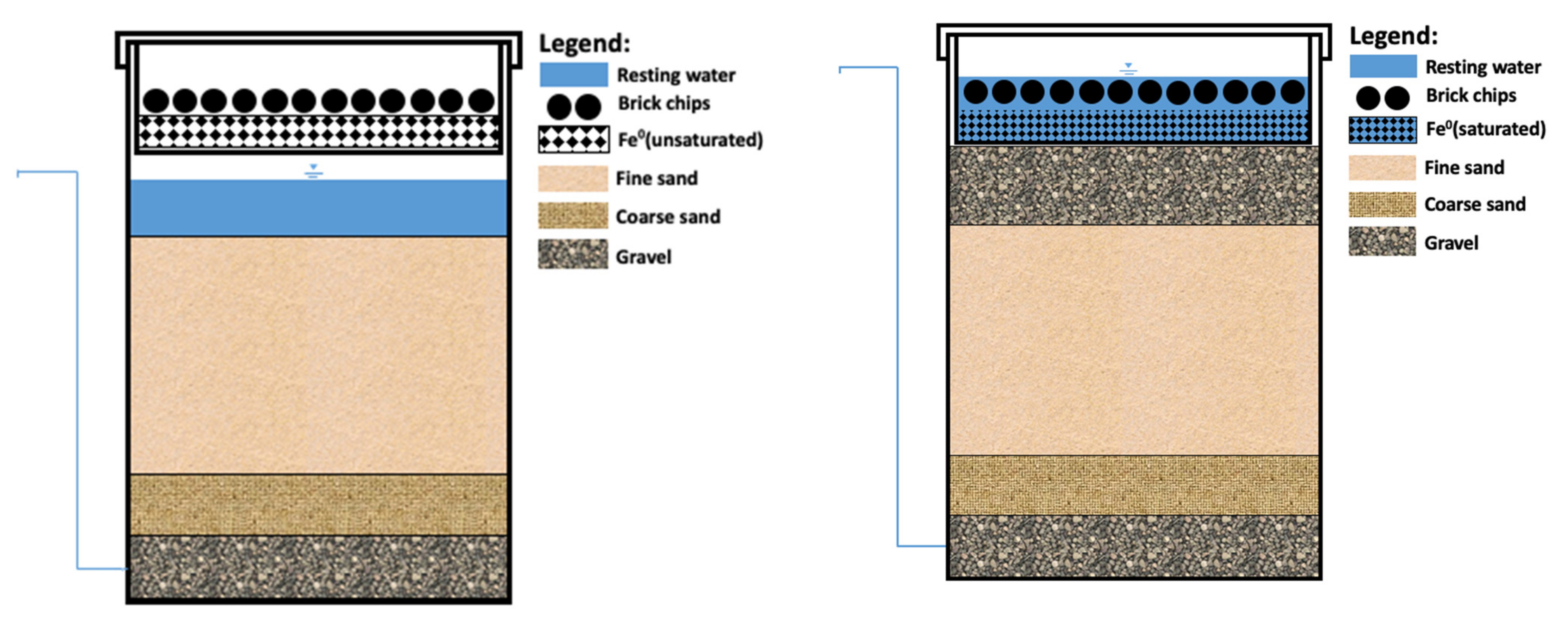
5. Questioning the Suitability of KAF
6. Recommendations for More Efficient KAFs
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mueller, B. First step concerning improvement of arsenic removal by adapted Kanchan filters in the lowlands of Nepal. J. Chem. Appl. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Mueller, B.; Dangol, B.; Ngai, T.K.K.; Hug, S.J. Kanchan arsenic filters in the lowlands of Nepal: Mode of operation, arsenic removal, and future improvements. Environ. Geochem. Health 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ogata, R.; Dangol, B.; Sakamoto, M. Sustainability assessment of long-term, widely used household Kanchan Arsenic Filters in Nepal. J. Environ. Sci. Health A 2020, 55, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health Part A 2007, 42, 1869–1878. [Google Scholar] [CrossRef]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Mak, M.S.H.; Rao, P.; Lo, I.M.C. Zero-valent iron and iron oxide-coated sand as a combination for removal of co-present chromate and arsenate from groundwater with humic acid. Environ. Pollut. 2011, 159, 377–382. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Heimann, S.; Ndé-Tchoupé, A.I.; Hu, R.; Licha, T.; Noubactep, C. Investigating the suitability of Fe0 packed-beds for water defluoridation. Chemosphere 2018, 209, 578–587. [Google Scholar] [CrossRef]
- Bretzler, A.; Nikiema, J.; Lalanne, F.; Hoffmann, L.; Biswakarma, J.; Siebenaller, L.; Demange, D.; Schirmer, M.; Hug, S.J. Arsenic removal with zero-valent iron filters in Burkina Faso: Field and laboratory insights. Sci. Total Environ. 2020, 737, 139466. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Ruppert, H.; Noubactep, C. Designing the next generation of Fe0-based filters for decentralized safe drinking water treatment. Processes 2020, 8, 745. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.H. Permeable reactive barriers: A sustainable technology for cleaning contaminated groundwater in developing countries. Desalination 2009, 248, 352–359. [Google Scholar] [CrossRef]
- Tuladhar, S.; Smith, L.S. SONO filter: An excellent technology for save water in Nepal. Sophen 2009, 7, 18–24. [Google Scholar]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freib. Online Geosci. 2015, 32, 1–80. [Google Scholar]
- Antia, D.D.J. Water treatment and desalination using the eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz[mH2O], and n-Fex[Cation]nOyHz[Anion]m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Thakur, A.K.; Vithanage, M.; Das, D.B.; Kumar, M. A review on design, material selection, mechanism, and modelling of permeable reactive barrier for community-scale groundwater treatment. Environ. Technol. Innov. 2020, 19, 100917. [Google Scholar] [CrossRef]
- Khan, A.H.; Rasul, S.B.; Munir, A.K.M.; Habibuddowla, M.; Alauddin, M.; Newaz, S.S.; Hussam, A. Appraisal of a simple arsenic removal method for ground water of Bangladesh. J. Environ. Sci. Health Part A 2000, 35, 1021–1041. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Murcott, S.; Shrestha, R.R.; Dangol, B.; Maharjan, M. Development and dissemination of Kanchan™ Arsenic Filter in rural Nepal. Water Sci. Technol. Water Supply 2006, 6, 137–146. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health Part A 2007, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Chiew, H.; Sampson, M.L.; Huch, S.; Ken, S.; Bostick, B.C. Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended BioSand filters. Environ. Sci. Technol. 2009, 43, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Smith, L.S.; Shrestha, S.; Maden, N. Efficacy of arsenic filtration by Kanchan Arsenic Filter in Nepal. J. Water Health 2014, 12, 596–599. [Google Scholar] [CrossRef]
- Wenk, C.B.; Kaegi, R.; Hug, S.J. Factors affecting arsenic and uranium removal with zero-valent iron: Laboratory tests with Kanchan-type iron nail filter columns with different groundwaters. Environ. Chem. 2014, 11, 547–557. [Google Scholar] [CrossRef]
- Smith, K.; Li, Z.; Chen, B.; Liang, H.; Zhang, X.; Xu, R.; Li, Z.; Dai, H.; Wei, C.; Liu, S. Comparison of sand-based water filters for point-of-use arsenic removal in China. Chemosphere 2017, 168, 155–162. [Google Scholar] [CrossRef]
- Śmiech, K.M.; Tolsma, A.; Kovács, T.; Dalbosco, V.; Yasadi, K.; Groendijk, L.; Agostinho, L.L.F. Comparing mixed-media and conventional slow-sand filters for arsenic removal from groundwater. Water 2018, 10, 119. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water treatment using metallic iron: A tutorial review. Processes 2019, 7, 622. [Google Scholar] [CrossRef]
- Hashmi, F.; Pearce, J.M. Viability of small-scale arsenic-contaminated water purification technologies for sustainable development in Pakistan. Sust. Dev. 2011, 19, 223–234. [Google Scholar] [CrossRef]
- Whitney, W.R. The corrosion of iron. J. Am. Chem. Soc. 1903, 25, 394–406. [Google Scholar] [CrossRef]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Dunn, D.S.; Bogart, M.B.; Brossia, C.S.; Cragnolino, G.A. Corrosion of iron under alternating wet and dry conditions. Corrosion 2000, 56, 470–481. [Google Scholar] [CrossRef]
- Kim, D.-K.; Muralidharan, S.; Ha, T.-H.; Bae, J.-H.; Ha, Y.-C.; Lee, H.-G.; Scantlebury, J.D. Electrochemical studies on the alternating current corrosion of mild steelunder cathodic protection condition in marine environments. Electrochim. Acta 2006, 51, 5259–5267. [Google Scholar] [CrossRef]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. Clean Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef]
- Baig, S.A.; Mahmood, Q.; Nawab, B.; Shafqat, M.N.; Pervez, A. Improvement of drinking water quality by using plant biomass through household biosand filter—A decentralized approach. Ecol. Eng. 2011, 37, 1842–1848. [Google Scholar] [CrossRef]
- Haig, S.J.; Collins, G.; Davies, R.L.; Dorea, C.C.; Quince, C. Biological aspects of slow sand filtration: Past, present and future. Water Sci. Technol. Water Supply 2011, 11, 468–472. [Google Scholar] [CrossRef]
- Elliott, M.; Stauber, C.E.; DiGiano, F.A.; de Aceituno, A.F.; Sobsey, M.D. Investigation of E. coli and virus reductions using replicate, bench-scale biosand filter columns and two filter media. Int. J. Environ. Res. Public Health 2015, 12, 10276–10299. [Google Scholar] [CrossRef]
- George, D.; Ahammed, A.M. Effect of zero-valent iron amendment on the performance of biosand filters. Water Supply 2019, 19, 1612–1618. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Etmannski, T.R. Accounting for Sustainability in Bengal: Examining Arsenic Mitigation Technologies Using Process Analysis Method. Ph.D. Thesis, University of Oxford, Oxford, UK, 2014. [Google Scholar]
- Etmannski, T.R.; Darton, R.C. A methodology for the sustainability assessment of arsenic mitigation technology for drinking water. Sci. Total Environ. 2014, 488–489, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Casentini, B.; Falcione, F.T.; Amalfitano, S.; Fazi, S.; Rossetti, S. Arsenic removal by discontinuous ZVI two steps system for drinking water production at household scale. Water Res. 2016, 106, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal. In Water and Sanitation in the New Millennium; Springer: Berlin/Heidelberg, Germany, 2017; pp. 127–137. [Google Scholar]
- Leupin, O.X.; Hug, S.J.; Badruzzaman, A.B.M. Arsenic removal from Bangladesh tube well water with filter columns containing zerovalent iron filings and sand. Environ. Sci. Technol. 2005, 39, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef]
- Kowalski, K.P.; Søgaard, E.G. Implementation of zero-valent iron (ZVI) into drinking water supply—Role of the ZVI and biological processes. Chemosphere 2014, 117, 108–114. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Crane, R.; Noubactep, C.; Nassi, A.; Ruppert, H. Testing metallic iron filtration systems for decentralized water treatment at pilot scale. Water 2015, 7, 868–897. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Stauber, C.E.; Casanova, L.M.; Brown, J.M.; Elliott, M.A. Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 2008, 42, 4261–4267. [Google Scholar] [CrossRef]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef]
- Elliott, M.A.; DiGiano, F.A.; Sobsey, M.D. Virus attenuation by microbial mechanisms during the idle time of a household slow sand filter. Water Res. 2011, 45, 4092–4102. [Google Scholar] [CrossRef]
- Reyneke, B.; Cloete, T.E.; Khan, S.; Khan, W. Rainwater harvesting solar pasteurization treatment systems for the provision of an alternative water source in peri-urban informal settlements. Environ. Sci. Water Res. Technol. 2018, 4, 291–302. [Google Scholar] [CrossRef]
- Domingos, M.; Sanchez, B.; Vieira-da-Motta, O.; Samarão, S.S.; Canela, M.C. A new automated solar disc for water disinfection by pasteurization. Photochem. Photobiol. Sci. 2019, 18, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Parisia, L.M.; Maranghia, S.; Manfrid, G.; Basosi, R.; Sinicropi, A. Environmental impact analysis applied to solar pasteurization systems. J. Clean. Prod. 2019, 212, 1368–1380. [Google Scholar] [CrossRef]
- Zaman, S.; Yousuf, A.; Begum, A.; Bariand, M.L.; Rabbani, K.S. Evaluation of adaptive low cost solar water pasteurization device for providing safe potable water in rural households. J. Water Health 2019, 17, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Hering, J.G.; Maag, S.; Schnoor, J.L. A call for synthesis of water research to achieve the sustainable development goals by 2030. Environ. Sci. Technol. 2016, 50, 6122–6123. [Google Scholar] [CrossRef]
- Naseri, E.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Nanseu-Njiki, C.P.; Noubactep, C.; Njau, K.N.; Wydra, K.D. Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 2017, 9, 1224. [Google Scholar] [CrossRef]
- Nanseu-Njiki, C.P.; Gwenzi, W.; Pengou, M.; Rahman, M.A.; Noubactep, C. Fe0/H2O filtration systems for decentralized safe drinking water: Where to from here? Water 2019, 11, 429. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a newly designed steel-wool-based household filter for safe drinking water provision: Hydraulic conductivity and efficiency for pathogen removal. Processes 2019, 7, 966. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Crane, R.A.; Mwakabona, H.T.; Noubactep, C.; Njau, K.N. Technologies for decentralized fluoride removal: Testing metallic iron-based filters. Water 2015, 7, 6750–6774. [Google Scholar] [CrossRef]
- Lilje, J.; Mosler, H.-J. Continuation of health behaviors: Psychosocial factors sustaining drinking water chlorination in a longitudinal study from Chad. Sustainability 2016, 8, 1149. [Google Scholar] [CrossRef]
- Amrose, S.E.; Cherukumilli, K.; Wright, N.C. Chemical contamination of drinking water in resource-constrained settings: Global prevalence and piloted mitigation strategies. Annu. Rev. Environ. Resour. 2020, 45, 195–226. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Xiao, M.; Qiu, P.; Lufingo., M.; Gwenzi, W.; Noubactep, C. Characterizing the suitability of granular Fe0 for the water treatment industry. Processes 2019, 7, 652. [Google Scholar] [CrossRef]
- Hildebrant, B.; Ndé-Tchoupé, A.I.; Lufingo, M.; Licha, T.; Noubactep, C. Steel wool for water treatment: Intrinsic reactivity and defluoridation efficiency. Processes 2020, 8, 265. [Google Scholar] [CrossRef]
- Francesco, C. Modeling the effects of material chemistry on water flow enhancement in nanotube membranes. MRS Bull. 2017, 42, 289. [Google Scholar]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination-development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Cao, V.; Nya, E.L.; Gwenzi, W.; Noubactep, C. Kanchan Arsenic Filters and the Future of Fe0-Based Filtration Systems for Single Household Drinking Water Supply. Processes 2021, 9, 58. https://doi.org/10.3390/pr9010058
Huang Z, Cao V, Nya EL, Gwenzi W, Noubactep C. Kanchan Arsenic Filters and the Future of Fe0-Based Filtration Systems for Single Household Drinking Water Supply. Processes. 2021; 9(1):58. https://doi.org/10.3390/pr9010058
Chicago/Turabian StyleHuang, Zhe, Viet Cao, Esther Laurentine Nya, Willis Gwenzi, and Chicgoua Noubactep. 2021. "Kanchan Arsenic Filters and the Future of Fe0-Based Filtration Systems for Single Household Drinking Water Supply" Processes 9, no. 1: 58. https://doi.org/10.3390/pr9010058
APA StyleHuang, Z., Cao, V., Nya, E. L., Gwenzi, W., & Noubactep, C. (2021). Kanchan Arsenic Filters and the Future of Fe0-Based Filtration Systems for Single Household Drinking Water Supply. Processes, 9(1), 58. https://doi.org/10.3390/pr9010058