Expression of the Thermobifida fusca β-1,3-Glucanase in Yarrowia lipolytica and Its Application in Hydrolysis of β-1,3-Glucan from Four Kinds of Polyporaceae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Construction of the β-1,3-Glucanase Expression Plasmid
2.3. Transformation and Screening of Y. lipolytica Transformant
2.4. Enzyme Expression and Purification
2.5. Determination of β-1,3-Glucanase Activity
2.6. Hydrolysis of Polyporaceae Glucans
2.7. Quantification of Reducing Sugars and Total Sugar
2.8. Assessment of Antioxidant Properties
2.9. Statistical Analysis
3. Results and Discussion
3.1. Amplification and Construction of the β-1,3-Glucanase Gene in a Y. lipolytica Expression System
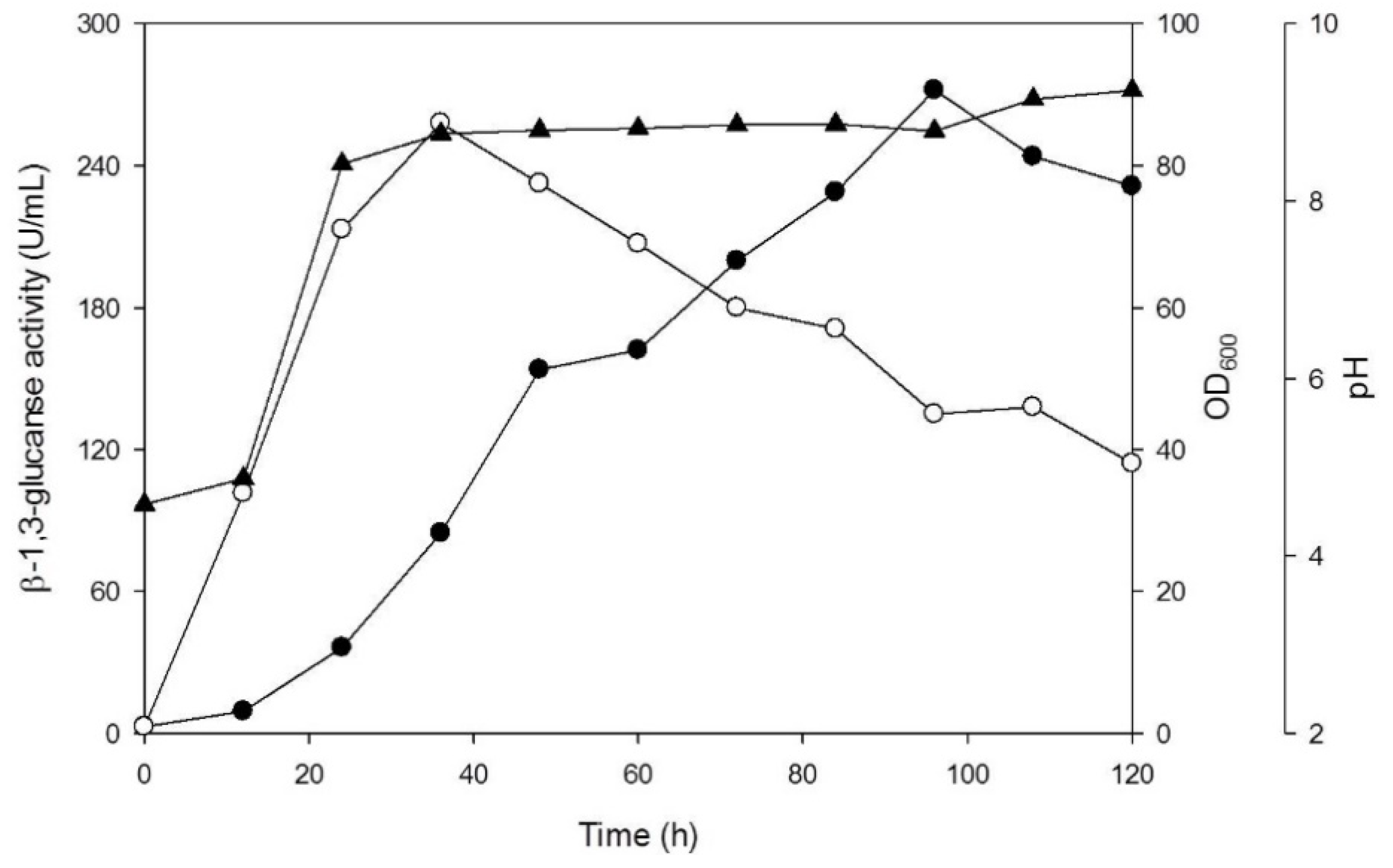
3.2. Production of β-1,3-Glucanase from Y. lipolytica Transformant (pYLSC1-13g)
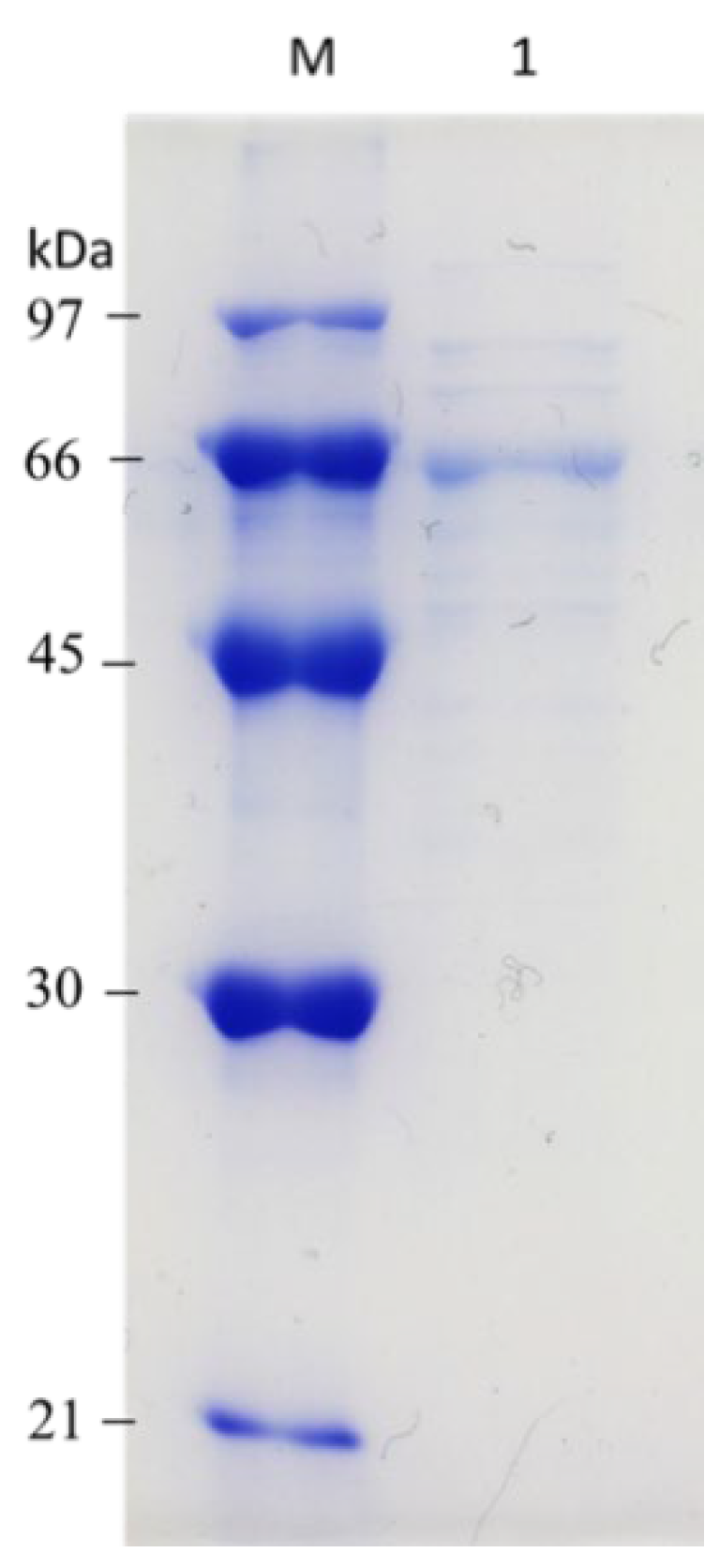
3.3. Purification of β-1,3-Glucanase from Y. lipolytica Transformant (pYLSC1-13g)
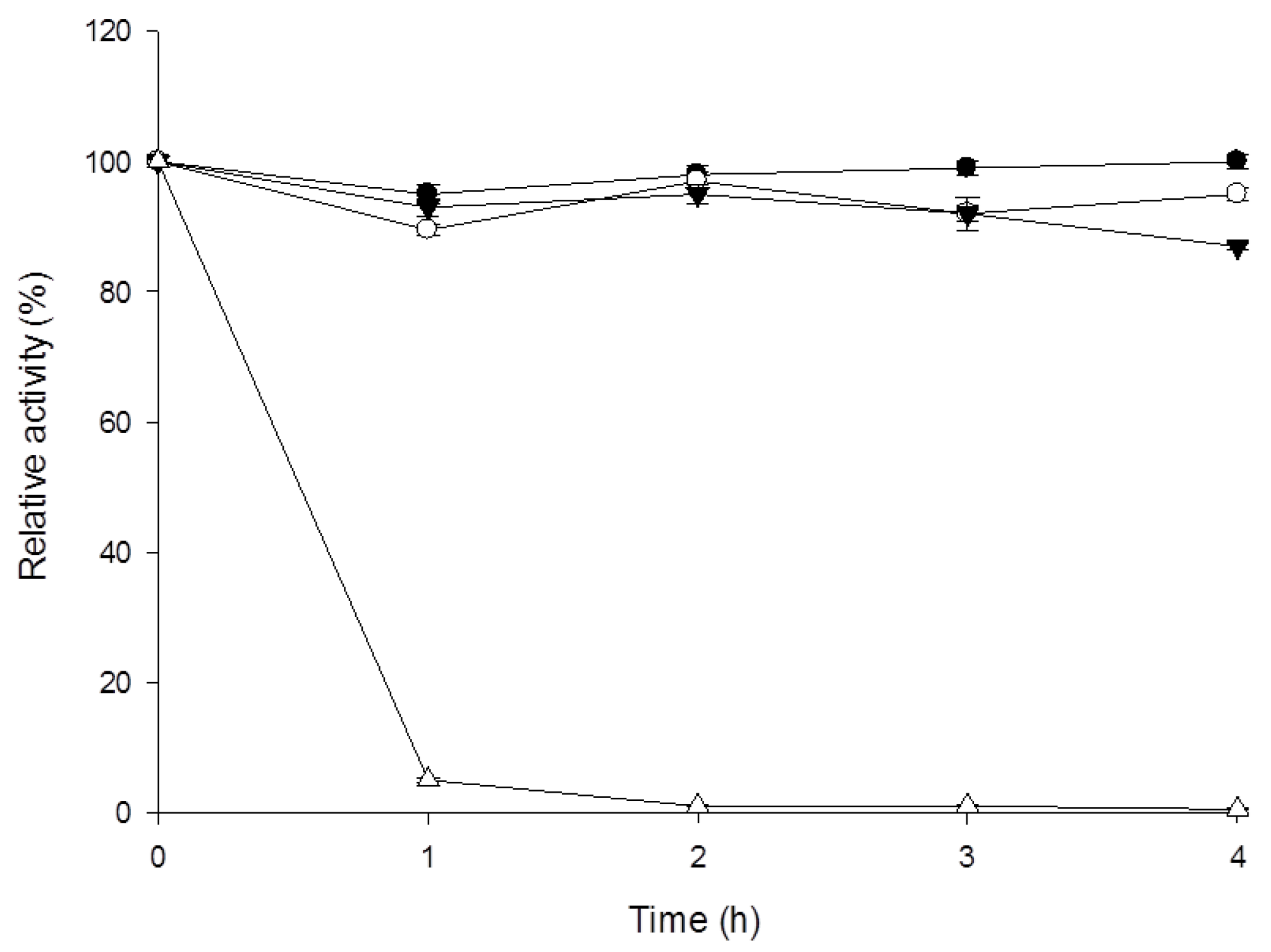
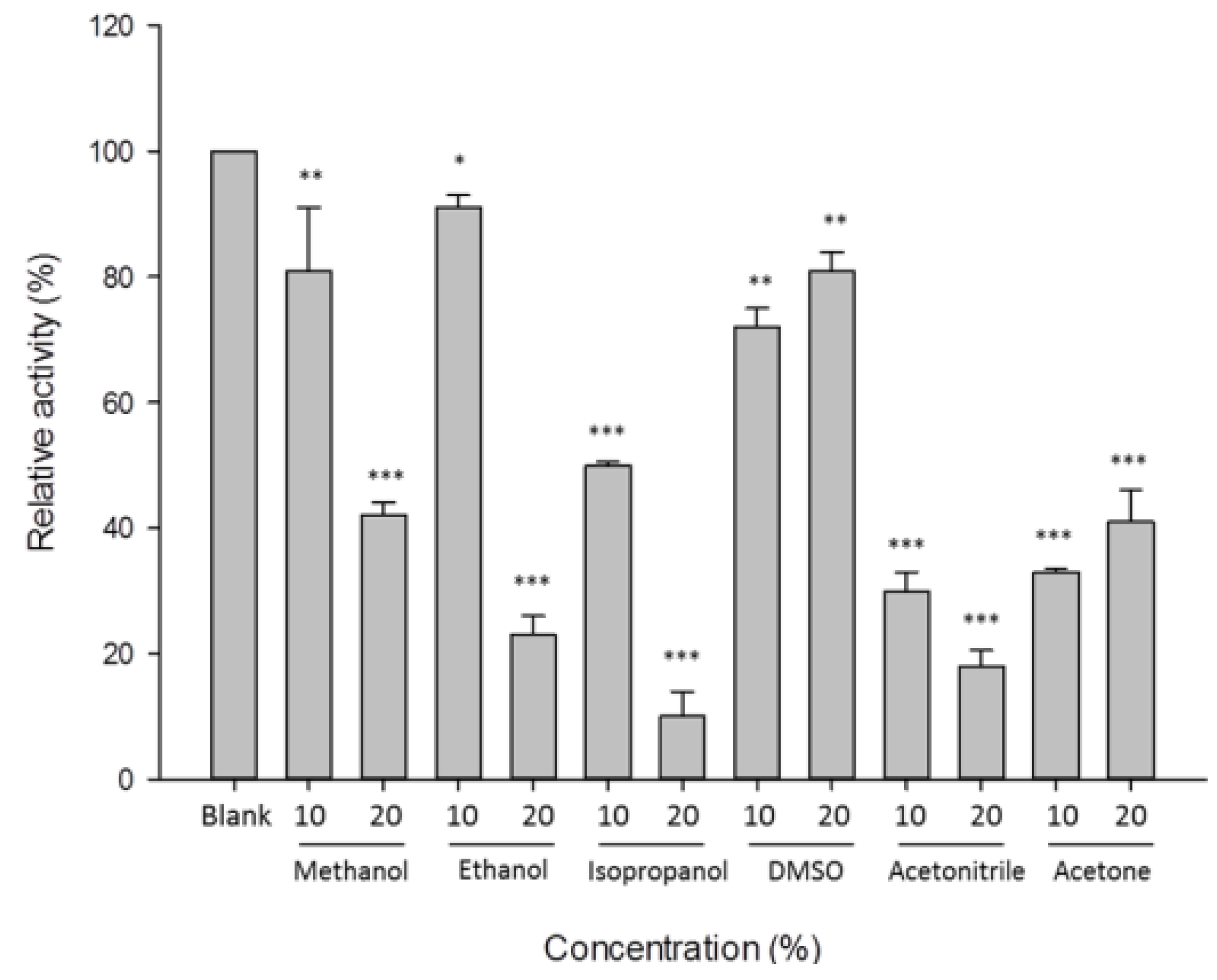
3.4. Characterization of β-1,3-Glucanase from Y. lipolytica Transformant (pYLSC1-13g)
3.5. Enzymatic Hydrolysis of Polyporaceae Glucans
3.6. Evaluation of the Antioxidant Activity of Polyporaceae Hydrolysates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, E.Y.; Lee, S.S.; Hyeon, J.Y.; Choe, S.H.; Keum, B.R.; Lim, J.M.; Park, D.C.; Chio, I.S.; Cho, K.K. Effects of β-glucan on the release of nitric oxide by macrophages stimulated with lipopolysaccharide. Asian-Australas J. Anim. Sci. 2016, 29, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Hori, T.; Kurita, K.; Takeo, K.; Hara, C.; Itoh, W.; Tabata, K.; Elgsaeter, A.; Stokke, B.T. An antitumor, branched (1→ 3)-β-D-glucan from a water extract of fruiting bodies of Cryptoporus volvatus. Carbohydr. Res. 1994, 263, 111–121. [Google Scholar] [CrossRef]
- Huiqin, F.; Qingyao, Y.; Xiaotong, Y.; Ke, M.; Lingyun, Z. Analysis of polysaccharide isolated from the fruit bodies and mycelia of Grifola frondosa. Huadong Shifan Daxue Xuebao J. East China Norm. Univ. 2001, 3, 9–96. [Google Scholar]
- Lei, N.; Wang, M.; Zhang, L.; Xiao, S.; Fei, C.; Wang, X.; Zhang, K.; Zheng, W.; Wang, C.; Yang, R. Effects of low molecular weight yeast β-glucan on antioxidant and immunological activities in mice. Int. J. Mol. Sci. 2015, 16, 21575–21590. [Google Scholar] [CrossRef]
- Choromanska, A.; Kulbacka, J.; Rembialkowska, N.; Pilat, J.; Oledzki, R.; Harasym, J.; Saczko, J. Anticancer properties of low molecular weight oat beta-glucan–An in vitro study. Int. J. Biol. Macromol. 2015, 80, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Tanioka, A.; Iwasawa, H.; Hatashima, K.; Shoji, Y.; Ishibashi, K. Structural characterisation and biological activities of a unique type β-D-glucan obtained from Aureobasidium pullulans. Glycoconj J. 2008, 25, 851–861. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Physiological effects of different types of β-glucan. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2007, 151, 225–231. [Google Scholar] [CrossRef]
- Tuong, T.D.; Chu, D.X.; Dieu, B.T.M. Antioxidant activity of fruiting body extracts from Pycnoporus sanguineus (L.: Fr.) Murrill mushroom. Vietnam J. Sci. Technol. 2020, 58, 143–151. [Google Scholar]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Dzhavakhiya, V.G.; Ozeretskovskaya, O.L.; Zinovyeva, S.V. Chapter 10-Immune response. In Comprehensive and Molecular Phytopathology; Dyakov, Y.T., Dzhavakhiya, V.G., Korpela, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 265–314. [Google Scholar]
- Wu, Q.; Dou, X.; Wang, Q.; Guan, Z.; Cai, Y.; Liao, X. Isolation of β-1,3-Glucanase-Producing Microorganisms from Poria cocos Cultivation Soil via Molecular Biology. Molecules 2018, 23, 1555. [Google Scholar] [CrossRef]
- Kao, P.F.; Wang, S.H.; Hung, W.T.; Liao, Y.H.; Lin, C.M.; Yang, W.B. Structural characterization and antioxidative activity of low-molecular-weights beta-1, 3-glucan from the residue of extracted Ganoderma lucidum fruiting bodies. J. Biomed. Biotechnol. 2012, 1, 673764. [Google Scholar]
- Wang, H.; Mukerabigwi, J.F.; Zhang, Y.; Han, L.; Jiayinaguli, T.; Wang, Q.; Liu, L.; Cao, Y.; Sun, R.; Huang, X. In vivo immunological activity of carboxymethylated-sulfated (1→ 3)-β-d-glucan from sclerotium of Poria cocos. Int. J. Biol. Macromol. 2015, 79, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Nie, J.; Li, D.; Zhu, W.; Zhang, S.; Ma, F.; Sun, Q.; Song, J.; Zheng, Y.; Chen, P. Characterization and antioxidant activities of degraded polysaccharides from Poria cocos sclerotium. Carbohydr. Polym. 2014, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- He, P.F.; Zhang, A.Q.; Wang, X.L.; Qu, L.; Li, G.L.; Li, Y.P.; Sun, P.L. Structure elucidation and antioxidant activity of a novel polysaccharide from Polyporus umbellatus sclerotia. Int. J. Biol. Macromol. 2016, 82, 411–417. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.W.; Yang, Z.L.; Bau, T.; Li, T.H.; Dai, Y.C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Yang, L.J.; Wang, R.T.; Liu, J.S.; Tong, H.; Deng, Y.Q.; Li, Q.H. The effect of Polyporus umbellatus polysaccharide on the immunosuppression property of culture supernatant of S180 cells. Cell Mol. Immunol. 2004, 20, 234–237. [Google Scholar]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory properties and antitumor activities of glucans. Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.J.; Xu, C.P. Culture characterization of exopolysaccharides with antioxidant activity produced by Pycnoporus sanguineus in stirred-tank and airlift reactors. J. Taiwan Inst. Chem. Eng. 2014, 45, 2075–2080. [Google Scholar] [CrossRef]
- Zhou, L.H.; Zhang, Y.Q.; Wang, R.J.; Shen, X.L.; Li, Y.Q.; Guan, W.J. Optimization of mycelial biomass and protease production by Laccocephalum mylittae in submerged fermentation. Afr. J. Biotechnol. 2009, 8, 1591–1601. [Google Scholar]
- Tuncer, M.; Ball, A.S.; Rob, A.; Wilson, M.T. Optimization of extracellular lignocellulolytic enzyme production by a thermophilic actinomycetes Thermomonospora fusca BD25. Enzyme Microb. Technol. 1999, 25, 38–47. [Google Scholar] [CrossRef]
- Yang, C.H.; Liu, W.H. Purification and properties of an acetylxylan esterase from Thermobifida fusca. Enzyme Microb. Technol. 2008, 42, 181–186. [Google Scholar] [CrossRef] [PubMed]
- McGrath, C.E.; Wilson, D.B. Characterization of a Thermobifida fusca β-1,3-glucanase (Lam81A) with a potential role in plant biomass degradation. Biochemistry 2006, 45, 14094–14100. [Google Scholar] [CrossRef] [PubMed]
- Bordes, F.; Fudalej, F.; Dossat, V.; Nicaud, J.M.; Marty, A. A new recombinant protein expression system for high-throughput screening in the yeast Yarrowia lipolytica. J. Microbiol. Methods 2007, 70, 393–502. [Google Scholar] [CrossRef] [PubMed]
- Madzak, C.; Treton, B.; Blanchin-Roland, S. Strong hybrid promoters and integrative expression/secretion vectors for quasi-constitutive expression of heterologous proteins in the yeast Yarrowia lipolytica. J. Mol. Microbiol. Biotechnol. 2000, 2, 207–216. [Google Scholar]
- Madzak, C.; Gaillardin, C.; Beckerich, J.M. Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica. J. Biotechnol. 2004, 109, 63–81. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, C.Y.; Chen, W.-L.; Ciou, Y.-P.; Yang, T.Y.; Yang, C.H. Production and antioxidant properties of the ferulic acid-rich destarched wheat bran hydrolysate by feruloyl esterases from thermophilic actinomycetes. BioResources 2013, 8, 4981–4991. [Google Scholar] [CrossRef]
- El-Katatny, M.; Gudelj, M.; Robra, K.H.; Elnaghy, M.; Gübitz, G. Characterization of a chitinase and an endo-β-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Appl. Microbiol. Biotechnol. 2001, 56, 137–143. [Google Scholar] [CrossRef]
- Schwentke, J.; Sabel, A.; Petri, A.; König, H.; Claus, H. The yeast Wickerhamomyces anomalus AS1 secretes a multifunctional exo-β-1,3-glucanase with implications for winemaking. Yeast 2014, 31, 349–359. [Google Scholar] [CrossRef]
- Fridlender, M.; Inbar, J.; Chet, I. Biological control of soilborne plant pathogens by a β-1,3-glucanase-producing Pseudomonas cepacia. Soil Biol. Biochem. 1993, 25, 1211–1221. [Google Scholar] [CrossRef]
- Abeles, F.B.; Forrence, L.E. Temporal and hormonal control of β-1,3-glucanase in Phaseolus vulgaris L. Plant Physiol. 1970, 45, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Noronha, E.F.; Ulhoa, C. Characterization of a 29-kDa β-1,3-glucanase from Trichoderma harzianum. FEMS Microbiol. Lett. 2000, 183, 119–123. [Google Scholar] [CrossRef]
- Woo, C.B.; Kang, H.N.; Lee, S.B. Molecular cloning and anti-fungal effect of endo-β-1,3-glucanase from Thermotoga maritima. Food Sci. Biotechnol. 2014, 23, 1243–1246. [Google Scholar] [CrossRef]
- Monteiro, V.N.; Ulhoa, C.J. Biochemical characterization of a β-1,3-glucanase from Trichoderma koningii induced by cell wall of Rhizoctonia solani. Curr. Microbiol. 2006, 52, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, S.; Mori, H.; Yamamoto, S. Characterization of Endo β-1,3-Glucanase IV from Flavobacterium dormitator var. glucanolyticae FA-5. Agric. Biol. Chem. 1981, 45, 2689–2694. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Xing, R.; Li, K.C.; Li, R.F.; Qin, Y.K.; Wang, X.Q.; Wei, Z.H.; Li, P.C. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996. [Google Scholar] [CrossRef]
- Guo, X.; Ye, X.Q.; Sun, Y.J.; Wu, D.; Wu, N.A.; Hu, Y.Q.; Chen, S.G. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J. Agric. Food Chem. 2014, 62, 1088–1095. [Google Scholar] [CrossRef]
- Zha, S.H.; Zhao, Q.S.; Zhao, B.; Ouyang, J.; Mo, J.L.; Chen, J.J.; Cao, L.L.; Zhang, H. Molecular weight controllable degradation of Laminaria japonica polysaccharides and its antioxidant properties. J. Ocean Univ. China 2016, 15, 637–642. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Sci. Technol. 2018, 196, 287–299. [Google Scholar]
- Imeh, U.; Khokhar, S. Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. J. Agric. Food Chem. 2002, 50, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Ann. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A high throughput colorimetric assay of β-1,3-d-glucans by Congo red dye. J. Microbiol. Methods 2015, 109, 140–148. [Google Scholar] [CrossRef] [PubMed]


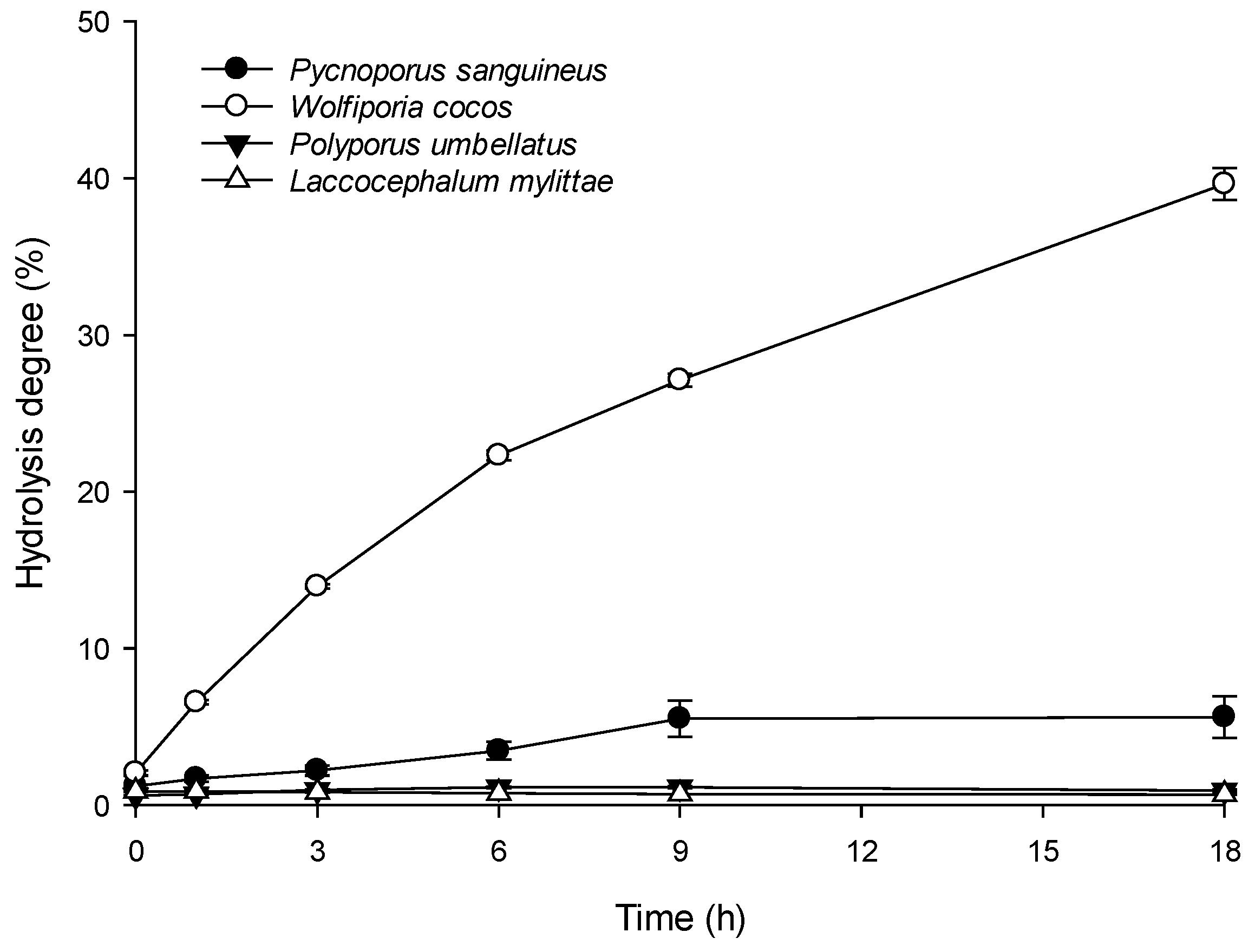
 Pycnoporus sanguineus;
Pycnoporus sanguineus;  Wolfiporia cocos. Data: mean ± SE from triplicate experiments (Student’s t-test p values: * p < 0.05; ** p < 0.01; *** p < 0.001).
Wolfiporia cocos. Data: mean ± SE from triplicate experiments (Student’s t-test p values: * p < 0.05; ** p < 0.01; *** p < 0.001).
 Pycnoporus sanguineus;
Pycnoporus sanguineus;  Wolfiporia cocos. Data: mean ± SE from triplicate experiments (Student’s t-test p values: * p < 0.05; ** p < 0.01; *** p < 0.001).
Wolfiporia cocos. Data: mean ± SE from triplicate experiments (Student’s t-test p values: * p < 0.05; ** p < 0.01; *** p < 0.001).
| Total Activity (U) | Total Protein (mg) | Specific Activity (U/mg) | Purification (Fold) | Yield (%) | |
|---|---|---|---|---|---|
| Culture supernatant | 101,341.38 | 12.36 | 8199.14 | 1 | 100 |
| Ni2+-NTA column | 29,661.15 | 0.92 | 32,240.38 | 3.93 | 29.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-L.; Hsu, J.-C.; Lim, C.-L.; Chen, C.-Y.; Yang, C.-H. Expression of the Thermobifida fusca β-1,3-Glucanase in Yarrowia lipolytica and Its Application in Hydrolysis of β-1,3-Glucan from Four Kinds of Polyporaceae. Processes 2021, 9, 56. https://doi.org/10.3390/pr9010056
Chen W-L, Hsu J-C, Lim C-L, Chen C-Y, Yang C-H. Expression of the Thermobifida fusca β-1,3-Glucanase in Yarrowia lipolytica and Its Application in Hydrolysis of β-1,3-Glucan from Four Kinds of Polyporaceae. Processes. 2021; 9(1):56. https://doi.org/10.3390/pr9010056
Chicago/Turabian StyleChen, Wei-Lin, Jo-Chieh Hsu, Chui-Li Lim, Cheng-Yu Chen, and Chao-Hsun Yang. 2021. "Expression of the Thermobifida fusca β-1,3-Glucanase in Yarrowia lipolytica and Its Application in Hydrolysis of β-1,3-Glucan from Four Kinds of Polyporaceae" Processes 9, no. 1: 56. https://doi.org/10.3390/pr9010056
APA StyleChen, W.-L., Hsu, J.-C., Lim, C.-L., Chen, C.-Y., & Yang, C.-H. (2021). Expression of the Thermobifida fusca β-1,3-Glucanase in Yarrowia lipolytica and Its Application in Hydrolysis of β-1,3-Glucan from Four Kinds of Polyporaceae. Processes, 9(1), 56. https://doi.org/10.3390/pr9010056





