1. Introduction
The removal of SO2 from exhaust gas using an absorptive magnesium-based slurry is a well-established method to control SO2 emissions and simultaneously recover Sulfur as a resource of further usage. The pulp production industry using magnesium bisulfite as cooking liquor can ensure a nearly full chemical recovery if applying the absorption process in an optimized way.
After combustion, magnesium oxide is recovered from the ash, hydrated to magnesium hydroxide, and serves subsequently as absorbent. The absorption of SO
2 from the exhaust gas with hydrated magnesium oxide forms magnesium bisulfite, which serves again as cooking liquor. Equations (1)–(3) show the simplified reactions leading to the recovered cooking liquor.
This process allows the full reuse of required chemicals leading to a nearly closed-loop process control.
However, besides the intended reactions, unwanted precipitation reactions can occur under unideal process conditions. The precipitation of salts in the system highly influences the efficiency of the process. A high precipitation rate causes chemical loss as the precipitated salts will be washed out in a regular cleaning step rather than recycled in the process [
1]. Uncontrolled precipitation of salts therefore means interruption of the closed-loop process leading to an increased usage of fresh chemicals and to more frequent cleaning operations. This challenges not only the ecological footprint but also the economic feasibility.
To prevent precipitation and provide a cleaner and more efficient production, it is essential to understand the precipitation reactions in the system. Advanced process modeling can help in optimizing process conditions. A model, which covers the complex matter of precipitation reactions in electrolyte systems, requires the inclusion of all necessary dependencies sufficiently. Reliable literature data are of vital importance to ensure accuracy of the developed model. This makes a comprehensive review of available solubility data in the literature necessary.
While MgO, SO2 and H2O are process elements, CaO and O2 can influence the reaction system as non-process elements. CaO can enter the process with process water and can consequently contribute to precipitation issues in the system. O2 enters the process as remaining oxygen in the exhaust gas stream and can influence precipitation due to oxidation. To provide a comprehensive understanding of the precipitation process in the system, this review studies the solubility of salts in the MgO-CaO-SO2-H2O-O2 system based on available literature data.
The following covers a comprehensive review of available solubility data for MgSO
3, CaSO
3, MgSO
4, CaSO
4, Mg(OH)
2 and Ca(OH)
2 as potential salts in the system. One major source for solubility data is the solubility data series of the International Union of Pure and Applied Chemistry (IUPAC) [
2,
3]. However, not all potential salts are covered in these evaluations. Therefore, this review includes additional data and also complements the data with newer studies to comprehensively review the state of the art of available solubility data. Furthermore, correlations describing the solubility reported in the literature are included and reviewed.
Based on the data, this paper recommends strategies for sufficiently accurate modeling of the precipitation. Although original data are partially given in molar units, this review gives all solubility data in g/100 g to provide consistency throughout the review.
2. Chemical System and Precipitation of Salts
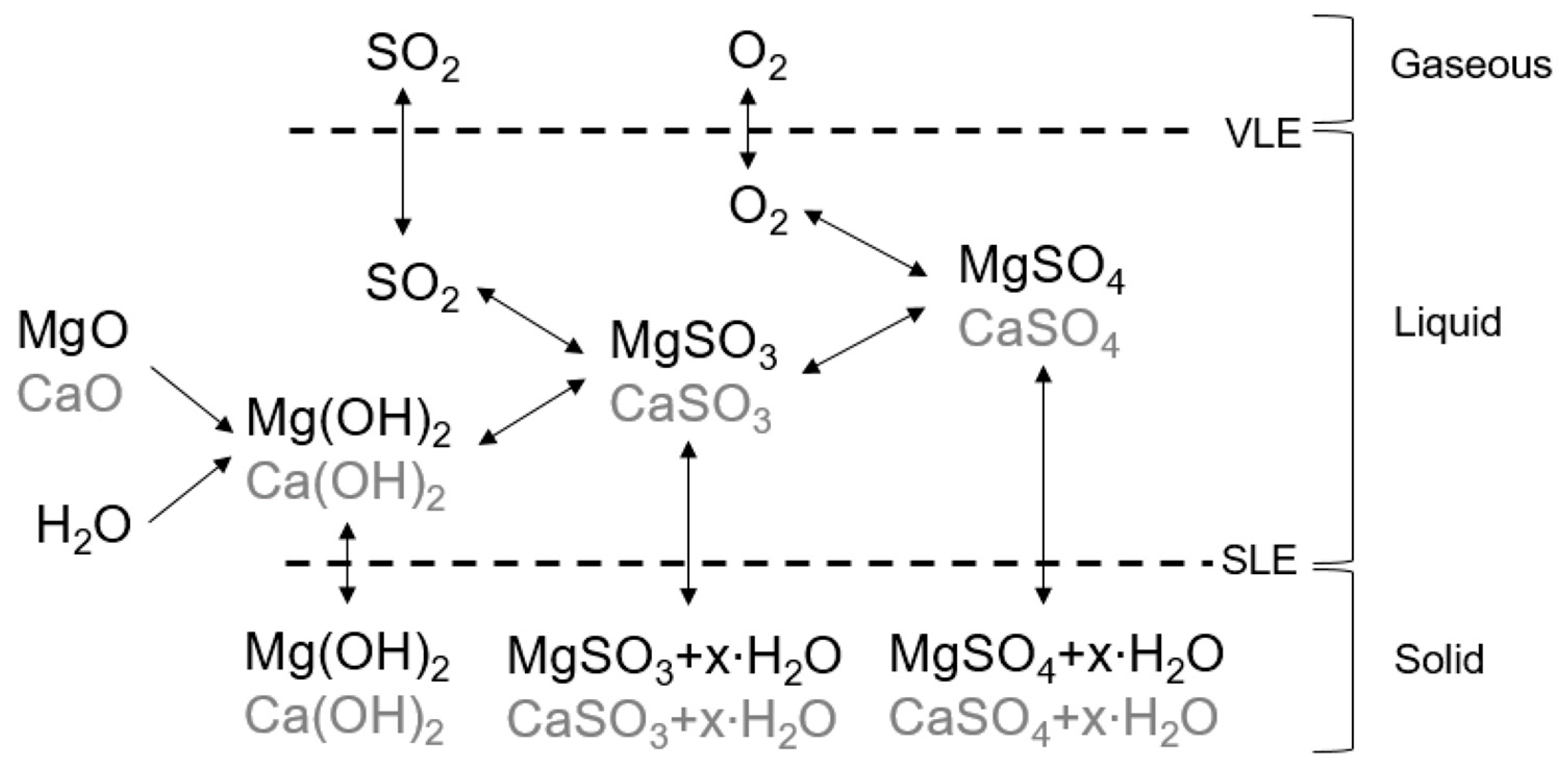
Figure 1 visualizes simplified the chemical system leading to the formation and precipitation of sulfites, sulfates, and hydroxides in the studies system.
SO
2 and O
2 from the gas phase dissolve into the liquid phase according to the Vapor-Liquid-Equilibrium (VLE). The presence of Mg
+ from dissolved Mg(OH)
2 (see
Section 2.5) and the presence of SO
3—from dissolved SO
2 in the liquid phase lead to the formation of MgSO
3. Mg(OH)
2 and MgSO
3 are intended products of the main process reactions (compare Equations (1)–(3)). Ca(OH)
2 and CaSO
3 are formed in the presence of CaO as a non-process-element in the same manner. The presence of oxygen in the exhaust gas can lead to oxidation of sulfites and the formation of MgSO
4 and CaSO
4 [
4]. Mg(OH)
2, Ca(OH)
2, MgSO
3, CaSO
3, MgSO
4 and CaSO
4 can precipitate in the system according to the Solid-Liquid-Equilibrium (SLE). The following focuses on the solubility of these potential salts.
Sulfites and Sulfates precipitate as different hydrates depending on process conditions [
2]. The solubility of the hydrate forms can differ greatly. Understanding the stability behavior of these hydrate forms is therefore of great practical interest when developing a reliable process model.
2.1. Magnesium Sulfite (MgSO3)
Equation (4) shows the solution equilibrium of MgSO
3.
Lutz’s evaluation in the Solubility Data Series of IUPAC reported that the stable hydrate form of MgSO
3 at temperatures lower than 37.85 °C is hexahydrate (MgSO
3*6H
2O). At temperatures higher than 37.85 °C it is trihydrate (MgSO
3*3H
2O) [
2]. This report is based on the studies of Hagisawa, Okabe et al., Pinaev et al., and Lutz et al. [
5]. However, a slow conversion rate from the metastable hexahydrate to trihydrate leads to the establishment of a metastable solubility equilibrium and the crystallization of hexahydrate even at temperatures higher than 37.85 °C [
6,
7].
The conversion of hexahydrate to trihydrate is a pH and temperature depending process. Steindl et al. monitored the conversion using Raman Spectroscopy [
1]. While at a temperature of 68 °C the conversion was completed after around 50 min, at a temperature of 53 °C the conversion was completed after around 580 min. The studies also showed an increasing conversion time with increasing starting pH value [
1]. Following Steindl et al. a low pH value and high temperatures enhance therefore the conversion of hexahydrate to trihydrate. At temperatures higher than 40 °C, magnesium hexahydrate has a higher solubility than trihydrate (see
Figure 2). Preventing the conversion of hexahydrate to trihydrate is therefore of practical interest to lower precipitation.
2.1.1. Solubility of MgSO3 Hydrates in Water
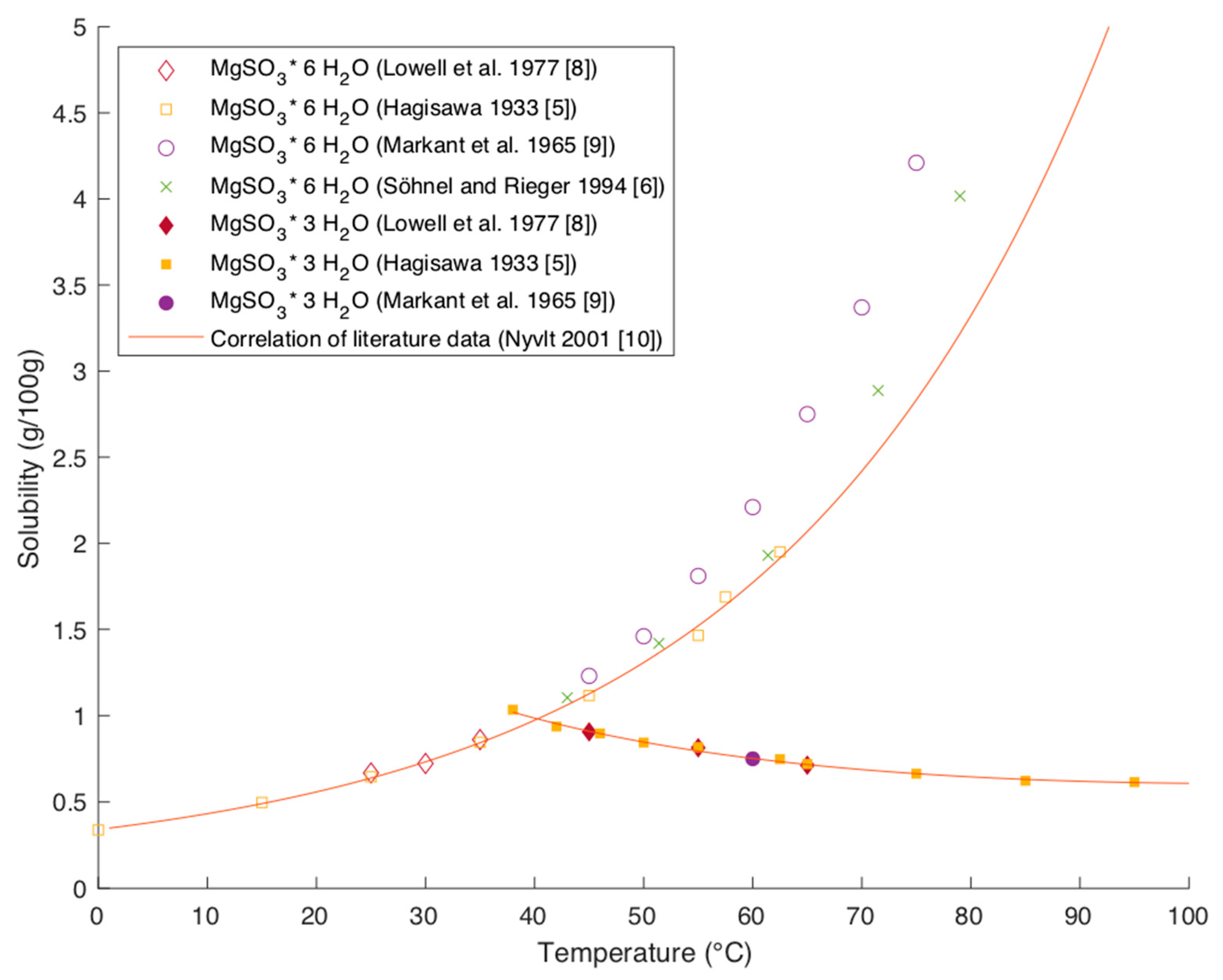
Figure 2 shows solubility data of magnesium hexa- and trihydrate in water.
The data found in the literature are mutually in very good agreement. The studies of Hagisawa and Markant were evaluated by Lutz in the IUPAC Solubility data series [
2,
5,
8,
9]. The data captured by Söhnel and Rieger and by Lowell et al. match those studies very well [
6,
8].
While the solubility of magnesium hexahydrate is increasing with temperature, the solubility of trihydrate is decreasing with temperature. This indicates that the dissolution of trihydrate is exothermic whereas the dissolution of hexahydrate is endothermic. Nyvlt was able to describe the temperature dependency of the solubility by correlating the data of Lowell, leading to the following equations with
being the molar fraction and
the temperature in K [
10]:
The plot of these equations in
Figure 2 shows a good correlation with existing literature data for trihydrate and hexahydrate at temperatures up to 60 °C. The correlation shows an increasing deviation from data points at higher temperatures.
The solubility of the two hydrate forms have an inverse dependency on temperature and the conversion times can be long. Consequently, occurring precipitation in the system is highly dependent on residence time and process conditions. While the given data provide a solid base for modeling purposes, a critical review of each given case is necessary to cover the change in solubility accurately.
However, the solubility of MgSO3 in the MgO-CaO-SO2-H2O-O2 system is not solely a function of temperature.
2.1.2. Influence of SO2 on Solubility of MgSO3 Hydrates
The solubility of MgSO
3 increases with increasing SO
2 content in the solution [
2].
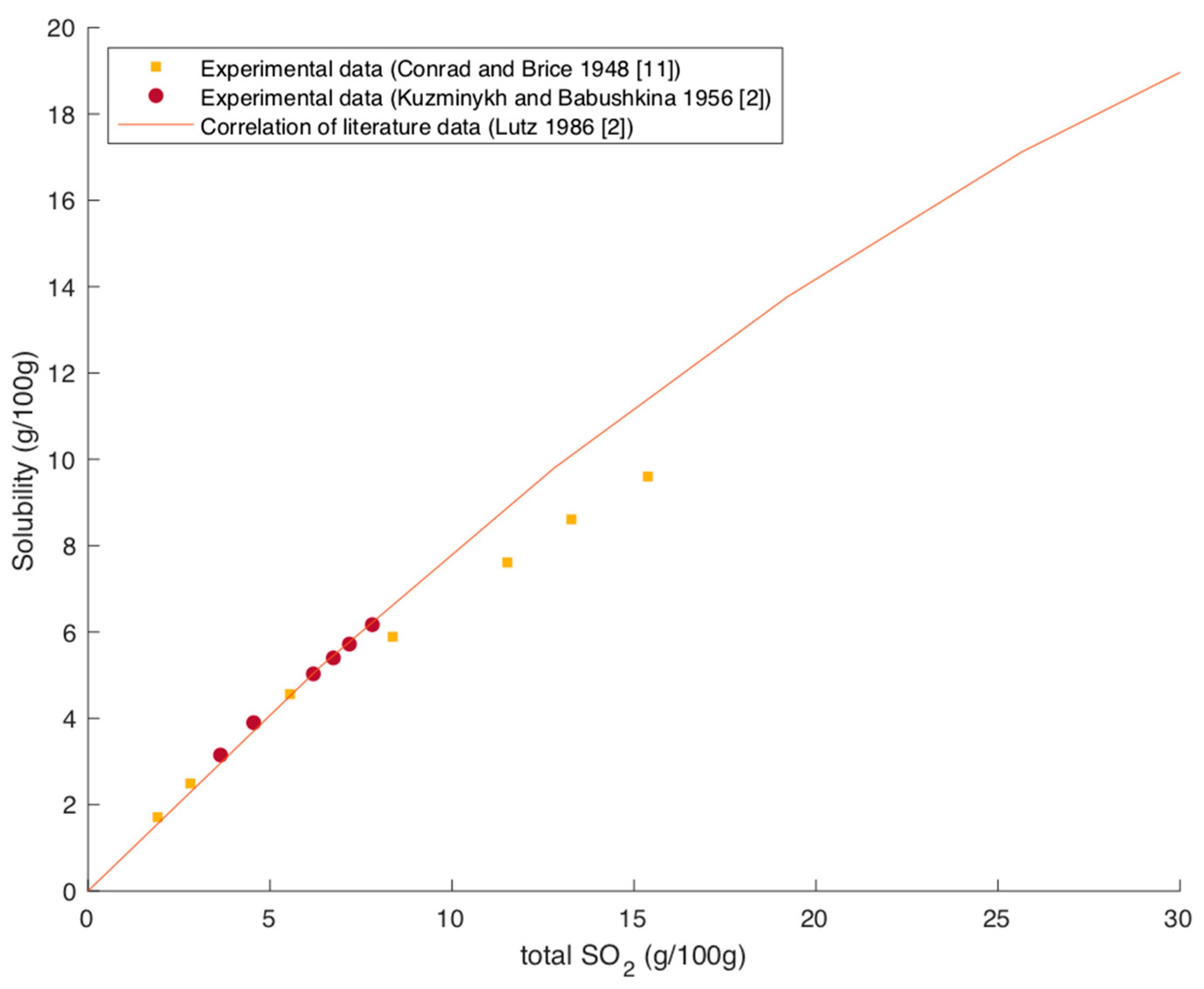
Figure 3 shows experimental data as well as a correlation of literature data of the solubility of magnesium sulfite over the SO
2 content in the solution at 25 °C [
2,
11].
Lutz recommends describing the change in solubility with the presence of SO
2 by following correlation with
being the Solubility of MgSO
3 in mol/kg and
the molality of SO
2 in mol/kg [
2]:
Figure 3 shows the good agreement of this correlation with the available experimental data by Conrad and Brice. The increasing solubility with an increasing SO
2 content can be explained with the formation of Mg(HSO
3)
2 (see Equation (3)). Several studies recorded an increasing amount of total dissolved magnesium sulfite with increasing amount of Mg(HSO
3)
2 in the MgSO
3-Mg(HSO
3)
2-H
2O system [
2].
2.1.3. Influence of MgSO4 on Solubility of MgSO3 Hydrates
Several authors reported the influence of MgSO
4 on the solubility of MgSO
3.
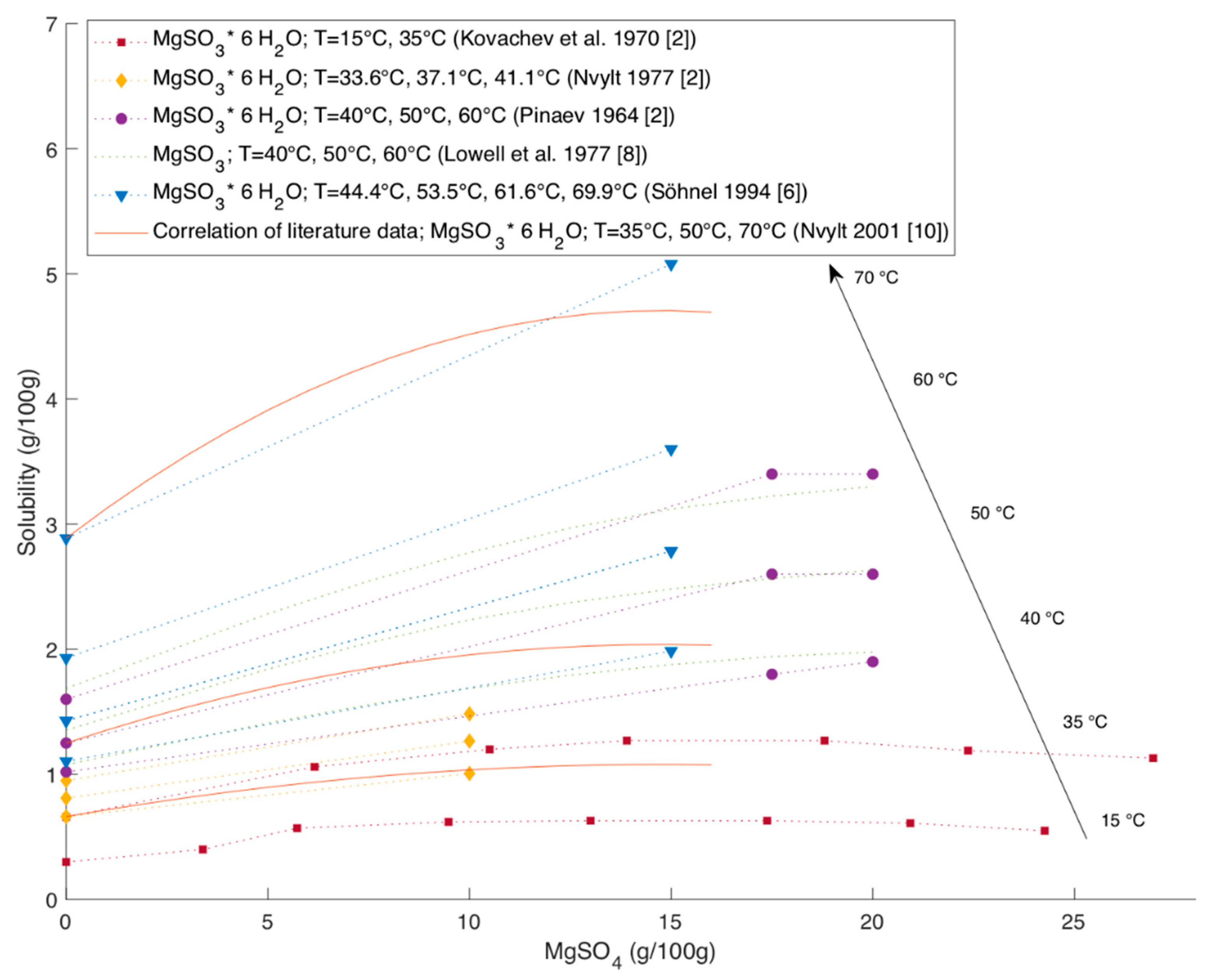
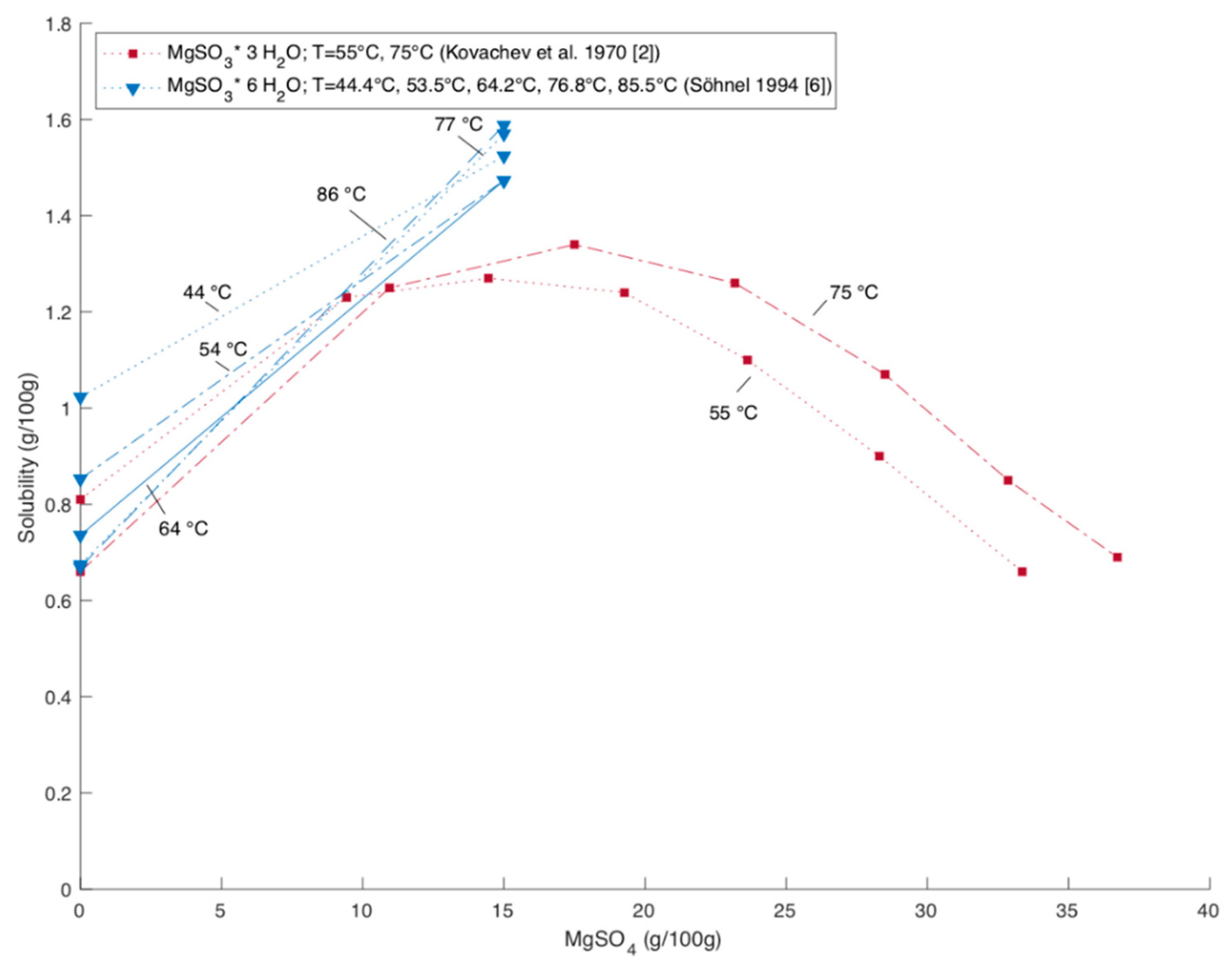
Figure 4 shows the solubility of MgSO
3 hexahydrate over the MgSO
4 content in the solution and
Figure 5 the solubility of MgSO
3 trihydrate respectively. The presence of MgSO
4 may also influence whether trihydrate or hexahydrate is formed as stable form [
6,
8]. Pinaev explains this by the effect of MgSO
4 on the solution temperature and viscosity [
8]. However, there are not enough studies found to confirm and characterize this influence sufficiently.
The data of Kovachev et al., 1970, Pinaev 1964 and Nvylt et al., 1977 are taken from the IPUAC solubility data series [
2]. The experimental data for trihydrate and hexahydrate are in relatively good agreement with each other. All studies report an increase in solubility with increasing MgSO
4 content. Kovachev et al. covered the widest range of MgSO
4 content. The study covers the whole range of MgSO
4 content in which only MgSO
3 hydrates precipitate. At higher MgSO
4 contents than plotted in
Figure 4 and
Figure 5, MgSO
4 hydrates precipitated besides MgSO
3 hydrates [
2].
The data of Kovachev et al. show a maximum in solubility of MgSO
3 at MgSO
4 content of around 13 to 17 g per 100 g for trihydrate as well as for the hexahydrate [
2]. The data for hexahydrate reported by Pinaev indicate as well that the solubility stabilizes when reaching a certain amount of MgSO
4 [
2]. Lowel et al. present a graph showing the dependency between MgSO
4 content and the solubility of MgSO
3 hexahydrate based on values reported by Pinaev and McGlamery et al. [
8].
The data of Söhnel and Kovachev et al. show that the effect of the presence of MgSO
4 on the solubility of MgSO
3 trihydrate is smaller for lower temperatures [
2,
6].
Nývlt correlated literature data to describe the influence of MgSO
4 on the solubility of hexahydrate with the following equation with
being the Solubility in kg/kg H
2O and
being the content of MgSO
4 in kg/kg H
2O [
10]:
Figure 4 shows the plot of this correlation for the solubility at 35 °C, 50 °C and 70 °C. While at 35 °C the correlation shows good agreements with experimental data, at higher temperatures the correlation increasingly underestimates the solubility of MgSO
3 with increasing MgSO
4 content.
2.2. Calcium Sulfite (CaSO3)
Equation (9) shows the solution equilibrium of CaSO
3 in water:
Lutz states in the IUPAC solubility series that CaSO
3 precipitates primarily as hemihydrate (CaSO
3*1/2H
2O). The existence of dihydrate (CaSO
3*2H
2O) and tetrahydrate (CaSO
3*4H
2O) was also reported but not confirmed for the CaSO
3-H
2O system [
2].
2.2.1. Solubility of CaSO3 Hydrates in Water
The solubility data of CaSO
3 in the literature are scarce and existing data scatter over a wide range. Lutz evaluated in the IUPAC solubility several studies found in the literature [
2]. Based on the evaluated data, Lutz recommends a solubility value of 0.0054 g/100 g of water at 25 °C. However, this value comes with a deviance of ±0.0012 g/100 g, which corresponds to around 22%. Lutz explains this strong scatter with the existence of different modifications of CaSO
3*1/2H
2O and its tendency to form supersaturated solutions [
2].
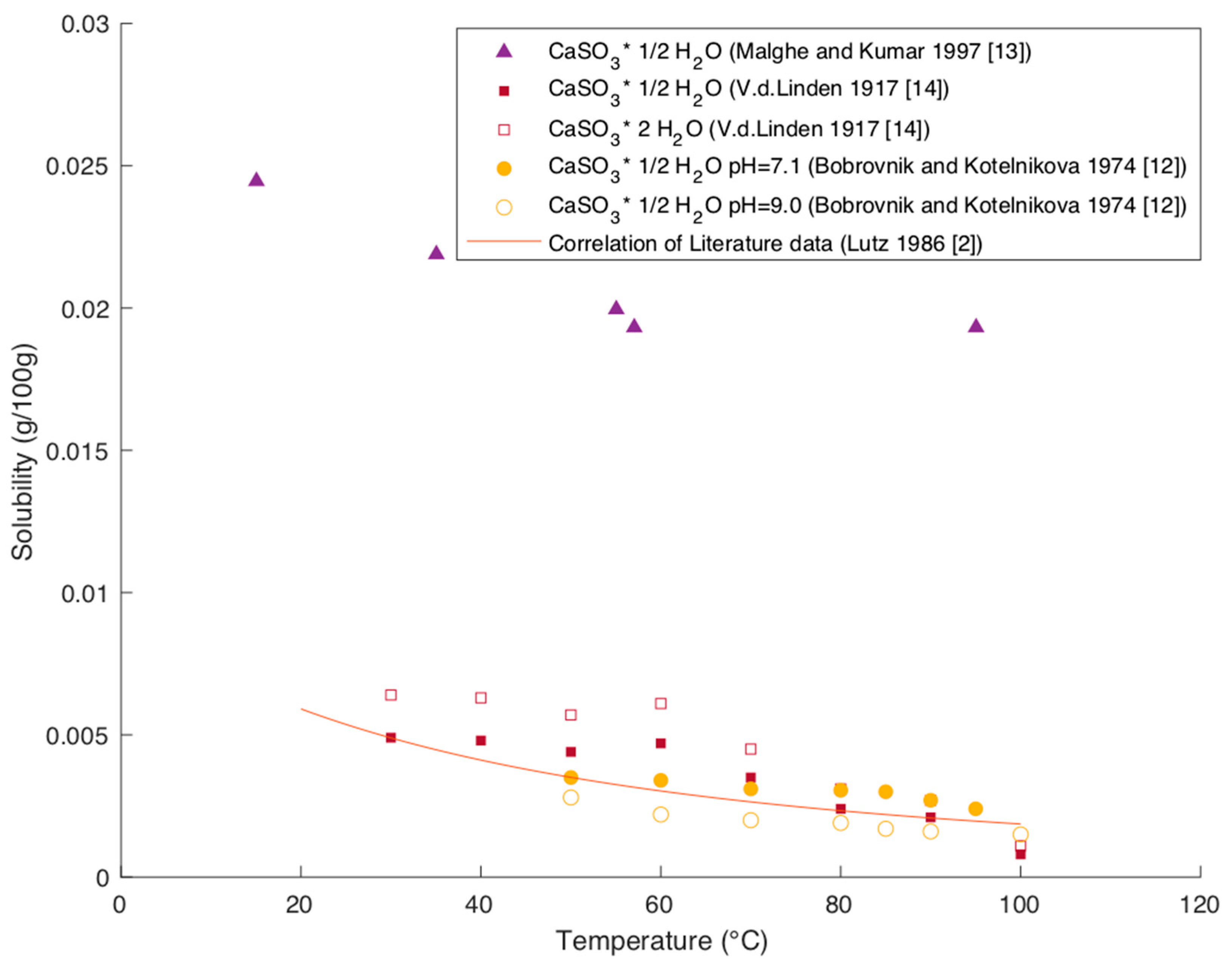
Figure 6 shows the literature data of Bobrovnik and Kotelnikova and van der Linden, which were identified as the most accurate by Lutz, as wells as the newer data of Malghe and Kumar [
2,
12,
13,
14].
The original data reported by Van Der Linden give the solubility for CaSO
3 dihydrate [
14]. Due to the conclusion that the stable form is the hemihydrate, the solubility for hemihydrate is given in the IUPAC solubility series as calculated values based on the data reported by Van der Linden [
2]. All data show a decrease in solubility with increasing temperature. The data from Malghe and Kumar strongly deviate from other literature data and greatly extend the deviance stated by Lutz. As the study does not specify the form of the analyzed CaSO
3 and due to the strong discrepancy, it is recommended to exclude these values for modeling purposes.
Lutz was able to correlate the literature data from Bobrovnik and Kotelnikova and van der Linden using the following equation with
as the solubility in mol/K and
as the temperature in Kelvin [
2]:
The plot of this correlation in
Figure 6 shows good agreement with the literature data when excluding the data by Malghe and Kumar [
13].
2.2.2. Influence of SO2 on Solubility of CaSO3 Hydrates
As for the MgSO
3-SO
2-H
2O system, the SO
2 content also has an increasing effect on the solubility of CaSO
3 [
2].
Figure 7 shows the solubility of CaSO
3 over the total SO
2 content in the solution at 25 °C.
The data from Kuzminykh and Babushkina in 1956 are taken from the IUPAC solubility data series [
2]. Lutz recommends describing the change in solubility with the presence of SO
2 by following correlation with
being the Solubility of CaSO
3 in mol/kg and
the molality of SO
2 in mol/kg.
Figure 7 shows the applicability of this correlation for an SO
2 content up to 10 g per 100 g water. For higher SO
2 contents, the correlation slightly overestimates the solubility compared to the data from Conrad and Brice.
As for MgSO3, the increasing solubility with increasing SO2 content can be explained with the formation of Ca(HSO3)2.
2.2.3. Influence of CaSO4 on Solubility of CaSO3 Hydrates
CaSO
4 has a decreasing effect on the solubility of CaSO
3 [
2].
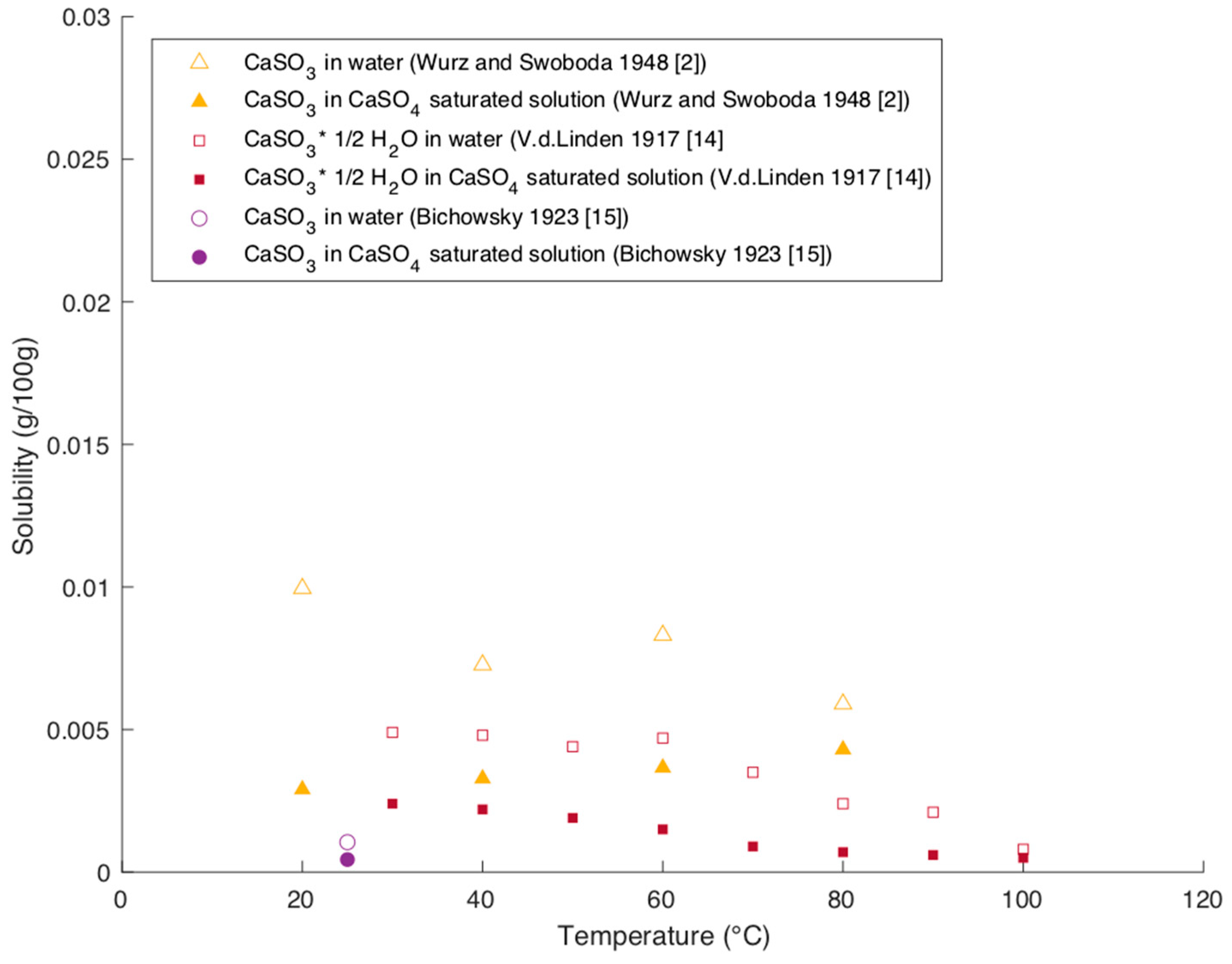
Figure 8 shows solubility data of CaSO
3 in water and in solution, which is saturated with CaSO
4 over temperature.
All reported data show a significant decrease in the solubility of CaSO3 in a solution, which is saturated with CaSO4 compared to the solubility in water. The effect of decreasing solubility is on average around −58% and maximum −75%. Unlike for MgSO3, there are no data reported, which analyze the effect of different sulfate concentrations on the solubility of CaSO3.
2.3. Magnesium Sulfate (MgSO4)
Equation (12) shows the solution equilibrium of MgSO
4:
Many studies have examined the stability of different hydrate forms of MgSO
4 [
16,
17,
18,
19,
20]. Following the study by Li et al. MgSO
4 exists stably as kieserite (MgSO
4*H
2O) and hexahydrate (MgSO
4*6H
2O) at 50 °C and only as kieserite at 75 °C in the MgCl
2-MgSO
4-H
2O system [
18]. Starkyite (MgSO
4*4H
2O) was recognized as metastable form at both temperatures and pentahydrate (MgSO
4*5H
2O) as metastable form at 50 °C. In a very recent study, Dongdong et al. have developed a thermodynamic model to predict the thermodynamic behavior of the Mg(OH)
2-MgSO
4-H
2O system. The model defines heptahydrate (MgSO
4*7H
2O) as stable form at temperatures lower than 45.85 °C, hexahydrate for the temperature range of around 45.85 to 71.85 °C and kieserite for temperatures higher than 71.85 °C [
20]. A solubility diagram of the MgSO
4-H
2O system presented by Steiger et al. supports this observation giving similar temperature windows for the existence of heptahydrate, hexahydrate and kieserite as stable hydrate forms of MgSO
4 [
17]. Nevertheless, several metastable hydrate forms, such as starkyite or pentahydrate, may coexist in this temperature range [
16,
17,
18] and different studies give partially contradictory new insides in the complicated reaction system [
18].
Solubility of MgSO4 Hydrates in Water
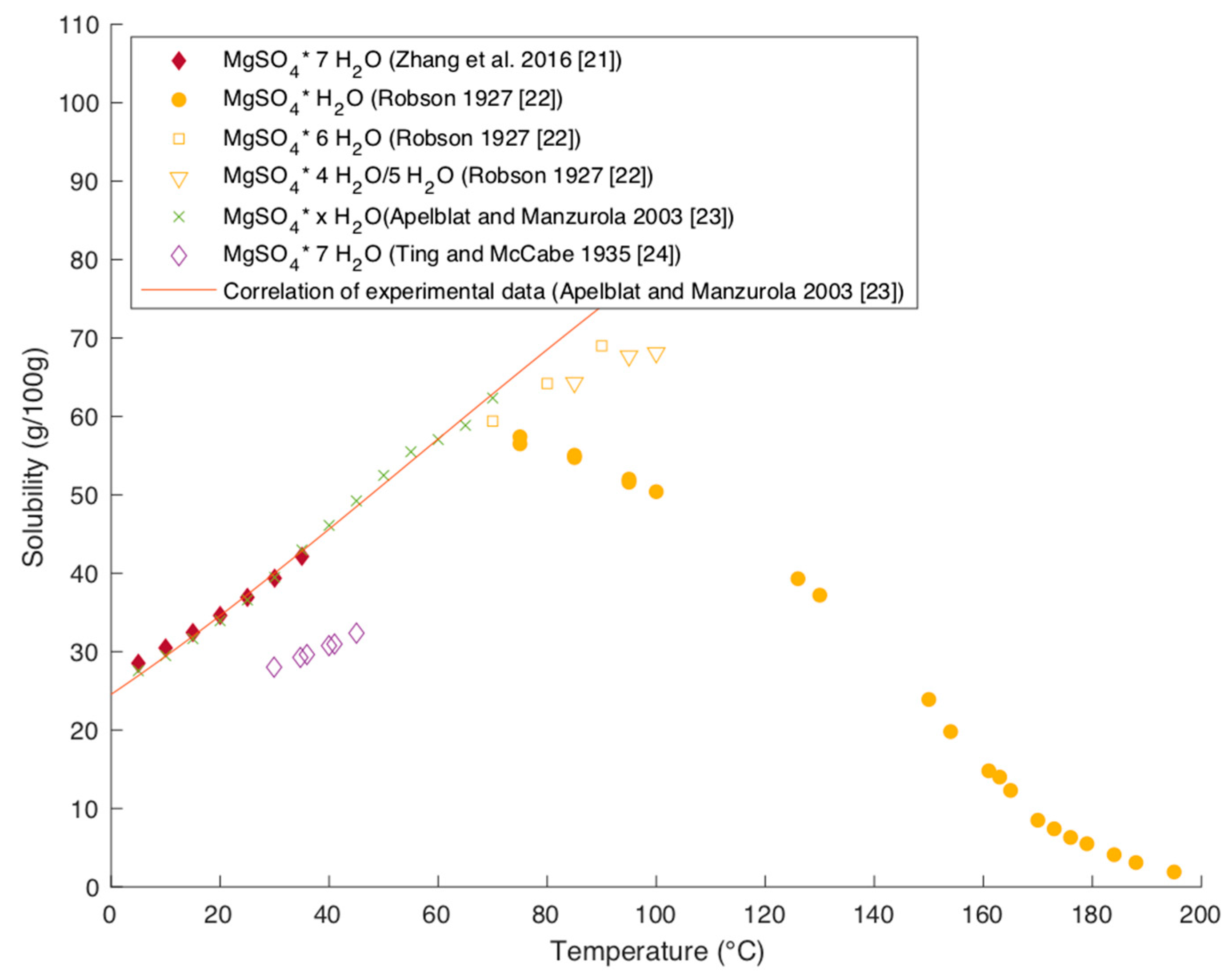
Figure 9 presents measured and correlated solubilities of the different hydrate forms over temperature.
The solubility of MgSO
4 salts in water is almost by factor 100 higher than the solubility of MgSO
3 hydrates (compare
Figure 2 and
Figure 9). Except for kieserite, the different hydrate forms show a similar solubility behavior. The data from Ting and McCabe report around 25% lower solubility than the other data. Otherwise, the data are in very good agreement with each other. The solubility of all hydrate forms except kieserite increases with increasing temperature. Apelblat and Manzurola correlated their data with the following equation with
being the Solubility in mol/kg and
being the temperature in K [
23]:
This correlation describes the solubility of all hydrate forms up to a temperature of 70 °C well when excluding the data from Ting and McCabe (see
Figure 9). At higher temperatures, the solubility decrease of kieserite reported by Robson needs to be taken into account and cannot be covered by the given correlation.
2.4. Calcium Sulfate (CaSO4)
Equation (14) describes the solution equilibrium of CaSO
4 in water:
Several studies report three main forms of CaSO
4, which is hemihydrate (CaSO
4*1/2H
2O), dihydrate (CaSO
4*2H
2O), and anhydrite (CaSO
4) [
25,
26]. While dihydrate is recognized as the stable form at temperatures lower than 40 °C and anhydrate at higher temperatures, hemihydrate is a metastable form and can coexist at temperatures around 90 to 130 °C [
27,
28,
29].
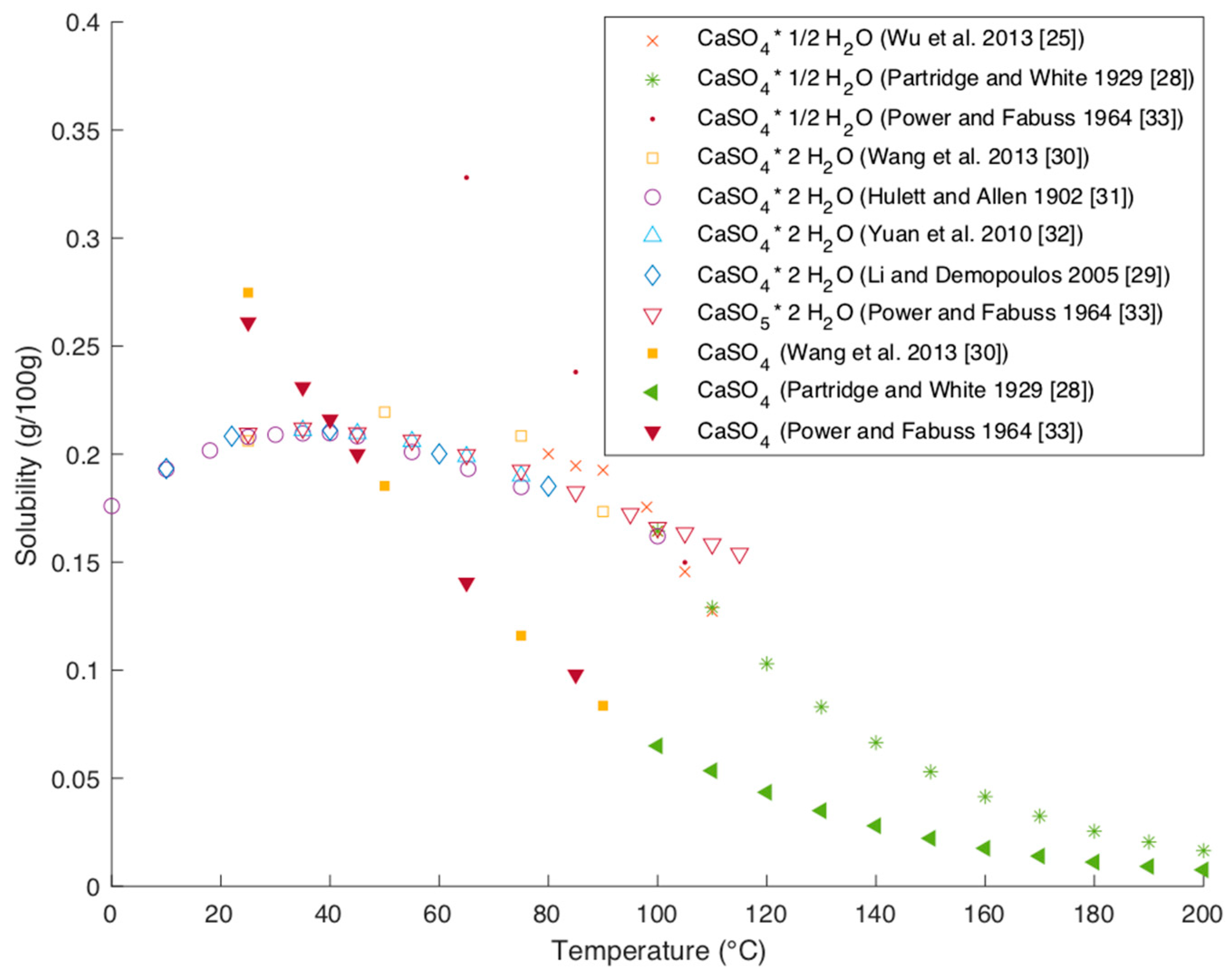
2.4.1. Solubility of CaSO4 Hydrates in Water
The solubility of the different CaSO
4 hydrates was studied by several researchers [
25,
28,
29,
30,
31,
32,
33]. The data are summarized in
Figure 10.
The newer data are in good agreement with older data, which indicates good accuracy of the data. The solubility curves are clearly distinguishable between the three different hydrate forms. While the solubility of dihydrate increases with temperature in the stable window to up to 40 °C, a further increase in temperature leads to a decrease in solubility for all hydrate forms. The phenomen that the solubility of dihydrate starts decreasing at around 40 °C can be explained thermodynamically with the change of the sign of the heat of solution [
31].
2.4.2. Influence of Mg++ on Solubility of CaSO4 Hydrates
The presence of magnesium and calcium can influence each other’s solubility. Several authors have reported the association effect of magnesium, which increases the solubility of CaSO
4 with an increasing presence of magnesium cations as the cation associates the partial sulfate ion to form stable MgSO
4 [
25,
34]. As a consequence of the formation of MgSO
4 the solubility of CaSO
4 increases. Wu et al. studied the influence of magnesium chloride on the solubility of CaSO
4. They report that at a MgCl
2 content of around 9 mol/kg the solubility of CaSO
4 dihydrate is around half the solubility at the same temperature in water [
25].
2.5. Magnesium Hydroxide (Mg(OH)2)
When describing the solubility of Mg(OH)
2 in an aqueous solution, two main equilibria need to be considered. One is the dissociation of Mg(OH)
2 and the other is the formation of the ion pair MgOH
+.
Therefore the solubility of Mg(OH)
2 is a distinctive function of the pH value of the solution [
3,
35]. Scholz and Kahlert studied the pH dependence of the solubility of metal hydroxides and established an equation describing the solubility
as a function of pH, the solubility product
and the autoprotolysis constant of water
[
35]:
This equation is based on the consideration that formed OH
− ions are part of the autoprotolysis reaction of water and takes the following definitions into account [
35]:
Knowing the solubility product of Mg(OH)2 this equation allows an easy calculation at which pH value the precipitation of Mg(OH)2 starts at a given temperature. While a lower pH value is leading to a higher solubility, Mg(OH)2 is almost insoluble at neutral pH or higher.
Solubility of Mg(OH)2 in Water
Lambert and Clever evaluated comprehensively available data in the IUPAC solubility data series [
3].
Figure 11 shows solubility data of Mg(OH)
2 in water.
The data from Näsänen (1942), Travers and Nouvel (1929), and Lambert (1982) were taken from the IUPAC solubility data series [
3]. Due to the low solubility, the direct measurement of a precise value is difficult. Therefore, several studies derived the solubility in water indirectly from the solubility product determined by measurements in a ternary system [
3]. These values are designated as indirect determination in
Figure 11. Literature data distinguish between active and inactive Mg(OH)
2. Active Mg(OH)
2 describes the amorphous form of Mg(OH)
2 after precipitation or hydration of MgO. Aging leads to a thermodynamic stable form with a lower solubility described as inactive Mg(OH)
2 [
3]. Several studies focused on the solubility change from active to inactive Mg(OH)
2 [
36,
37]. Although there is a general trend visible that active Mg(OH)
2 has a higher solubility, the spread of data points at room temperature makes it impossible to derive a systematic trend [
3]. While the direct measurements done by Busch show only a slight decrease of solubility with progressing aging, Hostetler observed a solubility decrease by almost 50% [
36,
37]. As the value for the active Mg(OH)
2 from Busch is taken after 8 h of equilibration time, the small decrease can be explained with an already progressed aging at this time.
While there are several studies analyzing the solubility at room temperature, there are only a few studies covering a wider temperature range. Travers and Nouvel performed direct measurements of the solubility in water, Lambert and Clever determined the solubility indirectly [
3]. Lambert and Clever explain the difference between the values of these two studies with insufficient aging of the Mg(OH)
2 by Travers and Nouvel [
3]. Correlating the literature data Lambert and Clever recommend the following equation to describe the tentative values for the solubility of Mg(OH)
2 in water as a function of temperature with
S being the solubility in mol/kg and
T the temperature in Kelvin [
3]:
The plot of this correlation in
Figure 11 shows a good agreement with experimental data for temperatures higher than 20 °C.
In a very recent study, Dongdong et al. developed several thermodynamic models to describe the Mg(OH)
2-H
2O system based on the CALPHAD methodology [
20]. The model describes the thermodynamic properties of the system through the Gibbs free energy. Adjustable parameters allow the optimization of the model by fitting it to literature data. They were able to describe the system by fitting the adjustable parameters to solubility data described in the literature. However, they found that using the previously described literature data to fit the model leads to inconsistent thermodynamic results. Whereas a model using the solubility product given by McGee and Hostetler to complement the determination of the Gibbs energy equation showed consistency at least in the temperature window from 0 °C to 50 °C [
20,
42]. The solubility product reported by McGee and Hostetler shows only a small temperature dependency with –log(K) = 10.89 at 10 °C and –log(K) = 11.10 at 90 °C [
42]. Especially at higher temperatures, the model based on these values shows a high deviation from solubility data found in the literature (see
Figure 11) [
20]. Dongdong et al. conclude that the limitations in modeling the Mg(OH)
2-H
2O system are due to the general lack of reliable experimental data of the solubility of Mg(OH)
2.
2.6. Calcium Hydroxide Ca(OH)2
Equation (23) shows the solution equilibrium of Ca(OH)
2:
Like Mg(OH)
2, the solubility of Ca(OH)
2 depends on the state of aging and the pH value of the solution. Following the study by Scholz and Kahlert, the pH dependency of the solubility can be expressed in the same way as for Mg(OH)
2 with the solubility product of Ca(OH)
2 respectively, leading to the following equation [
35]:
The evaluation of solubility data of Ca(OH)
2 by Lambert and Clever revealed that the difference in solubility between fresh and aged Ca(OH)
2 decreased with increasing temperature and was in general small [
3]. Which justifies the focus on the solubility of aged Ca(OH)
2 only.
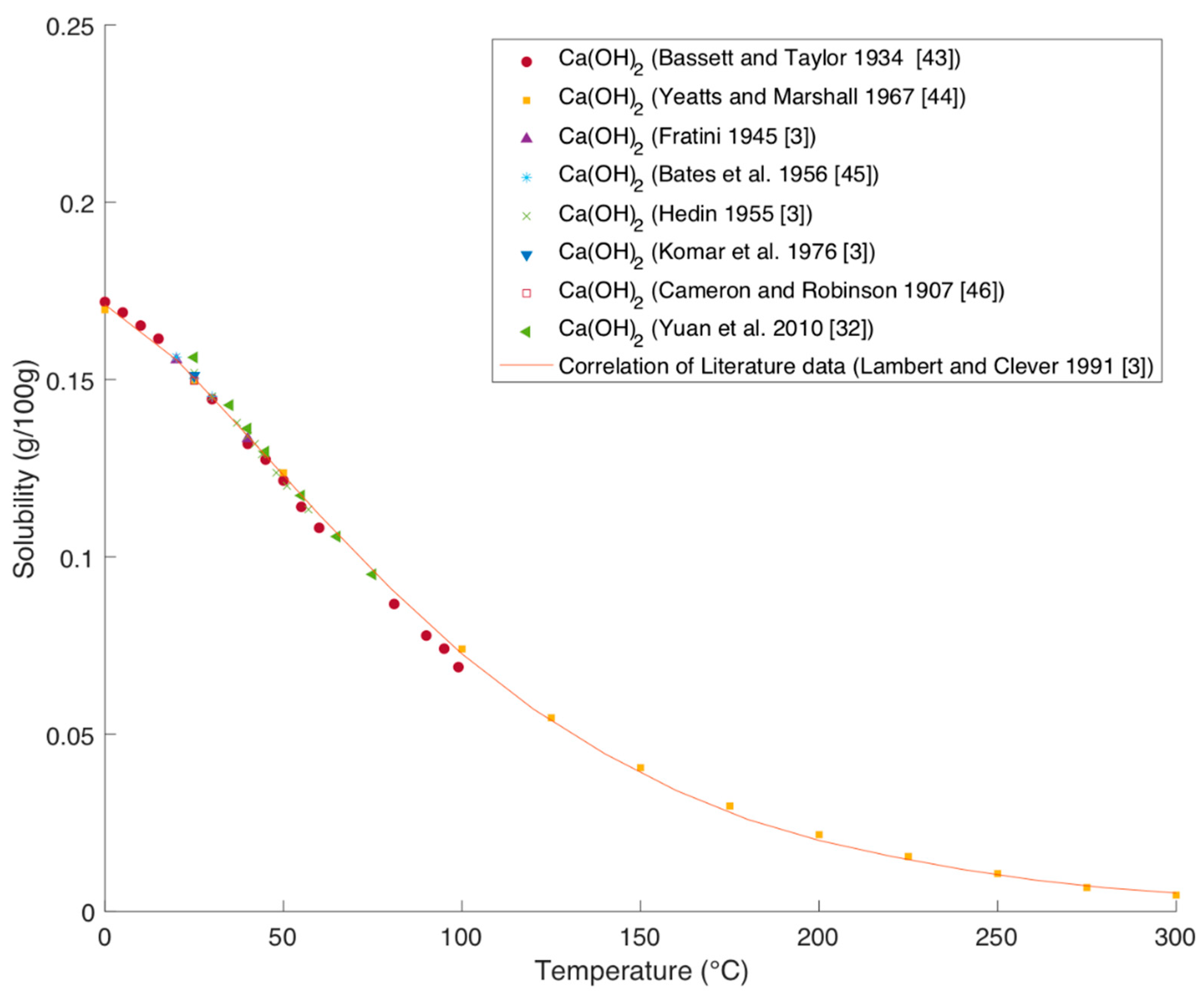
Solubility of Ca(OH)2 in Water
Figure 12 shows the solubility of Ca(OH)
2 over temperature. Except for the data of Yuan et al. all shown literature data were evaluated by Lambert and Clever in the IUPAC solubility data series [
3].
The data from Fratini (1945), Hedin (1955), and Komar et al. (1976) were taken from the IUPAC solubility series [
3]. The newer and older data are in very good agreement with each other. The solubility is around 100 times lower than for Mg(OH)
2 and decreases with increasing temperatures.
Lambert and Clever were able to correlate the literature data using the following equation with
S being the solubility in mol/kg and
T the temperature in Kelvin [
3]:
Also the younger experimental data of Yuan et al. fit the correlation very nicely, which confirms the applicability of that correlation [
3,
32].
3. Conclusions
Solubility data for MgSO3, CaSO3, MgSO4, CaSO4, Mg(OH)2 and Ca(OH)2 were summarized and reviewed to provide a solid base for modeling purposes.
To cover the precipitation of MgSO3 the solubility of its hexa- and trihydrate needs to be taken into account. The existence of these hydrate forms depends on the process conditions. Available solubility data for both hydrates cover a temperature range of 0 to 100 °C and are in good agreement with each other. The inverse dependency on temperature of the two hydrate forms makes it necessary to critical review, which hydrate form occurs under the given conditions in the process prior to its modeling.
The solubility data of CaSO3 are scarce, and only for a temperature range of 50 to 100 °C is more than one corresponding data source available. CaSO3 precipitates as different hydrate forms. However, the solubility behavior of these hydrate forms does not differ apparently. A differentiated consideration of the single hydrate forms is therefore not required to model precipitation accurately.
Much data reports the influence of the sulfates MgSO4 and CaSO4 on MgSO3 and CaSO3 respectively. To describe the precipitation accurately, it is essential to include this influence into a process model.
Investigations on the precipitation of MgSO
4 report multiple existences of metastable hydrate forms varying with temperature and relative humidity and sluggish kinetics [
16,
17,
19]. The available solubility data of MgSO
4 cover a temperature range of 0 to 200 °C. The available data for each single hydrate form are scarce. The solubility behavior of all studied hydrate forms except kieserite do not differ apparently. The available data imply that the different hydrate forms can be described together based on their combined data. The solubility of kieserite however shows an inverse temperature dependency and needs to be considered separately.
The precipitation and solubility of CaSO4 are well studied. To cover the solubility behavior accurately, the hemihydrate, the monohydrate and the dihydrate need to be taken into account. For all hydrate forms, more than one data source is available for a temperature range of 0 to 100 °C. All available data are in very good agreement with each other.
Studies of the solubility of Mg(OH)2 and Ca(OH)2 show that at pH value lower than 7, the precipitation of the hydroxides can be disregarded when modeling an industrial process. A change to higher pH values, however, can cause heavy precipitation. Mg(OH)2 is almost insoluble in water. The precise values of the solubility scatter at room temperature between around 0.75 × 10−3 and 2 × 10−3 g/100 g, partially explained by the solubility difference of active and inactive Mg(OH)2. Only two data sources are available for a wider temperature range of up to 200 °C, which show a corresponding trend. The solubility of Ca(OH)2 in water is well studied by many different researchers in a temperature range of 0 to 100 °C. All available data are in very good agreement with each other. For temperatures higher than 100 °C only one data set was found, which blends very well with the data for lower temperatures.
Author Contributions
Conceptualization, B.D.W. and M.H.; investigation, B.D.W.; writing—original draft preparation, B.D.W.; writing—review and editing, B.D.W. and M.H.; visualization, B.D.W.; supervision, M.H.; project administration, M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Competence Center CHASE GmbH. CHASE Competence Center is subsidized in the frame of COMET–Competence Centers for Excellent Technologies by BMVIT, BMWFW, Wirtschaftsagentur Wien, State of Upper Austria and its scientific partners. The COMET program is handled by FFG.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The support by the project partner Sappi Papier Holding GmbH is greatly acknowledged. Open Access Funding by TU Wien.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Steindl, M.; Röder, T.; Simharl, R.; Harasek, M.; Friedl, A.; Sixta, H. Online Raman monitoring of the phase transition of magnesium sulphite hydrate. Chem. Eng. Process. Process. Intensif. 2005, 44, 471–475. [Google Scholar] [CrossRef]
- Lutz, H.D. Sulfites, Selenites and Tellurites. Solubility Data Ser. 1986, 26, 153–193. [Google Scholar]
- Lambert, I.; Clever, H.L. Alkaline Earth Hydroxides in Water and aqueous solutions. Solubility Data Ser. 1992, 52, 49–226. [Google Scholar]
- Li, Q.; Yang, Y.; Wang, L.; Xu, P.; Han, Y. Mechanism and kinetics of magnesium sulfite oxidation catalyzed by multiwalled carbon nanotube. Appl. Catal. B Environ. 2017, 203, 851–858. [Google Scholar] [CrossRef]
- Hagisawa, H. Studies of Magnesium Sulphite. Bull. Inst. Phys. Chem. Res. 1933, 12, 976–983. [Google Scholar]
- Söhnel, O.; Rieger, A. Solubilites of Magnesium Sulfite Hydrates. J. Chem. Eng. Data 1994, 39, 161–162. [Google Scholar] [CrossRef]
- Söhnel, O.; Rieger, A. Phase Transition of Magnesium Sulphite Hydrates in Aqueous Suspension. Cryst. Res. Technol. 1993, 28, 487–493. [Google Scholar] [CrossRef]
- Lowell, P.S.; Meserole, F.P.; Parsons, T.B. Preciptiation Chemistry of Magnesium Sulfite Hydrates in Magnesium Oxide Scrubbing; Interagency Energy-Environment Research and Development Series; Radian Corp.: Austin, TX, USA, 1977; EPA-600/7-77/109. [Google Scholar]
- Markant, H.P.; Mcl LROY, R.A.; Matty, R.E. Absorption Studies—MgO-SO2 Systems. Tappi J. 1962, 45, 849–854. [Google Scholar]
- Nyvlt, J. Solubilities of Magnesium Sulfite. J. Therm. Anal. Calorim. 2001, 66, 509–512. [Google Scholar] [CrossRef]
- Conrad, F.H.; Brice, D.B. Some Equilibrium Relations in the System Magnesium Oxide—Sulfur Dioxide—Water (Acid Region) at Pressures below Atmospheric1. J. Am. Chem. Soc. 1948, 70, 2179–2182. [Google Scholar] [CrossRef]
- Bobrovnik, L.D.; Kotelnikova, L.P. Solublity of calcium sulphite in sugar solutions. Izv. Vyssh. Uchebn. Zaved. Pishch. Tekhnol. 1974, 4, 155–156. [Google Scholar]
- Malghe, Y.S.; Kumar, A. Solubility of CaSO3 in aqueous sucrose solutions from 288 to 368 K. Indian J. Chem. Sect. A 1998, 37, 56–58. [Google Scholar]
- Van der Linden, T. Solubility of calcium sulphite in water and in sugar solutions. J. Chem. Technol. Biotechnol. 1917, 36, 96. [Google Scholar] [CrossRef]
- Bichowsky, F.R. Free energy of the thiosulfate ion. J. Am. Chem. Soc. 1923, 45, 2225–2235. [Google Scholar] [CrossRef]
- Chipera, S.J.; Vaniman, D.T. Experimental stability of magnesium sulfate hydrates that may be present on Mars. Geochim. Cosmochim. Acta 2007, 71, 241–250. [Google Scholar] [CrossRef]
- Steiger, M.; Linnow, K.; Ehrhardt, D.; Rohde, M. Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4–H2O and Na+–Mg2+–Cl−–SO42−–H2O systems with implications for Mars. Geochim. Cosmochim. Acta 2011, 75, 3600–3626. [Google Scholar] [CrossRef]
- Li, H.; Zeng, D.; Yao, Y.; Gao, C.; Yin, X.; Han, H. Solubility Phase Diagram of the Ternary System MgCl2–MgSO4–H2O at 323.15 and 348.15 K. J. Chem. Eng. Data 2014, 59, 2177–2185. [Google Scholar] [CrossRef]
- Chou, I.-M.; Seal, R.R. Magnesium and calcium sulfate stabilities and the water budget of Mars. J. Geophys. Res. 2007, 112, 96. [Google Scholar] [CrossRef]
- Dongdong, L.; Dandan, G.; Yaping, D.; Wu, L. Modeling of phase relations and thermodynamics in the Mg(OH)2 + MgSO4 + H2O system with implications on magnesium hydroxide sulfate cement. Calphad 2019, 67, 101675. [Google Scholar] [CrossRef]
- Zhang, Y.; Asselin, E.; Li, Z. Solubility Measurement and Chemical Modeling of MgSO4·7H2O in the Ti(SO4)2–H2SO4–H2O System. J. Chem. Eng. Data 2016, 61, 2363–2370. [Google Scholar] [CrossRef]
- Robson, H.L. The System MgSO4-H2O from 68 TO 240°1. J. Am. Chem. Soc. 1927, 49, 2772–2783. [Google Scholar] [CrossRef]
- Apelblat, A.; Manzurola, E. Solubilites and vapour pressures of saturated aqueous solutions of sodium tetraborate, sodium carbonate, and magnesium sulfate at freezing-temperat ure lowerings of sodium tetraborate and sodium carbonate solutions. J. Chem. Thermodyn. 2003, 35, 221–238. [Google Scholar] [CrossRef]
- Ting, H.H.; McCabe, W.L. Solubility of Magnesium Sulfate Heptahydrate. Ind. Eng. Chem. 1934, 26, 1207–1208. [Google Scholar] [CrossRef]
- Wu, X.; Wang, K.; Xiong, Z.; Ye, X. Solubility of α-Calcium Sulfate Hemihydrate in Ca-Mg-K Chloride Salt Solution at (353.0 to 371.0) K. J. Chem. Eng. Data 2013, 58, 48–54. [Google Scholar] [CrossRef]
- Yang, L.; Guan, B.; Wu, Z.; Ma, X. Solubility and Phase Transitions of Calcium Sulfate in KCl Solutions between 85 and 100 °C. Ind. Eng. Chem. Res. 2009, 48, 7773–7779. [Google Scholar] [CrossRef]
- Shen, L.; Sippola, H.; Li, X.; Lindberg, D.; Taskinen, P. Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K. J. Chem. Eng. Data 2019, 64, 2697–2709. [Google Scholar] [CrossRef]
- Partridge, E.P.; White, A.H. The solubility of calcium sulfate from 0 to 200°. J. Am. Chem. Soc. 1929, 51, 360–370. [Google Scholar] [CrossRef]
- Li, Z.; Demopoulos, G.P. Solubility of CaSO4 Phases in Aqueous HCl + CaCl2 Solutions from 283 K to 353 K. J. Chem. Eng. Data 2005, 50, 1971–1982. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, D.; Chen, Q.; Yin, X. Experimental determination and modeling of gypsum and insoluble anhydrite solubility in the system CaSO4–H2SO4–H2O. Chem. Eng. Sci. 2013, 101, 120–129. [Google Scholar] [CrossRef]
- Hulett, G.A.; Allen, L.E. The solubility of gypsum. J. Am. Chem. Soc. 1902, 24, 667–679. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, J.; Li, Z. Measurement and modelling of solubility for calcium sulfate dihydrate and calcium hydroxide in NaOH/KOH solutions. Fluid Phase Equilibria 2010, 297, 129–137. [Google Scholar] [CrossRef]
- Power, W.H.; Fabuss, B.M. Transient Solubilities in the Calcium Sulfate-Water System. J. Chem. Eng. Data 1964, 9, 437–442. [Google Scholar] [CrossRef]
- Templeton, C.C.; Rodgers, J.C. Solubility of anhydrite in several aqueous salt solutions between 250.degree. and 325.degree. J. Chem. Eng. Data 1967, 12, 536–547. [Google Scholar] [CrossRef]
- Scholz, F.; Kahlert, H. The calculation of the solubility of metal hydroxides, oxide-hydroxides, and oxides, and their visualisation in logarithmic diagrams. ChemTexts 2015, 1, 7. [Google Scholar] [CrossRef]
- Busch, W. Über die Verwendbarkeit der elektrometrischen Titration zur Löslichkeitsbestimmung schwerlöslicher Oxyde. Z. Anorg. Allg. Chem. 1927, 161, 161–179. [Google Scholar] [CrossRef]
- Hostetler, P.B. The stability and surface energy of brucite in water at 25 degrees C. Am. J. Sci. 1963, 261, 238–258. [Google Scholar] [CrossRef]
- Remy, H.; Kuhlmann, A. Löslichkeitsbestimmungen an schwer löslichen Stoffen. II. Wasserlöslichkeit der Oxyde von Beryllium, Aluminium, Zink, Cadmium, Blei, Kupfer und Silber. Anal. Bioanal. Chem. 1924, 65, 161–181. [Google Scholar] [CrossRef]
- Dupré; Bialas, J. Zur Bestimmung der Löslichkeit von Magnesia und Zinkoxyd in Wasser auf Grund des elektrischen Leitvermögens. Angew. Chem. 1903, 16, 54–55. [Google Scholar] [CrossRef]
- Whipple, G.C.; Mayer, A. The Solubility of Calcium Carbonate and of Magnesium Hydroxide and the Precipitation of These Salts with Lime Water. J. Infect. Dis. 1906, 3, S151–S165. [Google Scholar] [CrossRef][Green Version]
- Einaga, H. The hydrolytic precipitation reaction of Mg(II) from aqueous NaNO3 solution. J. Inorg. Nucl. Chem. 1981, 43, 229–233. [Google Scholar] [CrossRef]
- McGee, K.A.; Hostetler, P.B. Activity-product constant of brucite from 10 °C to 90 °C. J. Res. US Geol. Surv. 1977, 227–233. [Google Scholar]
- Bassett, H.; Taylor, H.S. CLXXX.—Calcium nitrate. Part III. The three-component system: Calcium nitrate–lime–water. J. Chem. Soc. Trans. 1914, 105, 1926–1941. [Google Scholar] [CrossRef]
- Yeatts, L.B.; Marshall, W.L. Aqueous systems at high temperature. XVIII. Activity coefficient behavior of calcium hydroxide in aqueous sodium nitrate to the critical temperature of water. J. Phys. Chem. 1967, 71, 2641–2650. [Google Scholar] [CrossRef]
- Bates, R.G.; Bower, V.E.; Smith, E.R. Calcium hydroxide as a highly alkaline pH standard. J. Res. Natl. Inst. Stand. 1956, 56, 305. [Google Scholar] [CrossRef]
- Cameron, F.K.; Robinson, W.O. The System, Lime, Nitric Acid and Water. J. Phys. Chem. 1907, 11, 273–278. [Google Scholar] [CrossRef][Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).