Abstract
In order to obtain an environmentally friendly epoxy system, L-tryptophan and guanine were investigated as novel green curing agents for the cross-link of diglycidyl ether of Bisphenol A (DGEBA) as a generic epoxy resin model of synthetic and analogous bio-based precursors. In particular, L-tryptophan, which displays high reaction temperature with DGEBA, was used in combination with various bio-based molecules such as urea, theobromine, theophylline, and melamine in order to increase the thermal properties of the epoxy resin and to reduce the crosslinking reaction temperature. Later, in order to obtain similar properties using a single product, guanine, a totally heterocyclic molecule displaying amine functional groups, was tested as hardener for DGEBA. The thermal behavior of the precursor mixtures was evaluated by dynamic differential scanning calorimetry (DSC) leading to a preliminary screening of different hardening systems which offered a number of interesting hints in terms of bio-based compounds able to provide high Tg resins. These encouraging results pave the way for a further study of a new class of renewable, low-toxic, and sustainable curing agent systems for the production of fully bio-based epoxy resins.
1. Introduction
Polymeric thermosetting materials account for less than 20% of global plastic production, and epoxy resins make up 70% of the market for thermosetting resins [1]. Their global market was estimated at around $6.8 million in 2015 and is anticipated to reach $10.3 million by 2022 [2], and more than 60% of global production is used in the coatings industry. Among thermosets, epoxy resins are widely used in paints, adhesives, and as matrices for composite materials, since they can be easily and conveniently tailored to display a unique combination of characteristics such as excellent adhesion, chemical and heat resistance, good mechanical properties, and very good electrical insulating properties although their cost is still relevant [3]. In order to obtain the desired product properties, the nature and the structure of the curing agents (also known as hardeners) are crucial, since they are actually responsible for the reaction of the epoxy rings in the polymeric precursors, hence, governing the effective kinetics of the overall curing process and finally generating hard, infusible, thermosetting networks. It is thus essential to define important parameters in the hardener choice, which are decisive for the curing process as well as for the final resin structure, such as the initial viscosity of the hardener system, the crosslinking reaction pathway, and the curing temperature range in which the system is reactive. For all the above-mentioned characteristics, the chemical nature of the hardener, its structure, and the number of functional groups it possesses are crucial properties. The most commonly used curing agents for epoxies are synthetic amines, anhydrides, phenols, carboxylic acids, alcohols, and other compounds with labile hydrogens [3]. Industrially, the most widely used are amines, either aliphatic or aromatic [4], which can be very versatile. As an example, aliphatic amines are very reactive and can be used even at room temperature; on the other hand, the aromatic ones are less reactive but provide better mechanical and thermal properties (high Tg), in addition to higher resistance to chemicals [5]. It is known that fillers, in particular nanofillers, can be added to improve mechanical properties, with focus on the increase of the glass transition [6,7], however the structure and functionality of the hardener should still remain the key factors in the definition and achievement of the final properties of the thermoset. It is, then, of utmost importance to have access to the widest possible range of curing agents with various structures, functionality, and reactivity, in order to modulate the properties of epoxy thermosets.
While the need to go sustainable in practically all fields has recently been getting more and more attention, in the latest years this aim extends also to finding next generation feedstocks from non-food competitive resources, such as waste or low added-value supplies. Given their widespread use, thermosetting epoxy resins represent a great opportunity to develop environmentally sustainable products, and although many bio-based feedstocks for the production of the epoxy precursors are already available [8,9,10,11], such as vegetable oils and dicarboxylic acids, it is essential in the future that all components of the formulation could be obtained from renewable resources [12,13,14]. In literature, various examples of bio-based epoxy resins obtained from a wide range of sources, such as catechin [15,16], cardanol [17], and lignin [11,18], can be found. Indeed, concerning the curing agents, their toxicity and safety have not been deeply investigated and it was observed that all of them still present some environmental problems. In particular, many of the epoxy resin hardeners are low molecular weight toxic compounds [19,20]. Therefore, the development of new environmentally friendly fully bio-based epoxy systems is of great importance for designing green and sustainable materials, and this requires the development of bio-based curing agents [21]. To be accepted as valid solutions, bio-based formulations must offer the same versatility and performance reliability as conventional products. First of all, in order to modulate the final properties of epoxy thermosets as much as with fossil hardeners, it is necessary to increase the number of amine-based hardeners from bio-available sources. Moreover, it is also of utmost importance to compare the properties that these new hardeners provide with those obtained upon the use of existing systems. To date, there are only a few commercial solutions based on renewable sources, and some of these are only partially bio-based [10,21]. Until now, two types of amines from renewable resources had been deeply investigated [22]: the first class is synthesized from modified compounds such as fatty oils, sugar derivatives, and others [21,23,24]; while the second one is made up of bio-resources having amino groups, such as chitosan and amino acids [25].
In recent studies, lysine and L-tryptophan were proposed as novel green and biocompatible amine curing agents for epoxy resins [24,26]. It was observed that epoxy resins cross-linked with lysine exhibit Tg and degradation temperature significantly lower than those cross-linked with L-tryptophan. Such behavior can be ascribed to the aromatic heterocyclic structure of the latter hardener, which is intrinsically more rigid. In particular, it was demonstrated that L-tryptophan amino acid was able to react with diglycidyl ether of Bisphenol A (DGEBA) and interesting results were obtained [24].
In this context, the present study aims at further exploring the possibility of using L-tryptophan together with other bio-based compounds, as curing agent systems for epoxy resin precursors. A former work demonstrated promising performance of L-tryptophan in the presence of two commercial late curing agents (LCAs), 2-undecyl-1H-imidazole and 3,3′-(4-methyl-1,3-phenylene)bis(1,1-dimethylurea) [27], which have the purpose of lowering the activation energy of the process. DGEBA was used as a simple epoxy precursor, given that its well-defined chemical structure is helpful in the evaluation of the reacting system. Hence, starting from the data previously obtained from these commercial fossil-based systems, several bio-based and nontoxic amine compounds (urea, theobromine, theophylline, and melamine) were evaluated, adding them to the DGEBA–tryptophan mixture in order to study new fully bio-based curing systems.
Finally, in order to also evaluate a single component hardener system, guanine (G), which possesses a hetero-aromatic structure and displays amine functional groups, was also investigated. Such a compound, bearing both a secondary aromatic amine and primary amine functionality, could possibly trigger a fast reaction with the epoxy groups of the resin behaving as hardener. The chemical structures of all the investigated hardeners are shown in Figure 1.
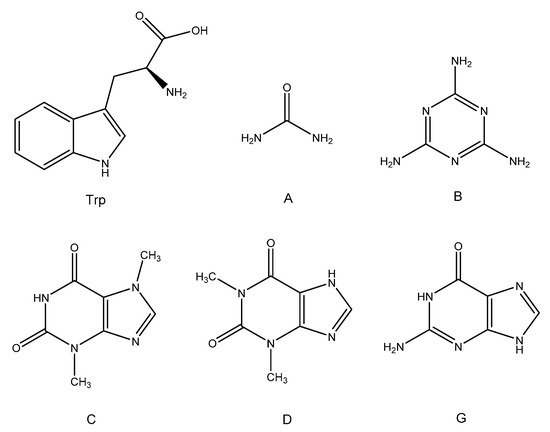
Figure 1.
Molecular structures of tryptophan (Trp), urea (A), melamine (B), theobromine (C), theophylline (D), and guanine (G).
Differential scanning calorimetry (DSC) was used to explore the cross-linking reactions of the epoxy with the different curing agent systems as well as to evaluate thermal performance in terms of Tg of the obtained thermosets, in order to highlight not only the ability of the system to react, but also to obtain a significant esteem of the final resin properties in a fast and approachable way.
2. Materials and Methods
Bisphenol A diglycidyl ether (DGEBA) with an epoxy equivalent weight (EEW) of 170 g/equivalent, urea (A), melamine (B), theobromine (C), and theophylline (D) were purchased from Sigma Aldrich. L-tryptophan (Trp) and was purchased from Fluka. Guanine (G) was purchased from Alfa Aesar.
2.1. Formulations Preparation
First, 0.5g (1.47 mmol) of DGEBA and 0.167 g (0.82 mmol) of L-tryptophan were mixed in a vial at 60 °C until complete homogenization to obtain the preliminary formulation called DT, that represented the 100 phr reference sample. Then, different amounts (1, 2.5, and 5 phr) of the investigated hardeners (urea (A), melamine (B), theobromine (C), or theophylline (D)) were added to DT mixture. The obtained formulations were magnetically stirred and kept at 60 °C in an oil bath until complete dissolution of all the components. Then, the mixtures were cooled to RT and used for the preparation of DSC samples. Different mixtures were produced and labelled according to the list reported in Table 1.

Table 1.
Compositions of the different diglycidyl ether of Bisphenol A (DGEBA)/L-tryptophan investigated thermoset formulations.
DG formulations (Table 2), containing DGEBA and guanine, were prepared by adding the desired amount of hardener (in the range 4–11% by weight) to DGEBA (1gr); the mixtures were magnetically stirred with a speed of 300 rpm at 75 °C until complete homogenization. Then, they were cooled to RT and used for the preparation of the DSC samples. Different mixtures were produced and labelled according to the list reported in Table 2.

Table 2.
Compositions of the investigated DGEBA/guanine formulations.
2.2. Material Thermal Characterization
The thermal behavior of the reacting mixtures was evaluated by differential scanning calorimetry (DSC, Q2000 TA Instruments) and the measurements were carried out in dynamic and isothermal mode. Dynamic DSC analysis was performed under nitrogen flow, heating the samples at a heating rate of 5 °C/min from ‒50 to 260 or 300 °C for formulations containing Tryptophan and guanine, respectively. After the first heating, the samples were cooled to 0 °C and then heated again with a heating rate of 20 °C/min from 0 to 260 °C for Tg evaluation. Isothermal DSC analysis was performed at 180 and 240 °C, introducing the samples in the pre-heated furnace. After the isothermal step, the samples were cooled to 0 °C and then heated again with a heating rate of 20 °C/min from 0 to 260 °C for Tg evaluation.
3. Results and Discussion
The investigation of a crosslinking system is often difficult, and owing to the lack of solubility of the obtained systems, many techniques are simply not exploitable. With the aim of evaluating the potential of different curing systems to cross-link epoxies, a wide screening of a number of different co-hardeners was undertaken, for which a fast and reliable approach was required. Hence, a calorimetric investigation was carried out via DSC measurements of different formulations. While this approach does not provide a full picture of the reactions proceeding, it offers crucial information in a single measure, allowing for a significant preliminary evaluation of the potential of the proposed hardeners. Indeed, DSC measurements are able to highlight the occurrence of the exothermic epoxy opening reaction via detection of an exothermic peak, allowing also the identification of the typical temperature range required for this reaction to proceed. Moreover, upon re-heating the same sample, it is also possible to obtain information on the glass transition temperature (Tg) reached by the thermoset. The latter is a significant parameter for understanding the potential of the proposed hardeners to positively cross-link the epoxy resin.
As reported in literature, DGEBA/L-tryptophan (DT) mixture displays a maximum cross-linking rate above 200 °C [24], a too high temperature for industrial application. With the aim of lowering the curing temperature, thus simplifying the process and making it technologically accessible, different bio-based amine hardeners were studied in mixture with L-tryptophan as novel curing agent systems for epoxy resins. In order to understand the reliability of such hardener systems, the simplest epoxy precursor possible was studied, specifically DGEBA.
Due to the low molecular weight of both DGEBA and the hardening system components, a highly cross-linked structure was expected. Nevertheless, a first reference attempt to cross-link DGEBA solely with Trp [27], i.e., DT formulation in Table 1, according to the literature [24], leads to a low Tg (Table 3). Such behavior could be due to the fast cross-linking kinetics of the system: once the reaction begins, the three-dimensional structure is quickly formed, hampering further reactivity of the residual moieties and leading to vitrification.

Table 3.
Relevant DSC data of bio-based DTA, DTB, DTC and DTD samples.
In a preliminary attempt to improve the DT thermoset performance, the cross-linking reaction of DGEBA was studied in the presence of Trp together with commercial fossil-based late curing agents (LCAs), undecyl-1H-imidazole (I) and 3,3′-(4-Methyl-1,3-phenylene)bis(1,1-dimethylurea) (U; Figure 1) [27]. All the investigated mixtures showed exotherms centered at temperatures lower than the reference DT, meaning that the addition of both compounds allowed the cross-linking reaction to take place at significant lower temperatures than in the plain DGEBA/L-tryptophan mixture. Additionally, in both cases, an increase of the final Tg of resins, determined in the second scan, is also observed. Given the promising results reported above, four bio-based late curing agents have been investigated for the first time with L-tryptophan. All the chosen molecules (Figure 1A–D) display NH groups of different natures (primary and secondary amines), able to react with epoxy moieties as well as aromatic nitrogen atoms. A recent paper by the authors [28] reports an investigation on the reaction mechanism via NMR spectroscopy of adenine, that displays both primary and secondary amines, with a mono epoxy model compound (glycidyl 2-methylphenyl ether). This study seems to hint at faster reactivity of the imidazole NH, followed by the primary amine that, however, appears unable to complete the reaction and form the three-substituted product in the applied conditions, probably because of steric hinderance. The latter effect can be invoked also for the observed lack of reactivity of the three aromatic nitrogen atoms present on the molecule. A similar concept can be applied to the present hardener coadjutants, thus assuming that imidazole NH are expected to react faster than primary and aromatic amines. Additionally, it is worth pointing out that all the selected molecules (Figure 1A–D) display a significant nitrogen atomic fraction, which, as previously reported [7,29], seems to be an effective way of improving epoxy resin flame retardancy.
On a preliminary basis, different ratios of DGEBA-tryptophan-LCA were tested. In particular, three different formulations were prepared by adding 1, 2.5, or 5 phr of each bio-based LCA to 100 phr of DT resin, named DTX-1, DTX-2.5, and DTX-5, respectively, where X is the bio-based co-hardener compound (Table 1). It is worth noting that the ratio between DGEBA and Trp was maintained constant, at 75 and 25% w/w, respectively [24]. The reactivity of all the formulations was evaluated by DSC in the same condition used for the samples containing the commercial LCAs. The first tested compound was urea (A), a linear molecule with four amine reactive hydrogens able to react with the epoxy groups and with no aromatic structures present (Figure 1A). It is worth noting that recently Centi et al. reported a new waste-to-urea (WtU) technology, which is economically valuable and environmentally advantageous in terms of saving resources and limiting carbon footprint, for the production of urea from municipal solid waste [30].
In Table 3 and Figure 2, the DSC experimental results are reported. The maximum temperature set in these tests was 260 °C in order to avoid the degradation of the resin-hardener systems.
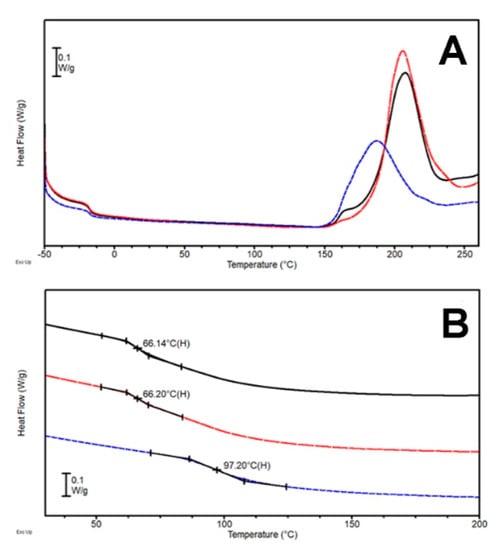
Figure 2.
(A) DSC first heating scan (heating rate 5 °C/min), and (B) second heating scan (heating rate 20 °C/min) of DTA-1 (−−−), DTA-2.5 (−− −− −−), and DTA-5 (− − −).
As reported in Figure 2A, all three formulations (DTA-1, DTA-2.5, and DTA-5) displayed a stepwise transition centered around −17 °C in the first DSC heating scan, which can be ascribed to the glass transition of the non-cured epoxy-hardening precursor system. Furthermore, in all formulations, an exothermic transition associated with the occurrence of the cross-linking reaction between the components of the mixture, was observed. This event is characterized by the presence of a main peak with different intensity, whose position shifts towards lower temperature values with greater fractions of added hardener, and an almost negligible shoulder; furthermore, the values of ΔH related to the total cross-linking enthalpy for formulations DTA-1, DTA-2.5, and DTA-5 (Table 3), show an increasing trend with the increasing amount of urea. Such behavior suggests that the cross-linking reaction occurs at lower temperatures and with higher conversion by increasing the hardener quantity. This is confirmed by the DSC second scans (Figure 2B) which displayed a shift in the Tg towards higher values by increasing the urea content. No residual cross-linking heat was detected. The increase in Tg and reaction enthalpy can be thus ascribed to the amount of urea in the system, since a greater quantity of such hardener brings in the reaction environment a higher number of groups able to react with the epoxy rings of DGEBA. However, tryptophan¬–urea systems do not show remarkable results with respect to the previously investigated fossil-based LCA formulations [31]. Indeed, no significant increase in the final Tg of the cured resin was registered, with respect to the plain DT. The cross-linking happens at almost the same temperature, apart from DTA-5. Furthermore, the cross-linking enthalpy is even lower, a sign that the reaction is not promoted with respect to the plain DT system. It is indeed worth pointing out that tryptophan-urea hardeners are not as well performing in all the principal analyzed parameters if compared with previously analyzed fossil-based LCA systems.
In order to increase the stiffness of the final system, other bio-based aromatic co-adjutants were investigated and mixed with Trp, such as melamine (Figure 1B). Melamine, being a heterocyclic aromatic compound with three primary amine groups (obtainable from urea) [31] and a total number of six active hydrogens able to open epoxy rings, should promote the cross-linking reaction and, thanks to the extreme stiffness of the aromatic central ring, should also help promote the increase of the Tg.
As shown in Figure 3, the presence of a stepwise transition around −18 °C in the first heating scan, convincingly ascribed to the glass transition of the non-cross-linked resin DTB, confirms once again that all the hardener coadjutants used in the present work do not seem to affect the behavior of the unreacted mixture and are not starting cross-linking reaction (at least to a significant extent) prior to the actual desired curing process.
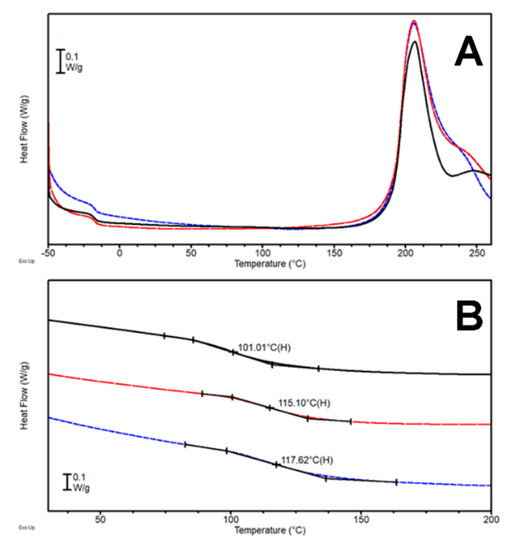
Figure 3.
(A) DSC first heating scan (heating rate 5 °C/min) and (B) second heating scan (heating rate 20 °C/min) for the systems DTB-1 (—), DTB-2.5 (---), and DTB-5 (---).
The cross-linking of the epoxy resin precursor triggered by the presence of the hardener system was confirmed by the clear exothermal transitions detected in each thermogram (Figure 4A). This time, the exotherms were characterized by a peculiar pattern, with the main signal displaying a high-T shoulder for every tested sample (DTB-1, DTB-2.5, and DTB-5). The cross-linking enthalpy keeps increasing with the increase of the melamine fraction in the formulations, and in turn, this behavior results in an increase of the glass transition of the mixture in the cured samples (Table 3). Conversely, the position of the reaction peak was not altered, temperature-wise, by the addition of the new hardener coadjutant. This event is consistent with the small dimensions of melamine molecules, which requires a high cross-linking temperature in order to accomplish the complete reaction of all the amine groups crowded onto the hardener. Comparing the results obtained with the previously tested DTA formulations, DTB samples display higher ΔH and Tg, even in comparison with previously published formulations [27]. The addition of a multifunctional amine molecule with an aromatic structure like melamine, appears to be able to increase the overall rigidity of the system. Such behavior is consistent with the intrinsic stiffness of the aromatic melamine ring and the high number of epoxy groups that can react with a single molecule of hardener, increasing the rigidity of the 3D cross-linked structure.
Figure 4.
(A) DSC first heating scan (heating rate 5 °C/min) and (B) second heating scan (heating rate 20 °C/min) for the systems DTD-1 (—), DTD-2.5 (---), and DTD-5 (---).
In order to exploit the aromatic ring stiffness, two additional bio-based molecules with a heterocyclic structure and amine functionalities (Figure 1C,D, Table 1) were tested, namely theobromine (C) and theophylline (D). The former molecule has just one reactive NH moiety and the trend in the rection exotherm position is negligible (Figure 5). It appears that a small quantity of theobromine hampers the reaction proceeding (lower enthalpy of reaction with respect to DT). When theobromine (C) fraction was increased, a high-T shoulder appears in the peak. Enthalpy of the reaction increased, suggesting the presence of some different process (with different kinetics) occurring when theobromine was present in a significant amount. Such hypothesis was also confirmed by the values related to the cross-linking enthalpy (Table 3) which is similar for DTC-1 and DTC-2.5, while it shows a significant increase for DTC-5.
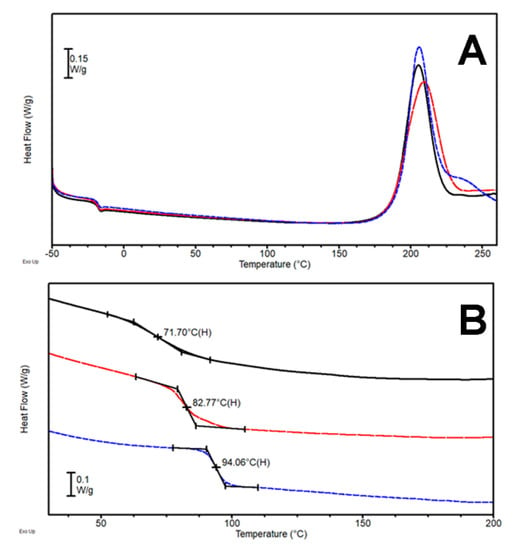
Figure 5.
(A) DSC first heating scan (heating rate 5 °C/min) and (B) second heating scan (heating rate 20 °C/min) for the systems DTC-1 (—), DTC-2.5 (---), and DTC-5 (---).
The tendency of the Tg value obtained is linearly growing, reaching a value close to 100 °C with DTC-5, 24 °C higher than plain DT. It is worth pointing out that theobromine has just one secondary amine group that is prone to react with the epoxy groups; on the other hand, the extreme stiffness of the heterocyclic aromatic compound definitely contributes to increasing the glass transition temperature of mixture, once the molecule is actively included in the network. While the cross-linking reaction temperature seems unaffected by the presence of theobromine, its concentration looks to be a critical parameter on the reactivity of additional functional groups that results in the appearance of the exothermal peak shoulder and increased glass transition of DTC-5 (Figure 5). Thus, the addition of theobromine, an aromatic compound, allows overall better results in comparison to DT and DTA samples, but are still worse than the DTB system and the reaction temperature is also still extremely high. Another bio-based aromatic hardener, theophylline, was thus investigated (Figure 1D, Table 1). This heterocyclic molecule is very similar to theobromine, but for the fact that it bears, among the others, a second amine functionality onto the aromatic ring.
The first heating scans (Figure 4A) show exothermal transitions present for each sample thermogram and the expected stepwise feature around −18 °C belonging to the Tg of the non-cross-linked resin. It is worth noting that the exothermal transition of DTD-1 sample is characterized by only one peak, while for formulation DTD-2.5 and DTD-5, shoulders appeared and peak maxima moved to lower temperatures (Figure 4A) when increasing the theophylline fraction in the starting mixture. A concomitant increase in the reaction enthalpy (Table 3) is observed with increasing theophylline content, that led to a higher Tg, as measured in the second heating scan (Figure 4B). The obtained results were very similar to those for theobromine-based systems (DTC): the attained ΔH and Tg were comparable and a slight decrease in the cross-linking temperature was displayed. These results support the previously hypothesized higher reactivity of imidazole NH with respect to other NH moieties [28].
Such behavior suggests that theophylline acts prevalently as catalyst coadjutant within the hardener system, lowering the cross-linking temperature and with a concomitant increase in the associated enthalpy. Theophylline based formulations, however, displayed lower cross-linking enthalpy and Tg, and higher reaction temperature when compared with previously analyzed fossil-based LCA systems [27]. Given the interesting results obtained for hetero-aromatic compounds such as melamine and theophylline, an attempt to obtain such combined properties in a single product was performed, introducing totally heterocyclic molecules displaying amine functional groups. Taking as a basis the above-mentioned products, a N-containing heterocyclic system free of any pendant aliphatic chain was sought. Guanine (Figure 1G), was directly tested as a single hardener, without the presence of L-tryptophan and other LCA systems. Guanine, thanks to its particular nitrogen rich heterocyclic aromatic structure, represents a potential starting point for the design of high-performance polymers from renewable resources. It bears both a secondary aromatic amine and primary amine functionality, and the presence of both functionalities in the same molecule could trigger a fast reaction between the epoxy group of the resin precursor and the hardener. On a preliminary basis, different DGEBA–guanine mixtures (DG samples) were tested. In particular, six formulations with guanine content in the range of 4–11% w/w (Table 2). However, owing to solubility limitation in DGEBA, no higher concentration than 11% w/w could be reached. DSC thermograms and the relevant data are reported in Figure 6 and Table 4.
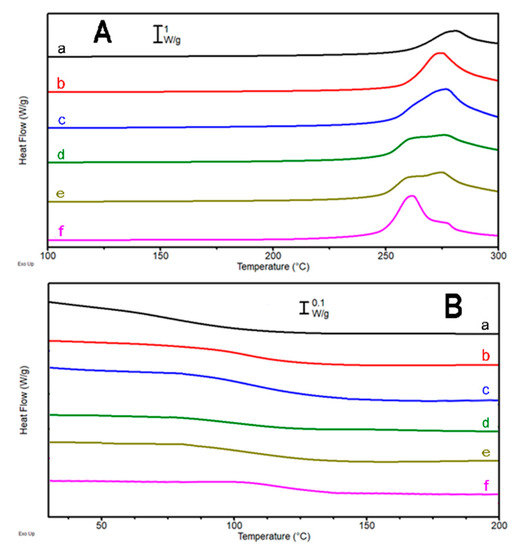
Figure 6.
(A) DSC thermograms of the first heating scan (heating rate 5 °C/min) and (B) second heating scan (heating rate 20 °C/min) for the systems DG-1 (a), DG-2 (b), DG-3 (c), DG-4 (d), DG-5 (e), and DG-6 (f).

Table 4.
Relevant DSC data of DEGBA/guanine (DG) samples.
The first heating scans (Figure 6A) show a clear exothermal transition, ascribed to the epoxy resin precursor reacting to provide the three-dimensional thermoset network. It is worth noting that DG-1 and DG-2 display only one peak at about 280 °C which, by increasing the amount of guanine, decreases in intensity. Such a drop is more evident in presence of higher amounts of hardener (DG-3, DG-4, DG-5, and DG-6), while an additional peak grows at lower temperature (Figure 6). Such behavior suggests the presence of some different processes (with different kinetics) occurring when higher amounts of guanine are present, with a potential autocatalytic effect. The cross-linking enthalpy keeps increasing with the increase of the guanine fraction in the formulations, resulting also in an increase of the glass transition in the cured samples (Table 4). Such behavior can be explained, taking into account that the higher the amount of added guanine, the more amine groups are present and able to react with the DGEBA, though it is still lower than the stoichiometric ratio due to solubility issues. Furthermore, guanine, displaying both primary and secondary amine groups, is prone to react with DGEBA in a way reminiscent of adenine behavior [28]. The great rigidity of the heterocyclic aromatic compound definitely contributes to increasing the glass transition temperature of the mixture once a 3D cross-linked network sets in. It is worth noting that the cross-linking peak temperature (262–280 °C), when compared to the formulations containing L-tryptophan, is sensibly higher (about 60–75 °C higher). This event is consistent with the small dimensions of the guanine molecule, which requires a high temperature in order to accomplish the complete reaction of the amine groups sterically hindered by the stiff aromatic structure, and the absence of a less hindered hardener molecule (like L-tryptophan) which allows the activation of the cross-linking reaction of the resin at lower temperature. Nevertheless, the studied system appears very promising since guanine is a biocompatible, non-toxic, and environmentally friendly molecule. By an addition of only 11% in weight of this hardener, satisfactory results have been obtained.
4. Conclusions
Four different bio-based curing agents coadjutants (urea, theobromine, theophylline, and melamine) mixed with L-tryptophan to produce a curing system fully based on renewable resources were used to crosslink DGEBA epoxy resin, and their action as hardeners were validated. Such a preliminary screening of different hardening systems offers a number of interesting hints in terms of bio-based hardening systems able to provide high-Tg resins. While unfortunately none of the applied bio-based curing agent coadjutants was able to achieve milder reaction conditions, good thermal results were obtained. The most relevant ones were with the addition of aromatic compounds; in particular with melamine, a heterocyclic molecule with six amine functionalities. Indeed, the use of melamine enables a reaction temperature (206 °C) similar to the plain L-tryptophan, higher than those in the sample where commercial fossil-based late cure agents were used. It allows the achievement of the highest final Tg (118 °C), in comparison to other samples reported in this study. On the other hand, the aromatic heterocycles appear to act more as catalytic helping promoters within the hardener system.
It is worth noting that this preliminary screening allowed collection of promising results which could be used to create new combinations of the different tested compounds, taking into account their different actions on the cross-linking reaction. As an example, melamine and theophylline could be used together in order to exploit the catalytic activity of the latter, together with the multifunctional primary amine structure and the rigidity of the former.
Finally, guanine, a nitrogenated heterocyclic aromatic system without lateral aliphatic chains, was tested alone as epoxy resin hardener and the results compared with respect to those of L-tryptophan formulations. Once again, the use of guanine allows a crosslinking reaction with the epoxy groups at high temperatures (260–280 °C). However, it is too high for industrial purposes. Nevertheless, the final high Tg obtained with only 11% in weight of this new bio-based hardener is very promising since guanine is a biocompatible, non-toxic, and environmentally friendly molecule.
Author Contributions
Conceptualization, L.G. and L.M.; methodology, L.M. and T.B.; formal analysis, S.M.; investigation, S.M.; resources, L.G.; data curation, S.M.; writing—original draft preparation, T.B.; writing—review and editing, L.M.; supervision, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PRIN 2015, “Smart composite laminates”, Project code 2015RT8Y45—PE8 and by project “TEAM SAVE—E91B18000460007” (PG/2018/632196) POR FESR 2014–2020 action by Regione Emilia Romagna.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to still ongoing studies.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.; De Lyon, U. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Epoxy Resin Research. Available online: https://www.alliedmarketresearch.com/epoxy-resins-market (accessed on 20 November 2020).
- Ellis, B. Chemistry and Technology of Epoxy Resins; Blackie Academic & Professional: London, UK, 1993. [Google Scholar]
- Pham, H.Q.; Marks, M.J. Epoxy resins. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Burton, D.L. Amine curing of epoxy resins: Options and key formulation considerations. Paint Coat. Ind. 2006, 22, 68–77. [Google Scholar]
- Mazzocchetti, L.; Benelli, T.; D’Angelo, E.; Ligi, S.; Minak, G.; Poodts, E.; Tarterini, F.; Palermo, V.; Giorgini, L. Managing heat phenomena in epoxy composites production via graphenic derivatives: Synthesis, properties and industrial production simulation of graphene and graphene oxide containing composites. 2D Mater. 2017, 4, 015020. [Google Scholar] [CrossRef]
- Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Lanzi, M.; Saraga, F.; Sambri, L.; Franchini, M.C.; Giorgini, L. New nitrogen-rich heterocycles for organo-modified bentonites as flame retardant fillers in epoxy resin nanocomposites. Polym. Eng. Sci. 2017, 57, 621–630. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Holladay, J.W.; Bozell, J.; Johnson, D. Top. Value-Added Chemicals from Biomass: Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; U.S. Department of Energy, Pacific Northwest National Laboratory: Richland, WA, USA, 2007.
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. Eur. Pol. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Mauck, J.R.; Yadav, S.K.; Sadler, J.M.; La Scala, J.J.; Palmese, G.R.; Schmalbach, K.M.; Stanzione III, J.F. Preparation and Characterization of Highly Bio-based Epoxy Amine Thermosets Derived from Lignocellulosics. Macrom. Chem. Phys. 2017, 218, 1700013. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Benelli, T.; D’Angelo, E.; Leonardi, C.; Zattini, G.; Giorgini, L. Validation of carbon fibers recycling by pyro-gasification: The influence of oxidation conditions to obtain clean fibers and promote fiber/matrix adhesion in epoxy composites. Compos. Part A 2018, 112, 504–514. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Brancolini, G.; Mazzocchetti, L. Recycling of carbon fiber reinforced composites waste to close their Life Cycle in a Cradle-to-Cradle approach. Curr. Opin. Green Sustain. Chem. 2020, 26, 100368. [Google Scholar] [CrossRef]
- Giorgini, L.; Leonardi, C.; Mazzocchetti, L.; Zattini, G.; Cavazzoni, M.; Montanari, I.; Tosi, C.; Benelli, T. Pyrolysis of Fiberglass/Polyester Composites: Recovery and Characterization of Obtained Products. FME Trans. 2016, 44, 405–414. [Google Scholar] [CrossRef]
- Boutevin, B.; Caillol, S.; Burguiere, C.; Rapior, S.; Fulcrand, H.; Nouailhas, H. Novel method for producing thermosetting epoxy resins. World Patent No. WO 2010136725, 2 December 2010. [Google Scholar]
- Nouailhas, H.; Aouf, C.; Le Guernevé, C.; Caillol, S.; Boutevin, B.; Fulcrand, H. Synthesis and properties of biobased epoxy resins. part 1. Glycidylation of flavonoids by epichlorohydrin. J. Polym. Sci. Part A 2011, 49, 2261–2270. [Google Scholar] [CrossRef]
- Fenn, D.; Webster, G.R.; McCollum, G.J. Modified epoxy resins comprising the reaction product of a biomass derived compound and an epoxy resin, and aqueous dispersions and coatings comprising such resins. World Patent No. US 7,812,101 B2, 12 October 2009. [Google Scholar]
- Hernandez, E.D.; Bassett, A.W.; Sadler, J.M.; La Scala, J.J.; Stanzione, J.F. Synthesis and Characterization of Bio-based Epoxy Resins Derived from Vanillyl Alcohol. ACS Sustain. Chem. Eng. 2016, 4, 4328–4339. [Google Scholar] [CrossRef]
- Bourne, L.B.; Milner, F.J.M.; Alberman, K.B. Health problems of epoxy resins and amine–curing agents. Br. J. Industr. Med. 1959, 16, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Epoxy Resins and Curing Agents: Toxicology, Health, Safety and Environmental Aspects; Plastics Europe Epoxy Resins Committee: Brussels, Belgium, 2006; pp. 1–28. Available online: https://epoxy-europe.eu/lang/en/resources/brochure-epoxy-resins-curing-agents-toxicology-health-safety-environmental-aspects/ (accessed on 26 December 2020).
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef]
- Metzger, J.O. Fats and oils as renewable feedstock for chemistry. Eur. J. Lipid Sci. Technol. 2009, 111, 865–876. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, F.; Wong, C.P. Novel, environmentally friendly crosslinking system of an epoxy using an amino acid: Tryptophan-cured diglycidyl ether of bisphenol A epoxy. J. Polym. Sci. Part A Pol. Chem. 2007, 45, 181–190. [Google Scholar] [CrossRef]
- Scott, E.; Peter, F.; Sanders, J. Biomass in the manufacture of industrial products—The use of proteins and amino acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, F.; Moon, K.-S.; Wong, C.P. Novel curing agent for lead-free electronics: Amino acid. J. Pol. Sci. Part A Pol. Chem. 2006, 44, 1020–1027. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Merighi, S.; Benelli, T.; Giorgini, L. Evaluation of L-Tryptophan—Late Curing Agents Systems as Hardeners for Epoxy Resins. AIP Conf. Proc. 2018, 1981, 020170. [Google Scholar]
- Merighi, S.; Mazzocchetti, L.; Benelli, T.; Giorgini, L. Adenine as epoxy resin hardener for sustainable composites production with recycled carbon fibers and cellulosic fibers. Polymers 2020, 12, 3054. [Google Scholar] [CrossRef]
- Cingolani, R.; Mazzoni, V.; Zanotti, M.; Sanviti, L.; Mazzocchetti, T.; Benelli, L. Giorgini. Bis-amino functionalized iron N-heterocyclic carbene as epoxy resins hardener and flame behaviour modifier. AIP Conf. Proc. 2019, 2196, 020035. [Google Scholar]
- Antonetti, E.; Iaquaniello, G.; Salladini, A.; Spadaccini, L.; Perathoner, S.; Centi, G. Waste-to-Chemicals for a Circular Economy: The Case of Urea Production (Waste-to-Urea). ChemSusChem 2017, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Kaasenbrood, P.J.C.; Van Nassau, P.J.M. Process for preparing Melamine from Urea. U.S. Patent US3682911, 8 August 1972. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).