Waste Wood Fly Ash Treatment in Switzerland: Effects of Co-Processing with Fly Ash from Municipal Solid Waste on Cr(VI) Reduction and Heavy Metal Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Origin, Sampling and Sample Preparation
2.2. Chemical Analysis
2.3. Mineralogical Analysis
2.4. Acid-Neutralizing Capacity
2.5. Water-Extractable Cr(VI)
2.6. Total Cr(VI)
2.7. Laboratory Experiments
2.8. Industrial-Scale Experiments
3. Results
3.1. Chemical Composition
3.2. Mineralogical Composition
3.3. Acid-Neutralizing Capacity
3.4. Water-Extractable and Total Cr(VI)
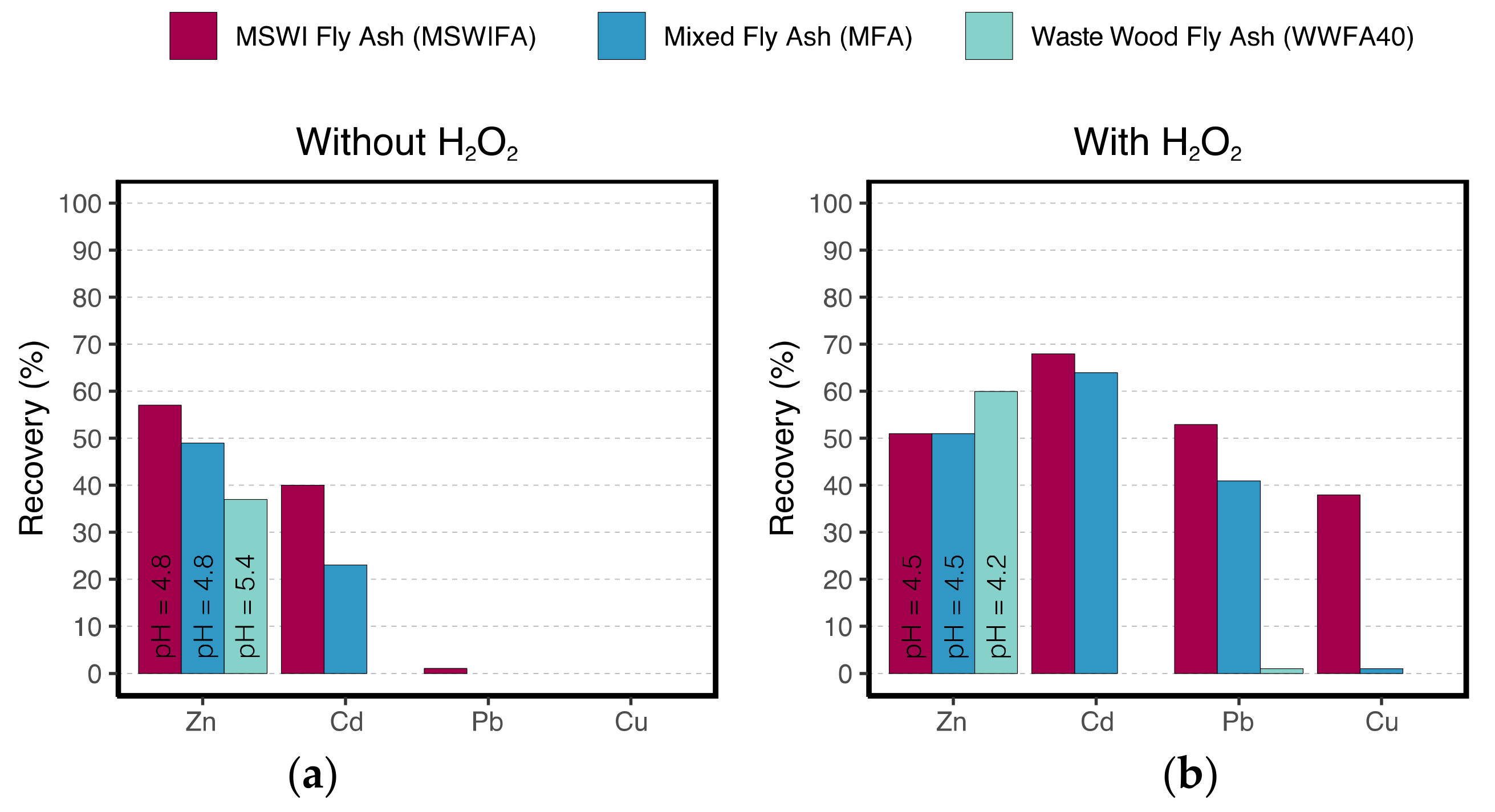
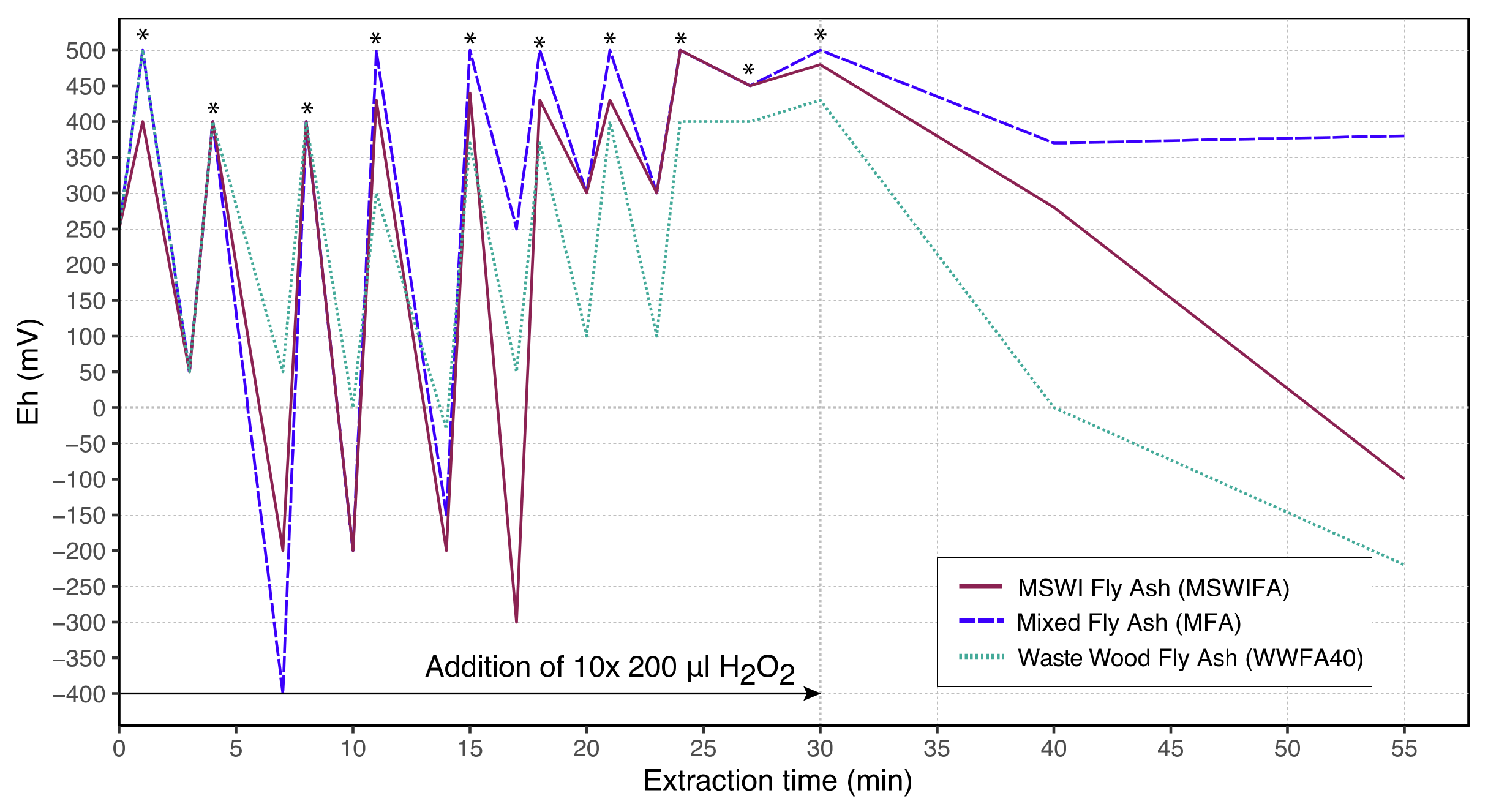
3.5. Laboratory-Scale Leaching Experiments
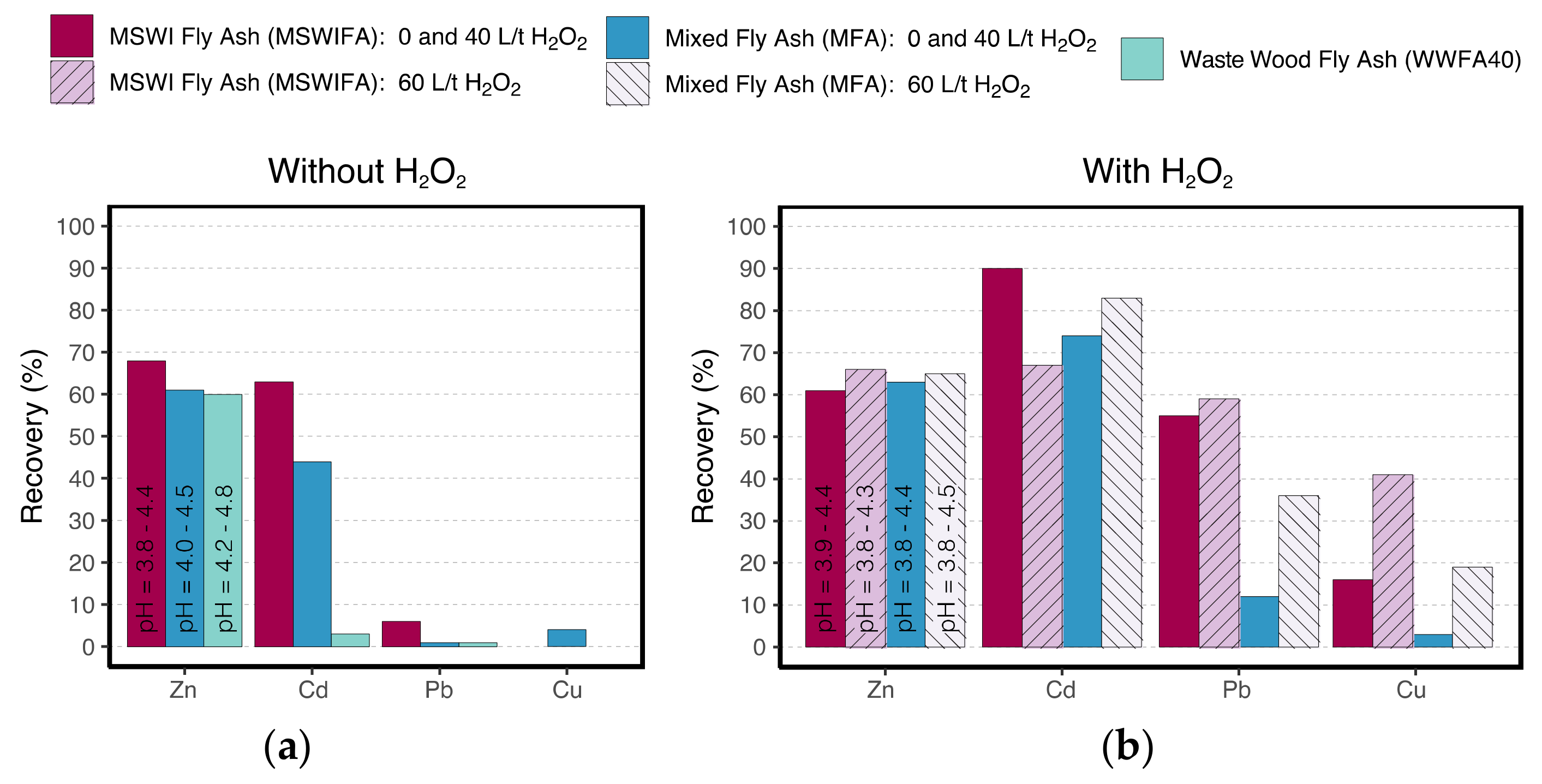
3.6. Industrial-Scale Leaching Experiments
4. Discussion
4.1. Chemical and Mineralogical Differences and its Effects on Acid-Neutralizing Capacity
4.2. Water-Extractable and Total Cr(VI) in WWFA and Filter Cakes
4.3. Leaching Experiments: Heavy Metal Recovery and Consumption of Neutralizing Chemicals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweizerische Eidgenossenschaft. Schweizerische Holzenergiestatistik—Erhebung für das Jahr 2019; Bundesamt für Energie: Bern, Switzerland, 2020.
- Obernberger, I. Nutzung fester Biomasse in Verbrennungsanlagen unter besonderer Berücksichtigung des Verhaltens aschebildender Elemente. In Schriftenreihe Thermische Biomassenutzung; dbv-Verlag: Graz, Austria, 1997. [Google Scholar]
- Zimmermann, S.; Hässig, J.; Landolt, W. Literaturreview Holzasche—Wald: Nährstoffentzug durch Holzernte, Ascherückführung in den Wald, abiotische und biotische Wirkungen; Swiss Federal Office of Environment: Birmensdorf, Schweiz, 2010.
- Tarelho, L.A.C.; Teixeira, E.R.; Silva, D.F.R.; Modolo, R.C.E.; Silva, J.J.F. Characteristics, management and applications of ashes from thermochemical conversion of biomass to energy. In Proceedings of the Conference Exhibition on Biomass for Energy, Jonkopin, Sweden, 29–31 May 2012. [Google Scholar]
- Etiégni, L.; Campbell, A.G. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Wunderli, S.; Zennegg, M.; Dolezal, I.S.; Gujer, E.; Moser, U.; Wolfensberger, M.; Hasler, P.; Noger, D.; Studer, C.; Karlaganis, G. Determination of polychlorinated dibenzo-p- dioxins and dibenzo-furans in solid residues from wood combustion by HRGC/HRMS. Chemosphere 2000, 40, 641–649. [Google Scholar] [CrossRef]
- Huron, M.; Oukala, S.; Lardière, J.; Giraud, N.; Dupont, C. An extensive characterization of various treated waste wood for assessment of suitability with combustion process. Fuel 2017, 202, 118–128. [Google Scholar] [CrossRef]
- Pohlandt-Schwandt, K. Treatment of wood ash containing soluble chromate. Biomass Bioenergy 1999, 16, 447–462. [Google Scholar] [CrossRef]
- Pohlandt-Schwandt, K.; Salthammer, T.; Marutzky, R. Reduction of soluble chromate in wood ash by formaldehyde. Biomass Bioenergy 2002, 22, 139–143. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Camões, A.; Branco, F.G. Valorization of wood fly ash on concrete. Resour. Conserv. Recycl. 2019, 145, 292–310. [Google Scholar] [CrossRef]
- Swiss Federal Council. Ordinance on the Avoidance and the Disposal of Waste (Waste Ordinance, ADWO); Bundesamt für Umwelt: Bern, Switzerland, 2015.
- Schlumberger, S.; Schuster, M.; Ringmann, S.; Koralewska, R. Recovery of high purity zinc from filter ash produced during the thermal treatment of waste and inerting of residual materials. Waste Manag. Res. 2007, 25, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Bühler, A.; Schlumberger, S. Schwermetalle aus der Flugasche zurückgewinnen «Saure Flugaschewäsche—FLUWA-Verfahren» ein zukunftsweisendes Verfahren in der Abfallverbrennung. KVA-Rückstände in der Schweiz—Der Rohstoff mit Mehrwert. Bundesamt für Umwelt BAFU 2010, 1, 185–192. [Google Scholar]
- Weibel, G.; Eggenberger, U.; Schlumberger, S. and Mäder, U.K. Chemical associations and mobilization of heavy metals in fly ash from municipal solid waste incineration. Waste Manag. 2017, 62, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Held, A.; Kramer, G.N.; Robouch, P.; Wätjen, U. The Certification of the Mass Fractions of As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sb, Se, Tl, V and Zn in Fly Ash BCR-176R; European Commission Directorate-General Joint Research Centre Institute for Reference Materials and Measurements: Luxembourg, 2007. [Google Scholar]
- Swiss Federal Office of Environment. Messmethoden im Abfall- und Altlastenbereich; Bundesamt für Umwelt: Bern, Switzerland, 2017.
- US Environment Protection Agency. EPA Method 3060A. Alkaline Digestion for Hexavalent Chromium; EPA: Washington, DC, USA, 1996.
- National Institute of Standards & Technology. Certificate of Analysis Standard Reference Material 2701. Certificate of Analysis Standard Reference Material 2701; National Institute of Standards & Technology: Gaithersburg, MD, USA, 2009.
- Weibel, G.; Waber, H.N.; Eggenberger, U.; Mäder, U.K. Influence of sample matrix on the alkaline extraction of Cr(VI) in soils and industrial materials. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Weibel, G. Optimized Metal Recovery from Fly Ash from Municipal Solid Waste Incineration. Ph.D. Thesis, University of Bern, Bern, Switzerland, 2017. [Google Scholar]
- Hartinger, L. Handbuch der Abwasser- und Recyclingtechnik, 2nd ed.; Fachbuchverlag Leipzig: Deutschland, Germany, 1991. [Google Scholar]
- Gehrmann, H.J.; Mätzing, H.; Nowak, P.; Baris, D.; Seifert, H.; Dupont, C.; Defoort, F.; Peyrot, M.; Castagno, F. Waste wood characterization and combustion behaviour in pilot lab scale. J. Energy Inst. 2020, 93, 1634–1641. [Google Scholar] [CrossRef]
- Belevi, H.; Moench, H. Factors determining the element behavior in municipal solid waste incinerators. 1. Field studies. Environ. Sci. Technol. 2000, 34, 2501–2506. [Google Scholar] [CrossRef]
- Morf, L.S.; Brunner, P.H.; Spaun, S. Effect of operating conditions and input variations on the partitioning of metals in a municipal solid waste incinerator. Waste Manag. Res. 2000, 18, 4–15. [Google Scholar] [CrossRef]
- Luan, J.; Li, R.; Zhang, Z.; Li, Y.; Zhao, Y. Influence of chlorine, sulfur and phosphorus on the volatilization behavior of heavy metals during sewage sludge thermal treatment. Waste Manag. Res. 2013, 31, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Bayuseno, A.P.; Schmahl, W.W. Characterization of MSWI fly ash through mineralogy and water extraction. Resour. Conserv. Recycl. 2011, 55, 524–534. [Google Scholar] [CrossRef]
- European Commission DG ENV E3. Heavy Metals in Waste—Final Report; Project ENV.E.3/ETU/2000/0058; COWI: Copenhagen, Denmark, 2002. [Google Scholar]
- Gad, S.C. Antimony. In Encyclopedia of Toxicology; Gad Consulting Services: Cary, NC, USA, 2014; Volume 1. [Google Scholar] [CrossRef]
- Gras, B. Untersuchung von Holzaschen aus Kleinfeuerungsanlagen: Erkennen von Brennstoffmissbrauch; Institut für Hygiene und Umwelt: Hamburg, Germany, 2006. [Google Scholar]
- Holmberg, S.L.; Claesson, T. Mineralogy of granulated wood ash from a heating plant in Kalmar, Sweden. Environ. Geol. 2001, 40, 820–828. [Google Scholar] [CrossRef]
- Anthony, E.J.; Jia, L.; Laursen, K. Strength development due to long term sulfation and carbonation/sulfation phenomena. Can. J. Chem. Eng. 2001, 79, 356–366. [Google Scholar] [CrossRef]
- Park, J.H.; Eom, J.H.; Lee, S.L.; Hwang, S.W.; Kim, S.H.; Kang, S.W.; Yun, J.J.; Cho, J.S.; Lee, Y.H.; Seo, D.H. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci. Total Environ. 2020, 740, 140205. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, P.R.; Palmer, C.D. Reduction of Cr(VI) in the Presence of Excess Soil Fulvic Acid. Environ. Sci. Technol. 1995, 29, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Pettine, M.; Campanella, L.; Millero, F.J. Reduction of Hexavalent Chromium by H2O2 in Acidic Solutions. Environ. Sci. Technol. 2002, 36, 901–907. [Google Scholar] [CrossRef]
- Vitale, R.J.; Mussoline, G.R.; Petura, J.C.; James, B.R. Hexavalent Chromium Extractions from Soils: Evaluation of an Alkaline Digestion Method. J. Environ. Qual. 1994, 23, 1249–1256. [Google Scholar] [CrossRef]
- Weibel, G.; Eggenberger, U.; Kulik, D.A.; Hummel, W.; Schlumberger, S.; Klink, W.; Fisch, M.; Mäder, U.K. Extraction of heavy metals from MSWI fly ash using hydrochloric acid and sodium chloride solution. Waste Manag. 2018, 76, 457–471. [Google Scholar] [CrossRef] [PubMed]

| Laboratory-Scale | Industrial-Scale | ||||||
|---|---|---|---|---|---|---|---|
| Ash Type | MSWIFA | MFA | WWFA40 | MSWIFA | MFA | WWFA40 | |
| Without H2O2 | Extraction pH | 3.8 | 3.8 | 3.8 | 3.7–4.1 | 3.8–4.2 | 3.7–4.3 |
| Leachate pH | 4.8 | 4.8 | 5.4 | 3.8–4.4 | 4.0–4.5 | 4.2–4.8 | |
| Experiment duration (h) | 1 | 1 | 1 | 8 | 4 | 5 | |
| 40 L H2O2 /t ash | Extraction pH | 2.5 | 2.5 | 2.5 | 3.3–3.8 | 3.5–4.2 | - |
| Leachate pH | 4.5 | 4.5 | 4.2 | 3.9–4.4 | 3.8–4.4 | - | |
| Experiment duration (h) | 1 | 1 | 1 | 21 | 18 | - | |
| 60 L H2O2 /t ash | Extraction pH | - | - | - | 3.5–3.8 | 3.7–4.3 | - |
| Leachate pH | - | - | - | 3.8–4.3 | 3.8–4.5 | - | |
| Experiment duration (h) | - | - | - | 24 | 6 | - | |
| MSWIFA | WWFA40 | WWFA100 | |||||
|---|---|---|---|---|---|---|---|
| mg/kg | mg/kg | mg/kg | |||||
| MSWIFA_1 | MSWIFA_2 | MSWIFA_3 | WWFA40_1 | WWFA40_2 | WWFA40_3 | WWFA100 | |
| Al | 39,315 | 45,558 | 37,210 | 46,912 | 44,985 | 42,520 | 32,200 |
| Ba | 1928 | 1996 | 1679 | 5091 | 4681 | 5107 | 5370 |
| Ca | 165,984 | 170,617 | 163,200 | 288,884 | 285,400 | 307,600 | 235,650 |
| Cd | 277 | 248 | 311 | 26 | 23 | 28 | 71 |
| Cl | 82,120 | 85,100 | 114,500 | 31,140 | 32,800 | 32,530 | 36,050 |
| Cr | 360 | 350 | 323 | 582 | 597 | 443 | 1221 |
| Cu | 2131 | 3411 | 1771 | 602 | 683 | 581 | 1131 |
| Fe | 18,930 | 20,123 | 12,980 | 22,562 | 22,350 | 20,890 | 26,265 |
| K | 52,045 | 46,290 | 60,030 | 61,974 | 71,182 | 70,050 | 41,475 |
| Mg | 2529 | 3024 | 901 | 20,685 | 20,218 | 20,390 | 11,075 |
| Mn | 820 | 811 | 876 | 4274 | 5327 | 4296 | 4856 |
| Na | 75,190 | 76,720 | 91,410 | 6905 | 7590 | 7940 | 8485 |
| Ni | 94 | 92 | 74 | 95 | 91 | 96 | 76 |
| P | 4774 | 4531 | 4485 | 9383 | 8730 | 9107 | 3798 |
| Pb | 8143 | 8688 | 8204 | 7876 | 7590 | 5152 | 21,015 |
| S | 80,762 | 70,840 | 72,500 | 25,845 | 25,042 | 29,250 | 65,215 |
| Sb | 3150 | 3533 | 2988 | <3 | <3 | <3 | 22 |
| Si | 101,232 | 107,883 | 86,750 | 144,334 | 148,950 | 126,300 | 129,000 |
| Ti | 11,712 | 11,472 | 8606 | n.a. | n.a. | n.a. | 16,535 |
| Zn | 44,607 | 39,810 | 39,570 | 12,569 | 13,047 | 13,330 | 21,550 |
| TOC | 5190 | 5750 | 6010 | 10,150 | 17,043 | 12,030 | 74,400 |
| MSWIFA | WWFA40 | WWFA100 | ||||||
|---|---|---|---|---|---|---|---|---|
| wt % | wt % | wt % | ||||||
| Phases | Formula | MSWIFA_1 | MSWIFA_2 | MSWIFA_3 | WWFA40_1 | WWFA40_2 | WWFA40_3 | WWFA100 |
| Chlorides | ||||||||
| Halite | NaCl | 9 | - | 14 | <1 | <1 | 1 | 1 |
| Sylvite | KCl | 3 | 3 | 5 | 4 | 4 | 5 | - |
| K2ZnCl4 | K2ZnCl4 | 4 | 3 | 3 | - | - | - | - |
| Sulfates | ||||||||
| Anhydrite | CaSO4 | 10 | 10 | 11 | - | - | - | 8 |
| Silicates | ||||||||
| Quartz | SiO2 | 3 | 2 | 2 | 10 | 10 | 7 | 5 |
| Gehlenite | Ca2Al(AlSi)O7 | 7 | 5 | 3 | 4 | 2 | 4 | 9 |
| Alpha belite | Ca2SiO4 | 4 | 3 | - | 5 | 5 | 3 | 3 |
| Albite | NaAlSi3O8 | 4 | 3 | 3 | 5 | 3 | 5 | 2 |
| Sanidine | KAlSi3O8 | - | - | - | - | - | - | 5 |
| Microcline | KAlSi3O8 | 3 | 4 | 2 | - | - | - | - |
| Carbonates | ||||||||
| Calcite | CaCO3 | 5 | 6 | 6 | 17 | 28 | 20 | 8 |
| Magnesite | MgCO3 | <1 | 2 | 1 | - | - | - | 7 |
| Ankerite | CaFeCO3 | - | - | - | - | 1 | - | - |
| Oxides | ||||||||
| Lime | CaO | - | - | - | 2 | - | 4 | - |
| Hematite | Fe2O3 | <1 | 1 | - | 1 | <1 | <1 | 1 |
| Rutile | TiO2 | - | - | - | 2 | 1 | 3 | 1 |
| Periclase | MgO | 1 | - | - | 3 | 4 | 3 | - |
| Mayenite | Ca12Al14O33 | 2 | 2 | 3 | - | - | - | - |
| Perovskite | CaTiO3 | 2 | 2 | 2 | - | - | - | - |
| Phosphates | ||||||||
| Monetite | CaHPO4 | 5 | 4 | 3 | 5 | 6 | 5 | 2 |
| Amorphous | 37 | 41 | 43 | 42 | 35 | 39 | 49 | |
| WWFA40 | WWFA100 | ||||||
|---|---|---|---|---|---|---|---|
| mg/kg | mg/kg | ||||||
| WWFA40_1 | WWFA40_2 | WWFA40_3 | WWFA40_4 | WWFA40_5 | WWFA40_6 | WWFA100 | |
| Water-extractable Cr(VI) | 58 | 117 | 95 | 83 | 96 | 110 | 1 * |
| Water-extractable Cr(VI) of filter cake | - | <0.05 | - | <0.05 | <0.05 | 0.22 | - |
| Total Cr(VI) | - | 1 * | - | - | - | - | 87 |
| Experiment without H2O2 | Experiments with H2O2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| mg/kg | mg/kg | ||||||||
| Zn | Cd | Pb | Cu | Zn | Cd | Pb | Cu | ||
| MSWIFA | 44,710 | 220 | 6070 | 1360 | 43,700 | 260 | 10,700 | 2500 | * |
| 57,350 | 320 | 12,910 | 3090 | ** | |||||
| MFA | 31,950 | 170 | 6760 | 2010 | 31,330 | 190 | 6460 | 1970 | * |
| 31,830 | 160 | 5710 | 1770 | ** | |||||
| WWFA40 | 13,890 | 30 | 5520 | 630 | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolffers, M.; Weibel, G.; Eggenberger, U. Waste Wood Fly Ash Treatment in Switzerland: Effects of Co-Processing with Fly Ash from Municipal Solid Waste on Cr(VI) Reduction and Heavy Metal Recovery. Processes 2021, 9, 146. https://doi.org/10.3390/pr9010146
Wolffers M, Weibel G, Eggenberger U. Waste Wood Fly Ash Treatment in Switzerland: Effects of Co-Processing with Fly Ash from Municipal Solid Waste on Cr(VI) Reduction and Heavy Metal Recovery. Processes. 2021; 9(1):146. https://doi.org/10.3390/pr9010146
Chicago/Turabian StyleWolffers, Mirjam, Gisela Weibel, and Urs Eggenberger. 2021. "Waste Wood Fly Ash Treatment in Switzerland: Effects of Co-Processing with Fly Ash from Municipal Solid Waste on Cr(VI) Reduction and Heavy Metal Recovery" Processes 9, no. 1: 146. https://doi.org/10.3390/pr9010146
APA StyleWolffers, M., Weibel, G., & Eggenberger, U. (2021). Waste Wood Fly Ash Treatment in Switzerland: Effects of Co-Processing with Fly Ash from Municipal Solid Waste on Cr(VI) Reduction and Heavy Metal Recovery. Processes, 9(1), 146. https://doi.org/10.3390/pr9010146





