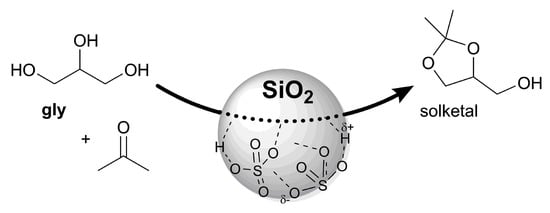
An Expedient Catalytic Process to Obtain Solketal from Biobased Glycerol
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. General Procedure for “Wet” Preparation of Supported Catalysts
2.3. Preparation of SSANa (3.0 mmol/g) Catalyst
2.4. Solketal Preparation (Small Scale)
2.5. Solketal Preparation (Bigger Scale)
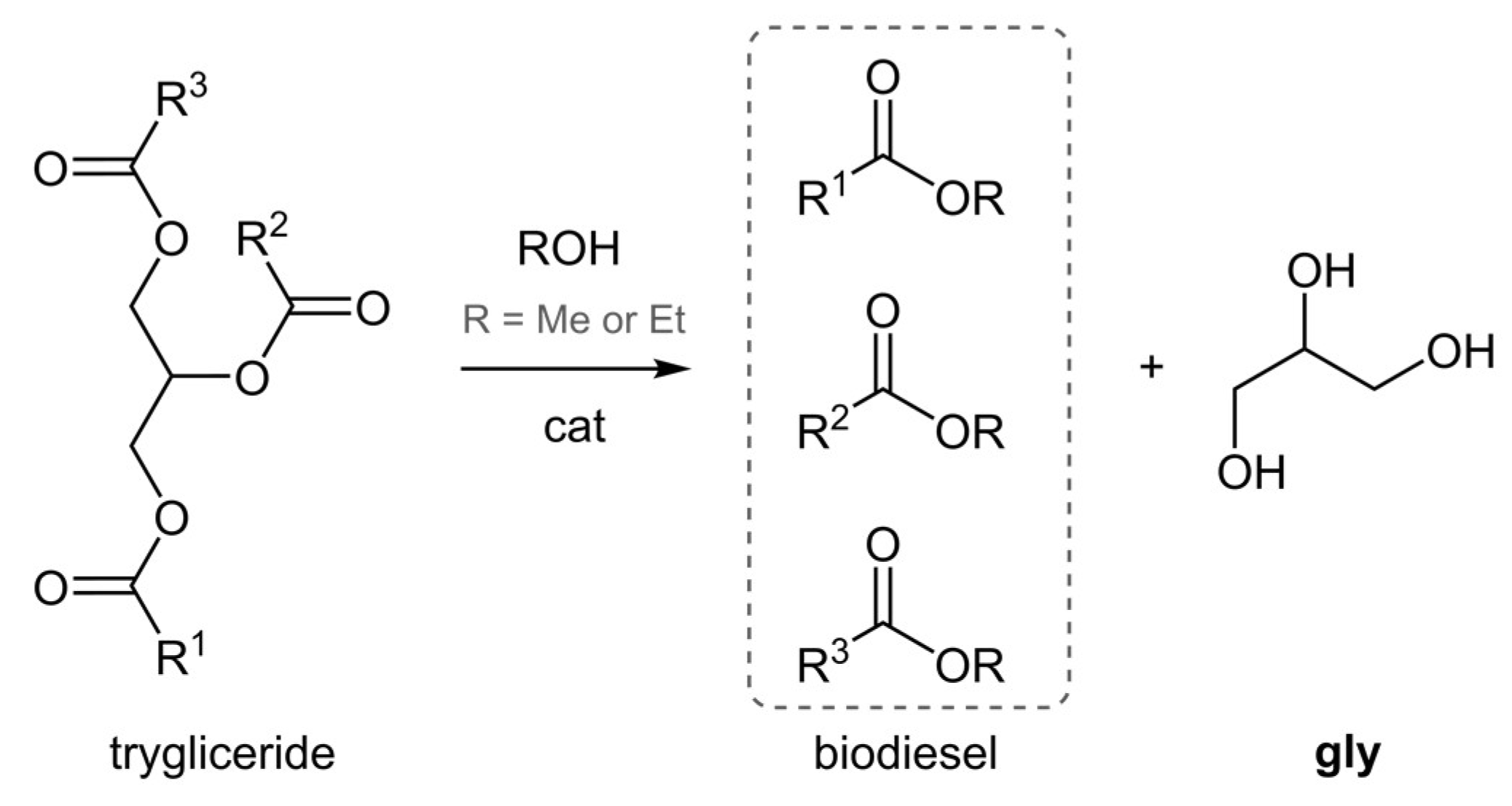
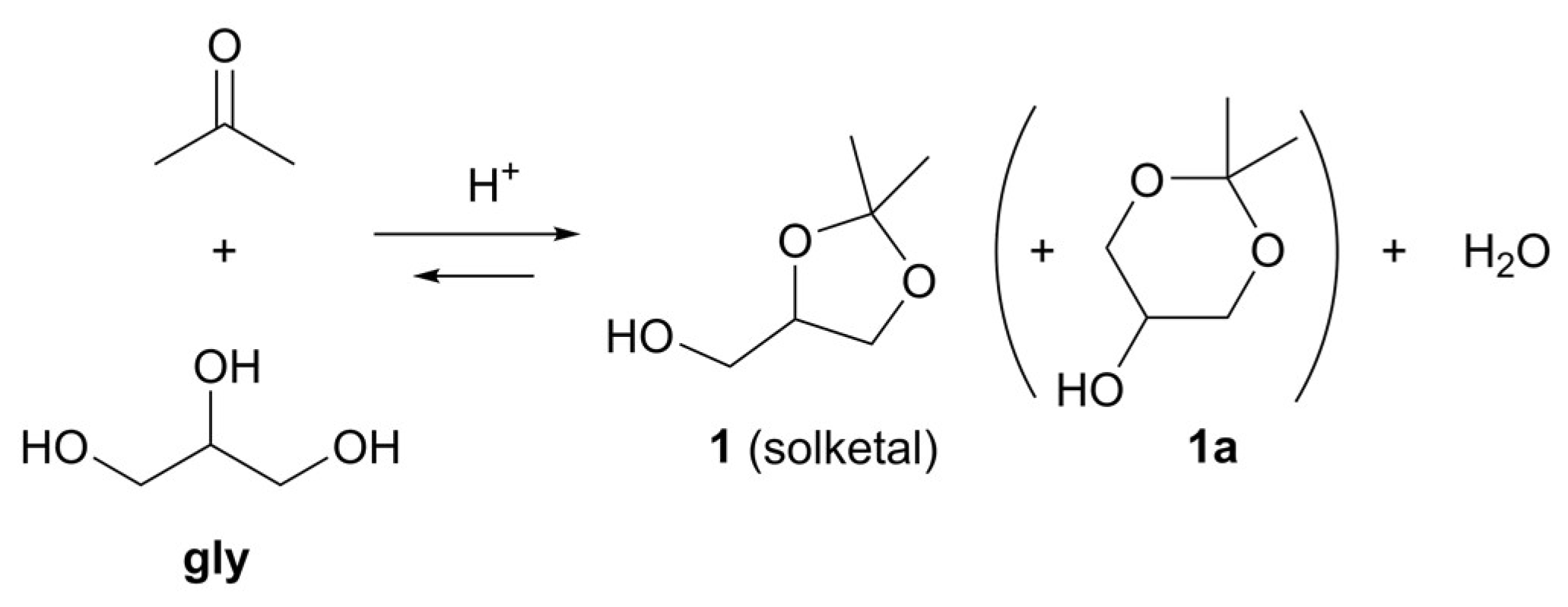
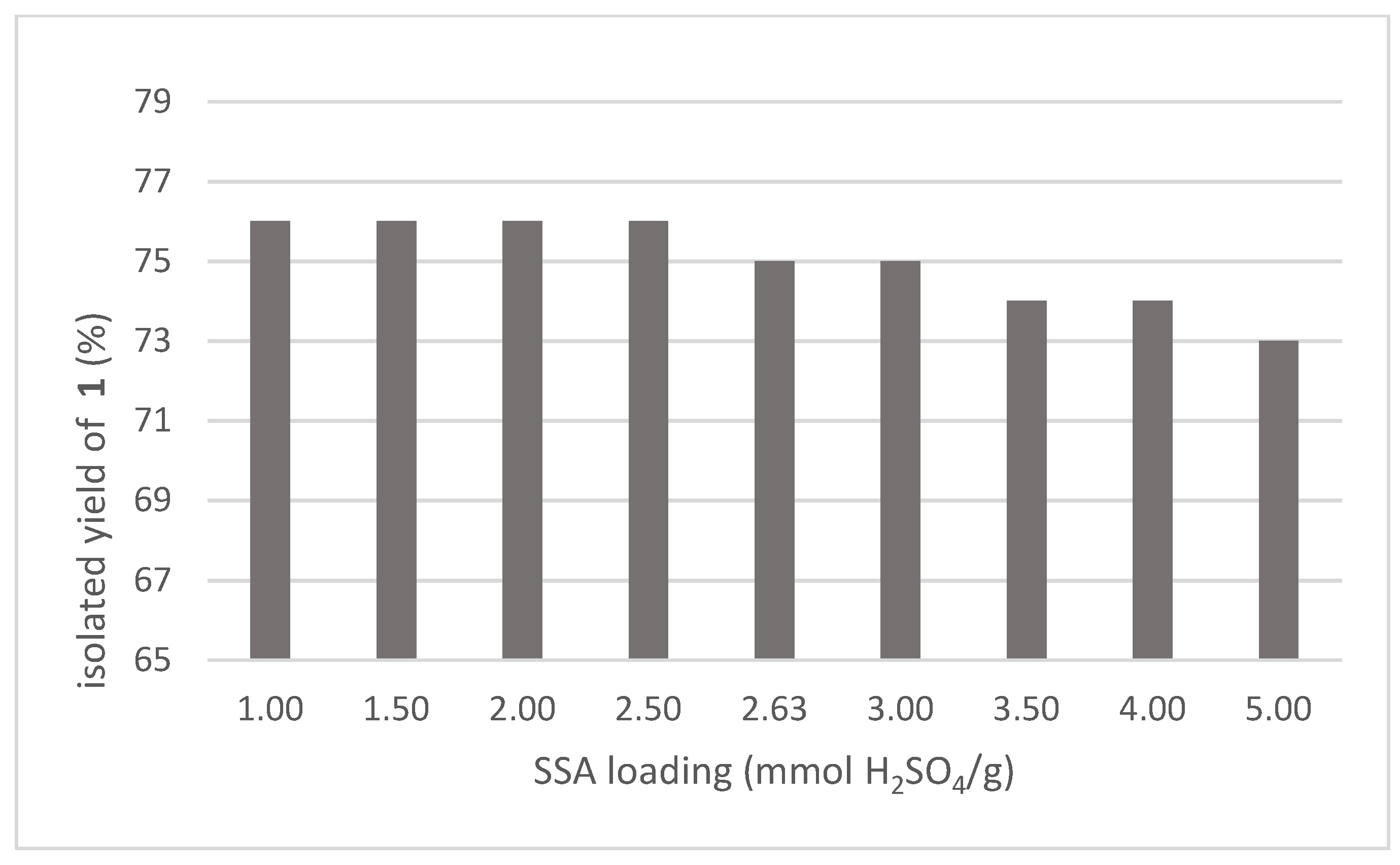
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadhukhan, J.; Ng, K.S.; Hernandez, E.M. (Eds.) Biorefineries and Chemical Processes: Design, Integration, and Sustainability Analysis; John Wiley & Sons Ltd.: Chichester, UK, 2014; ISBN 9781119990864. [Google Scholar]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022. [Google Scholar] [CrossRef]
- Nemoto, H.; Hattori, H.; Matsushita, T.; Yoshitomi, K.; Katagiri, A. An Efficient Method for the Refinement of 1,3-Methyleneglycerol via Bridged Acetal Exchange and the Synthesis of a Symmetrically Branched Glycerol Trimer. Synthesis 2012, 44, 2365–2373. [Google Scholar] [CrossRef][Green Version]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. A new continuous-flow process for catalytic conversion of glycerol to oxygenated fuel additive: Catalyst screening. Appl. Energy 2014, 123, 75. [Google Scholar] [CrossRef]
- Vasantha, V.T.; Venkatesha, N.J.; Shamshuddin, S.J.M.; D’Souza, J.Q.; Vijayasimha Reddy, B.G. Sulphated Zirconia Supported on Cordierite Honeycomb Monolith for Effective Synthesis of Solketal from Acetalisation of Glycerol with Acetone. ChemistrySelect 2018, 3, 602. [Google Scholar] [CrossRef]
- Melero, J.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Boot, M.; Frijters, P.P.; Luijten, C.C.; Somers, L.B.; Baert, R.R.; Donkerbroek, A.; Klein-Douwel, R.J.H.; Dam, N.N. Cyclic Oxygenates: A New Class of Second-Generation Biofuels for Diesel Engines? Energy Fuels 2009, 23, 1808–1817. [Google Scholar] [CrossRef]
- Zhen, Y.; Wan, S.; Liu, Y.; Yan, H.; Shi, R.; Wang, C. Atom Transfer Radical Polymerization of Solketal Acrylate Using Cyclohexanone as the Solvent. Macromol. Chem. Phys. 2005, 206, 607. [Google Scholar] [CrossRef]
- da Silva, C.X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38. [Google Scholar] [CrossRef]
- Ozorio, L.P.; Pianzolli, R.; Mota, M.B.S.; Mota, C.J.A. Reactivity of Glycerol/Acetone Ketal (Solketal) and Glycerol/Formaldehyde Acetals toward Acid-Catalyzed Hydrolysis. J. Braz. Chem. Soc. 2012, 23, 931. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Guimaraes, M.D.O.; Júlio, A.A. A Highly Regioselective and Solvent-Free Sn(II)-Catalyzed Glycerol Ketals Synthesis at Room Temperature. Catal. Lett. 2015, 145, 769–776. [Google Scholar] [CrossRef]
- Menezes, F.D.L.; Guimaraes, M.D.O.; da Silva, M.J. Highly Selective SnCl2-Catalyzed Solketal Synthesis at Room Temperature. Ind. Eng. Chem. Res. 2013, 52, 16709. [Google Scholar] [CrossRef]
- Nowicki, J. Oligomerization of glycerol over modified molecular sieves. Przemyls Chem. 2012, 91, 1164. [Google Scholar]
- da Silva, C.X.A.; Mota, C.J.A. The influence of impurities on the acid-catalyzed reaction of glycerol with acetone. Biomass Bioenergy 2011, 35, 3547–3551. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- Vinu, A.; Justus, J.; Balasubramanian, V.V.; Halligudi, S.B.; Ariga, K.; Mori, T. Synthesis of fructone and acylal using hexagonally ordered mesoporous aluminosilicate catalyst. Collect. Czech. Chem. Commun. 2008, 73, 1112. [Google Scholar] [CrossRef]
- Rodrigues, R.; Gonçalves, M.; Mandelli, D.; Pescarmona, P.P.; Carvalho, W.A. Solvent-free conversion of glycerol to solketal catalysed by activated carbons functionalised with acid groups. Catal. Sci. Technol. 2014, 4, 2293. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Yang, S.; Sun, Q.; Zhu, L.; Wu, Q.; Zhang, H.; Meng, X.; Xiao, F.-S. Sulfonated hollow sphere carbon as an efficient catalyst for acetalisation of glycerol. J. Mater. Chem. A 2013, 1, 9422–9426. [Google Scholar] [CrossRef]
- Klahr, D. Learning Sciences Research and Pasteur’s Quadrant. J. Learn. Sci. 2019, 28, 153–159. [Google Scholar] [CrossRef]
- Stokes, D.E. Pasteur’s Quadrant. Basic Science and Technological Innovation; Brookings Institution Press: Washington, DC, USA, 1997. [Google Scholar]
- Kautz, J.; Feltrin, W.; Sales, E.S.; Eifler-Lima, V.L.; Merlo, A.A. Reaíção de condensação do glicerol com compostos carbonílicos. Síntese, caracterização e aplicação em cristais líquidos. Quim. Nova 2015, 38, 1053. [Google Scholar]
- Barbosa, S.L.; Ottone, M.; de Almeida, M.T.; Lage, G.L.C.; Almeida, M.A.R.; Nelson, D.L.; dos Santos, W.T.P.; Clososki, G.C.; Lopes, N.P.; Klein, S.I.; et al. Ketalization of Ketones to 1,3-Dioxolanes and Concurring Self-Aldolization Catalyzed by an Amorphous, Hydrophilic SiO2-SO3H Catalyst under Microwave Irradiation. J. Braz. Chem. Soc. 2018, 29, 1663. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Lima, P.C.; Dos Santos, W.T.; Klein, S.I.; Clososki, G.C.; Caires, F.J. Oxygenated biofuels: Synthesis of fatty acid solketal esters with a mixture of sulfonated silica and (Bu4N)(BF4) catalyst. Catal. Commun. 2019, 120, 76–79. [Google Scholar] [CrossRef]
- Mukhopadhyay, B. Sulfuric acid immobilized on silica: An efficient promoter for one-pot acetalation–acetylation of sugar derivatives. Tetrahedron Lett. 2006, 47, 4337. [Google Scholar] [CrossRef]
- Chavez, F.; Suárez, S.; Diaz, M.A. Sulfuric acid adsorbed on silica gel. A multipurpose acid catalyst. Synth. Commun. 1994, 24, 2325. [Google Scholar] [CrossRef]
- Manna, J.; Roy, B.; Sharma, P. Efficient hydrogen generation from sodium borohydride hydrolysis using silica sulfuric acid catalyst. J. Power Sources 2015, 275, 727–733. [Google Scholar] [CrossRef]
- Montazeri, N. Sulfuric Acid Impregnated on Silica Gel (H2SO4/SiO2): A Versatile and Reusable Catalyst for the Synthesis of 1,2,4-Triazolo [5,1-b][1,3]thiazin-7-ones. Asian J. Chem. 2010, 22, 7432. [Google Scholar]
- Riego, J.M.; Sedin, Z.; Zaldívar, J.; Marziano, N.C.; Tortato, C. Sulfuric acid on silica-gel: An inexpensive catalyst for aromatic nitration. Tetrahedron Lett. 1996, 37, 513–516. [Google Scholar] [CrossRef]
- Zolfigol, M.A. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron 2001, 57, 9509–9511. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Madrakian, E.; Ghaemi, E. Silica Sulfuric Acid/NaNO2 as a Novel Heterogeneous System for the Nitration of Phenols under Mild Conditions. Molecules 2002, 7, 734. [Google Scholar] [CrossRef]
- Pathak, S.; Debnath, K.; Pramanik, A. Silica sulfuric acid: A reusable solid catalyst for one pot synthesis of densely substituted pyrrole-fused isocoumarins under solvent-free conditions. Beilstein J. Org. Chem. 2013, 9, 2344. [Google Scholar] [CrossRef]
- Khan, A.T.; Choudhury, L.H.; Ghosh, S. Silica supported perchloric acid (HClO4-SiO2): A highly efficient and reusable catalyst for geminal diacylation of aldehydes under solvent-free conditions. J. Mol. Catal. A 2006, 255, 230. [Google Scholar] [CrossRef]
- Khan, A.T.; Parvin, T.; Choudhury, L.H. Silica-Supported Perchloric Acid (HClO4-SiO2): A Versatile Catalyst for Tetrahydropyranylation, Oxathioacetalization and Thioacetalization. Synthesis 2006, 15, 2497. [Google Scholar] [CrossRef]
- Chanu, L.V.; Singh, T.P.; Devi, L.R.; Singh, O.M. Synthesis of bioactive heterocycles using reusable heterogeneous catalyst HClO4–SiO2 under solvent-free conditions. Green Chem. Lett. Rev. 2018, 11, 352. [Google Scholar] [CrossRef]
- Henderson, M.P.; Miasek, V.I.; Swaddle, T.W. Kinetics of Thermal Decomposition of Aqueous Perchloric Acid. Can. J. Chem. 1971, 49, 317. [Google Scholar] [CrossRef]
- Liu, P.N.; Xia, F.; Wang, Q.W.; Ren, Y.J.; Chen, J.Q. Triflic acid adsorbed on silica gel as an efficient and recyclable catalyst for the addition of β-dicarbonyl compounds to alcohols and alkenes. Green Chem. 2010, 12, 1049. [Google Scholar] [CrossRef]
- Karimi, B.; Ghoreishi-Nezhad, M. Highly chemoselective acetalization of carbonyl compounds catalyzed by a novel recyclable ammonium triflate-functionalized silica. J. Mol. Catal. A 2007, 277, 262. [Google Scholar] [CrossRef]
- Shirini, F.; Nahzomi, H.T.; Zolfigol, M.A. Silica Triflate as an Efficient Reagent for the Chemoselective Formylation of Alcohols. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1245. [Google Scholar] [CrossRef]
- Sriram, Y.H.; Fatima, T.; Kumar, M.S.; Rajanna, K.C.; Venkateswarlu, M.; Sudhakar, M.S.; Raju, R.M. Reusable silica supported perchloric acid and potassium bisulphate as green catalysts for thiocyanation of aromatic compounds under solvent free conditions. Iran. Chem. Comm. 2017, 5, 352. [Google Scholar]
- Gupta, P.; Paul, S. Solid acids: Green alternatives for acid catalysis. Catal. Today 2014, 236, 153. [Google Scholar] [CrossRef]
- Li, L.; Korányi, T.I.; Sels, B.F.; Pescarmona, P.P. Highly-efficient conversion of glycerol to solketal over heterogeneous Lewis acid catalysts. Green Chem. 2012, 14, 1611. [Google Scholar] [CrossRef]
- Huang, F.; Su, Y.; Long, Z.; Chen, G.; Yao, Y. Enhanced Formation of 5-Hydroxymethylfurfural from Glucose Using a Silica-Supported Phosphate and Iron Phosphate Heterogeneous Catalyst. Ind. Eng. Chem. Res. 2018, 57, 10198. [Google Scholar] [CrossRef]
- Stawicka, K.; Díaz-Álvarez, A.E.; Calvino-Casilda, V.; Trejda, M.; Bañares, M.A.; Ziolek, M. The Role of Brønsted and Lewis Acid Sites in Acetalization of Glycerol over Modified Mesoporous Cellular Foams. J. Phys. Chem. C 2016, 120, 16699. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Ottone, M.; Santos, M.C.; Junior, G.C.; Lima, C.D.; Glososki, G.C.; Lopes, N.P.; Klein, S.I. Benzyl benzoate and dibenzyl ether from of benzoic acid and benzyl alcohol under microwave irradiation using a SiO2–SO3H catalyst. Catal. Commun. 2015, 68, 97. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Roman, V.K.; Maletina, E.A. Bis[trimethylsilyl]sulfate as an organosilicon synthon. Synthesis 1982, 4, 277. [Google Scholar] [CrossRef]
- Wittenberg, D.; Wu, T.C.; Gilman, H. Cleavage of Diphenyl Sulfone and Diphenyl Sulfide by Triphenylsilyllithium. J. Org. Chem. 1958, 23, 1898. [Google Scholar] [CrossRef]
- Haas, A. The Chemistry of Silicon-Sulfur Compounds. Angew. Chem. Int. Ed. 1965, 4, 1014. [Google Scholar] [CrossRef]
- Eisenbraun, E.J.; Payne, K.W. Dean-Stark Apparatus Modified for Use with Molecular Sieves. Ind. Eng. Chem. Res. 1999, 38, 4521. [Google Scholar] [CrossRef]
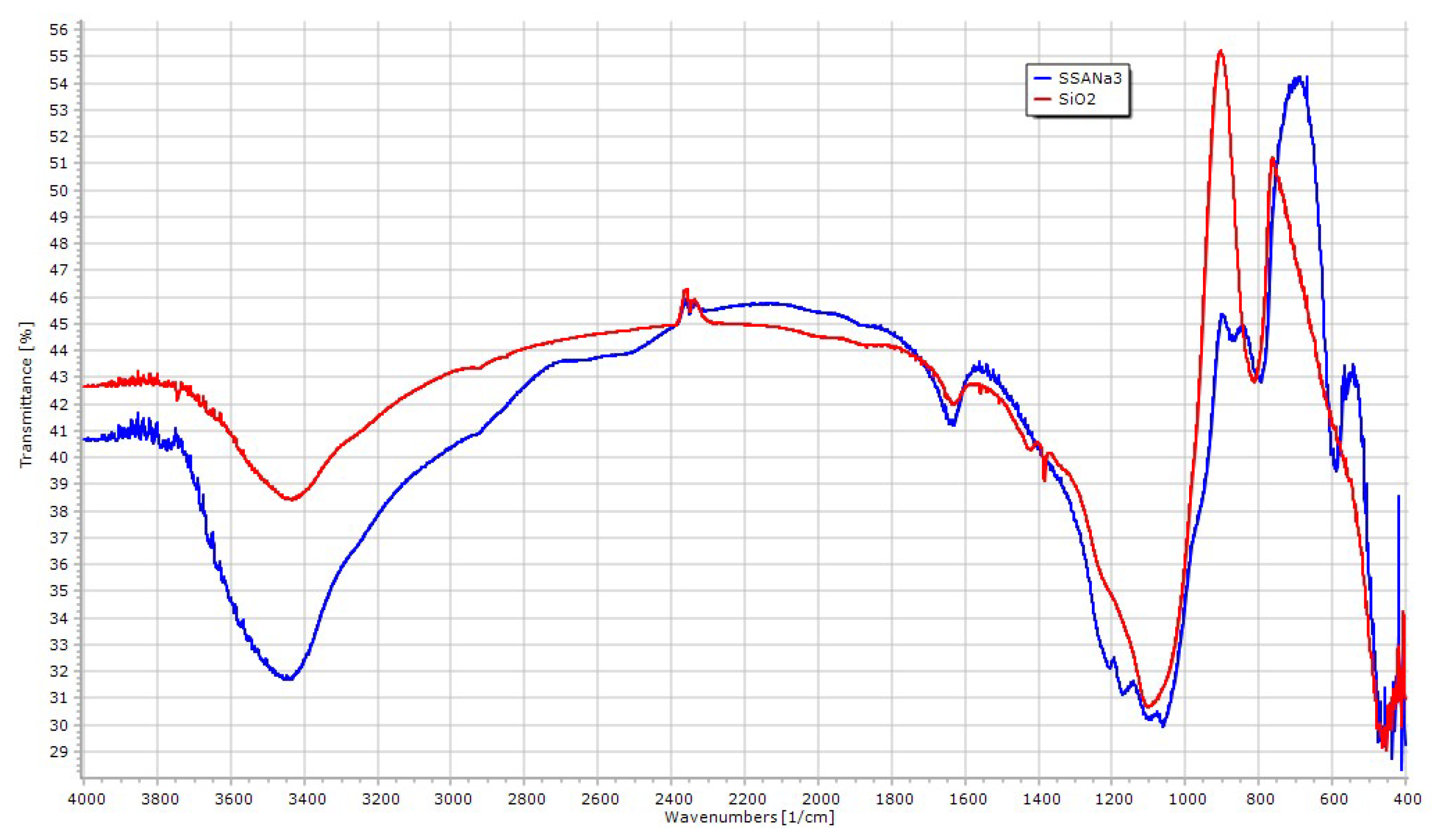

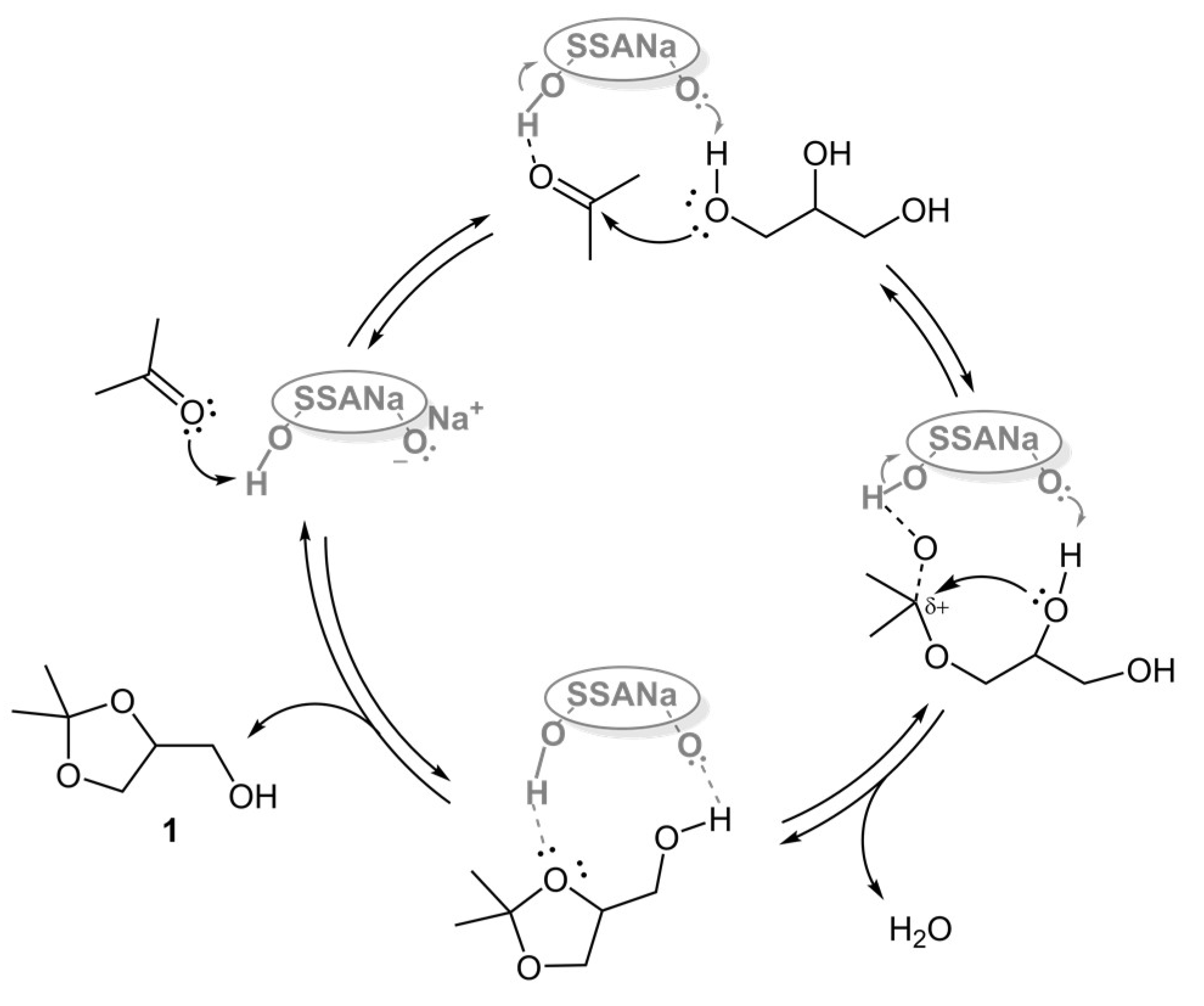
| Entry | Catalyst | Acetone Anhyd. a | Added Desiccant | Isolated Yield of 1 (%) |
|---|---|---|---|---|
| 1 | SnCl2·2H2O | n | - | 25 |
| 2 | SnCl2·2H2O | y | - | 41 |
| 3 | SnCl2·2H2O | y | MS (after 30′) | 70 |
| 4 | - | y | MS | 0 |
| 5 | “dry” SSA (2.63 mmol/g) | y | MS (after 30′) | 75 |
| 6 | “wet” SSA (2.63 mmol/g) | y | MS (after 30′) | 75 |
| 7 | SiO2 | y | MS (after 30′) | 0 |
| Entry | Catalyst | Isolated Yieldof 1 (%) |
|---|---|---|
| 1 | SPCA (0.75 mmol/g) | 76 |
| 2 | STA (1.5 mmol/g) | 76 |
| 3 | SSANa (3.0 mmol/g) | 76 |
| 4 | SSAK (3.0 mmol/g) | 76 |
| 5 | SSAN (3.0 mmol/g) | 75 |
| 6 | SSAI (3.0 mmol/g) | 27 |
| Entry | Catalyst Amount (mol%) a | Desiccant (mg) | Isolated Yield of 1 (%) |
|---|---|---|---|
| 1 | 1.25 | MS powder (120) | 70 |
| 2 | 1.50 | MS powder (120) | 75 |
| 3 | 1.75 | MS powder (120) | 76 |
| 4 | 1.50 | - | 15 |
| 5 | 1.50 | CaCl2 (150) | 31 |
| 6 | 1.50 | MgSO4 (150) | 50 |
| 7 | 1.50 | MgSO4 (300) | 51 |
| 8 | 1.50 | Na2SO4 (150) | 30 |
| Entry | supported Catalyst (mmol/g) | Isolated Yield of 1 (%) |
|---|---|---|
| 1 | SiO2·SnCl2 (2.5) | 32 |
| 2 | SiO2·FeCl3 (2.5) | 21 |
| 3 | SiO2·ZnCl2 (2.5) | 0 |
| 4 | SiO2·H3PO4(3.0)·SnCl2 (1.5) | 24 |
| 5 | SiO2·H3PO4(3.0)·FeCl3 (1.5) | 18 |
| 6 | SiO2·H2SO4(3.0)·SnCl2 (1.5) | 74 |
| 7 | SiO2·H2SO4 (3.0)·FeCl3 (1.5) | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roncaglia, F.; Forti, L.; D’Anna, S.; Maletti, L. An Expedient Catalytic Process to Obtain Solketal from Biobased Glycerol. Processes 2021, 9, 141. https://doi.org/10.3390/pr9010141
Roncaglia F, Forti L, D’Anna S, Maletti L. An Expedient Catalytic Process to Obtain Solketal from Biobased Glycerol. Processes. 2021; 9(1):141. https://doi.org/10.3390/pr9010141
Chicago/Turabian StyleRoncaglia, Fabrizio, Luca Forti, Sara D’Anna, and Laura Maletti. 2021. "An Expedient Catalytic Process to Obtain Solketal from Biobased Glycerol" Processes 9, no. 1: 141. https://doi.org/10.3390/pr9010141
APA StyleRoncaglia, F., Forti, L., D’Anna, S., & Maletti, L. (2021). An Expedient Catalytic Process to Obtain Solketal from Biobased Glycerol. Processes, 9(1), 141. https://doi.org/10.3390/pr9010141