In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals
Abstract
1. Introduction
2. Catalytic Aquathermolysis
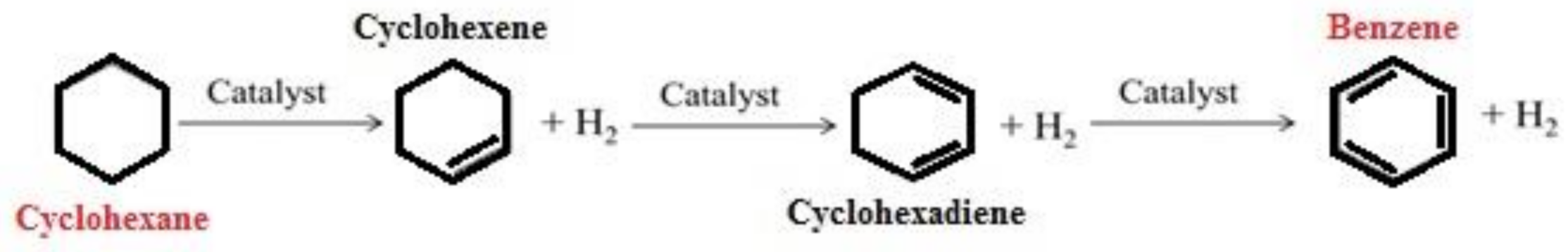
2.1. Use of Hydrogen Donors
2.2. Use of Aquathermolysis Catalysts
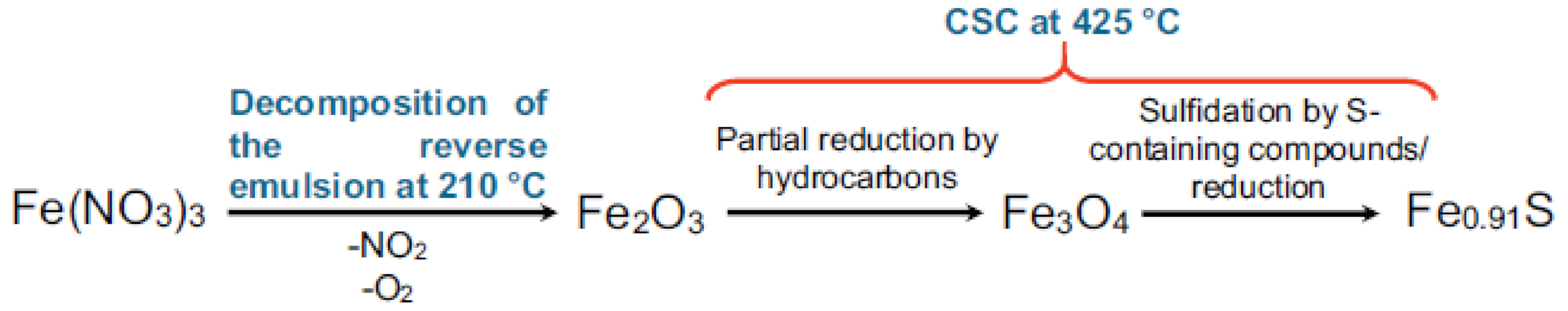
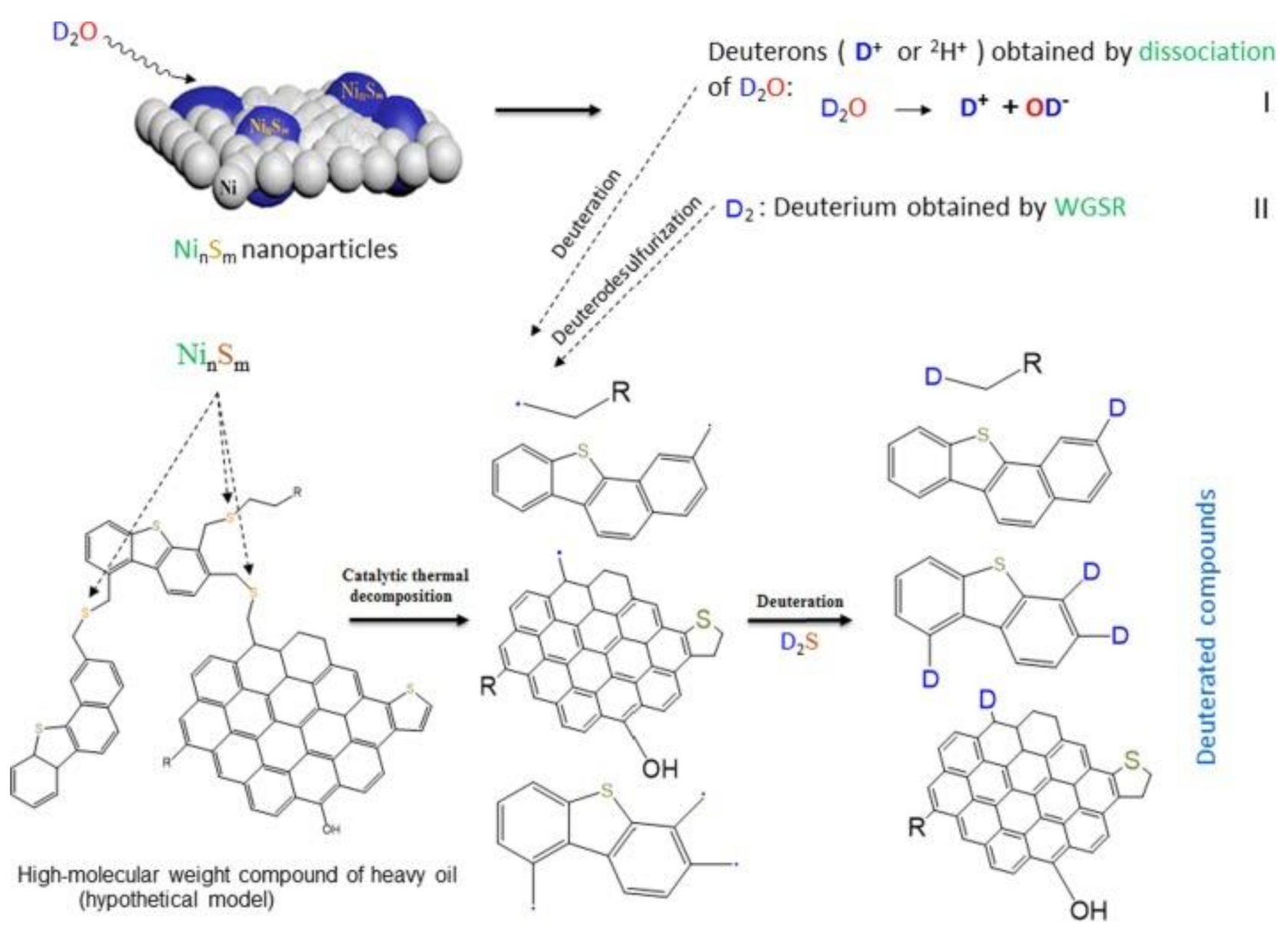
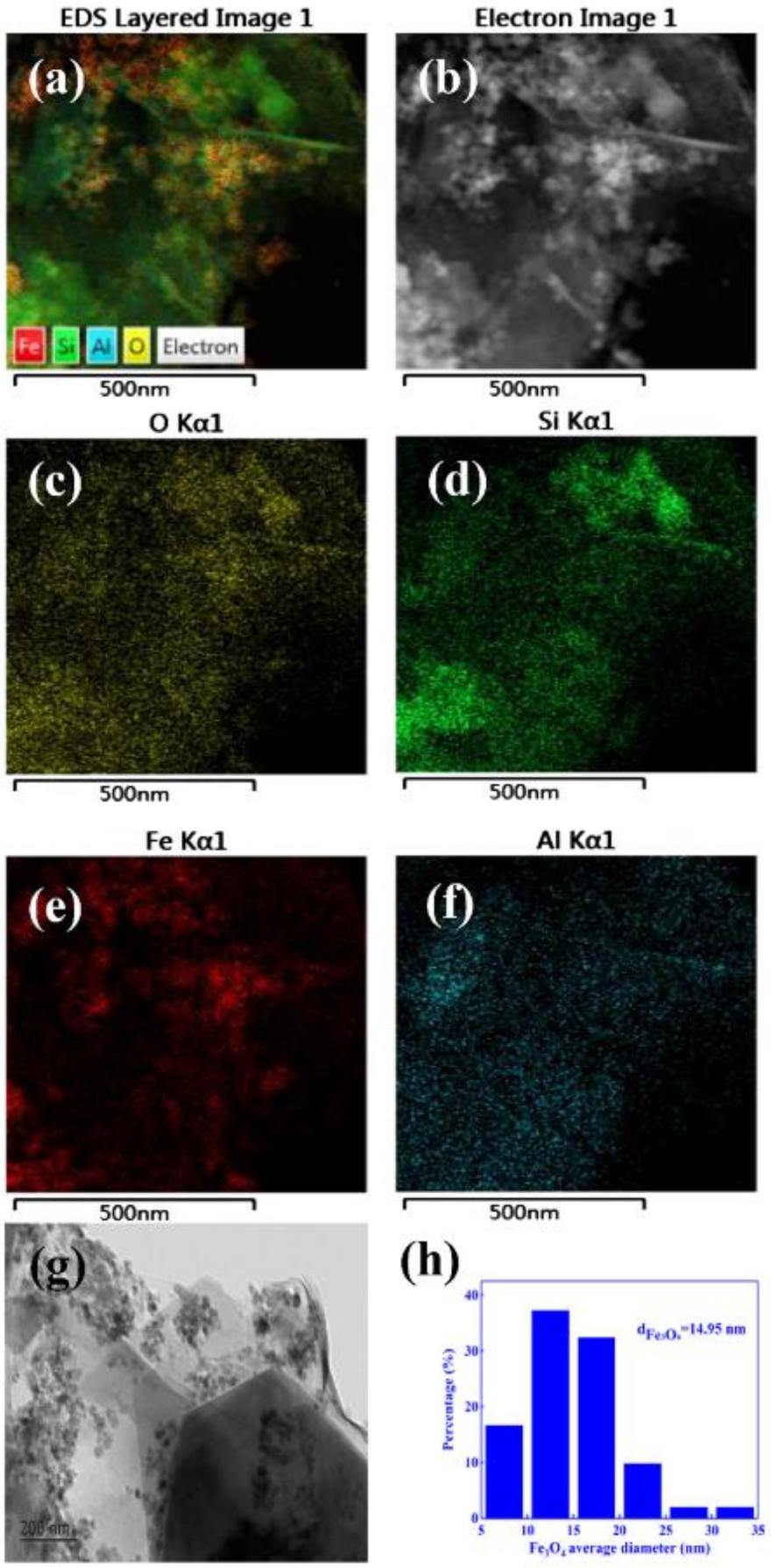
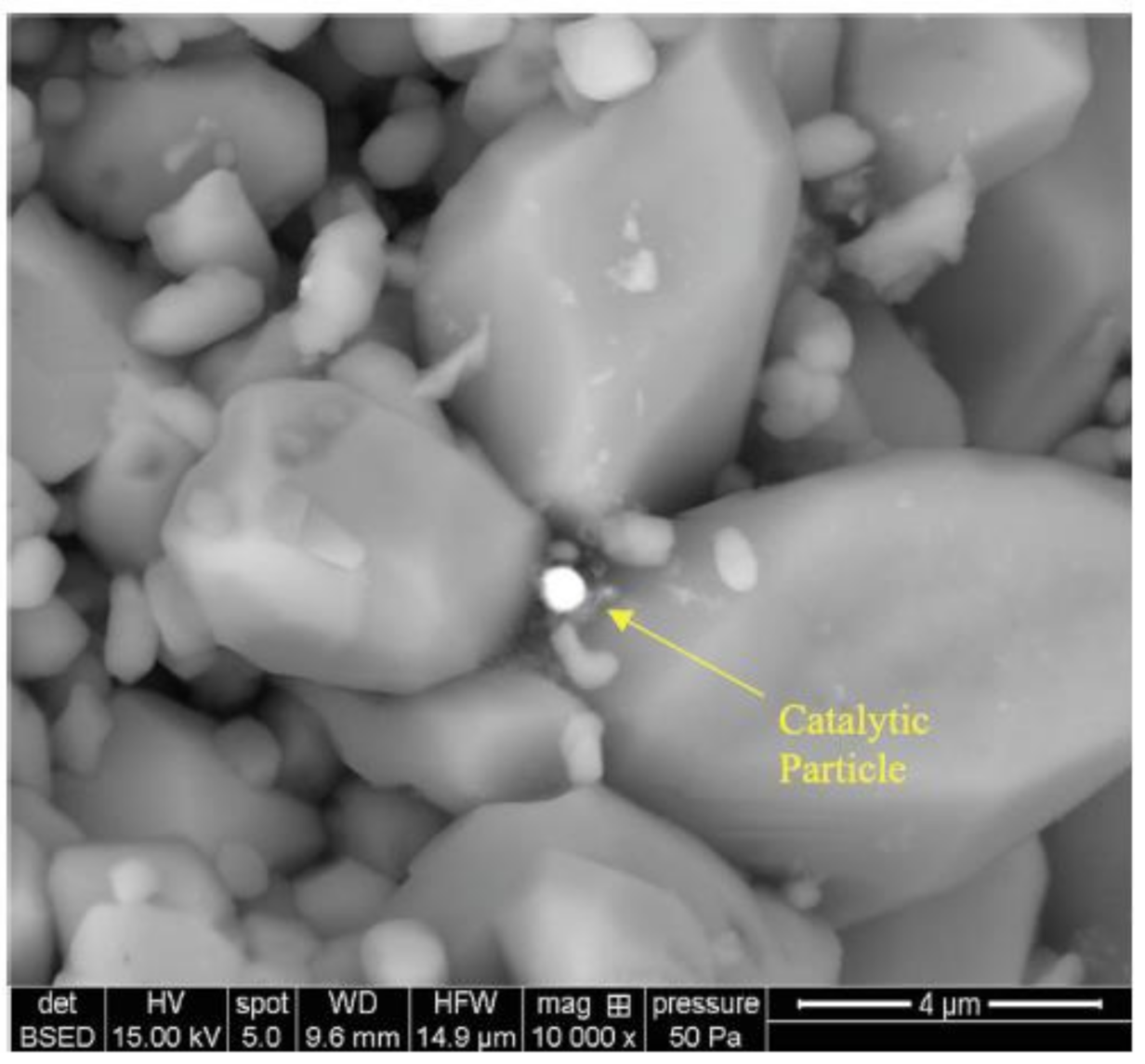
2.2.1. Nanocatalysts
2.2.2. Water-Soluble Catalysts
2.2.3. Oil-Soluble Catalysts
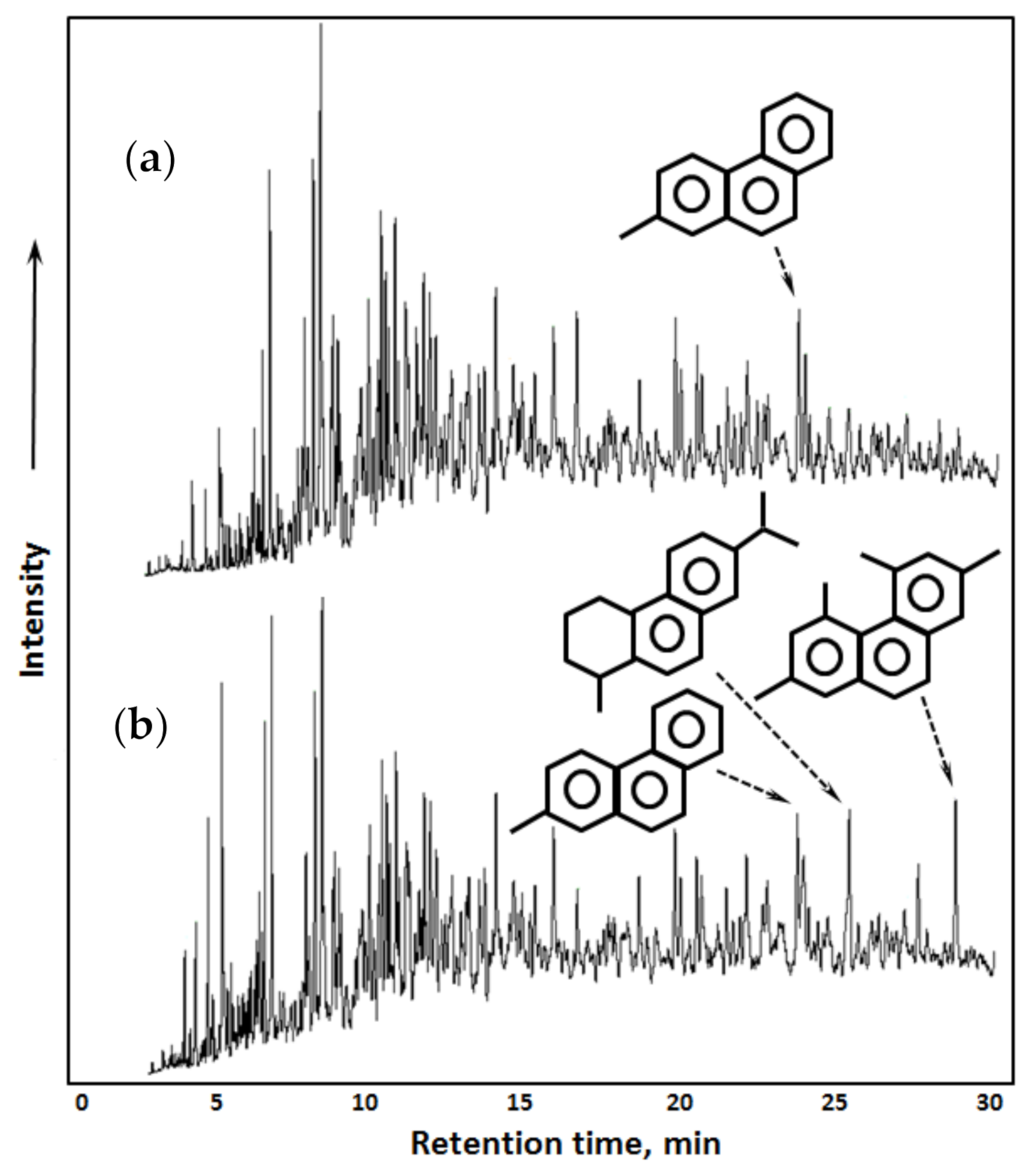
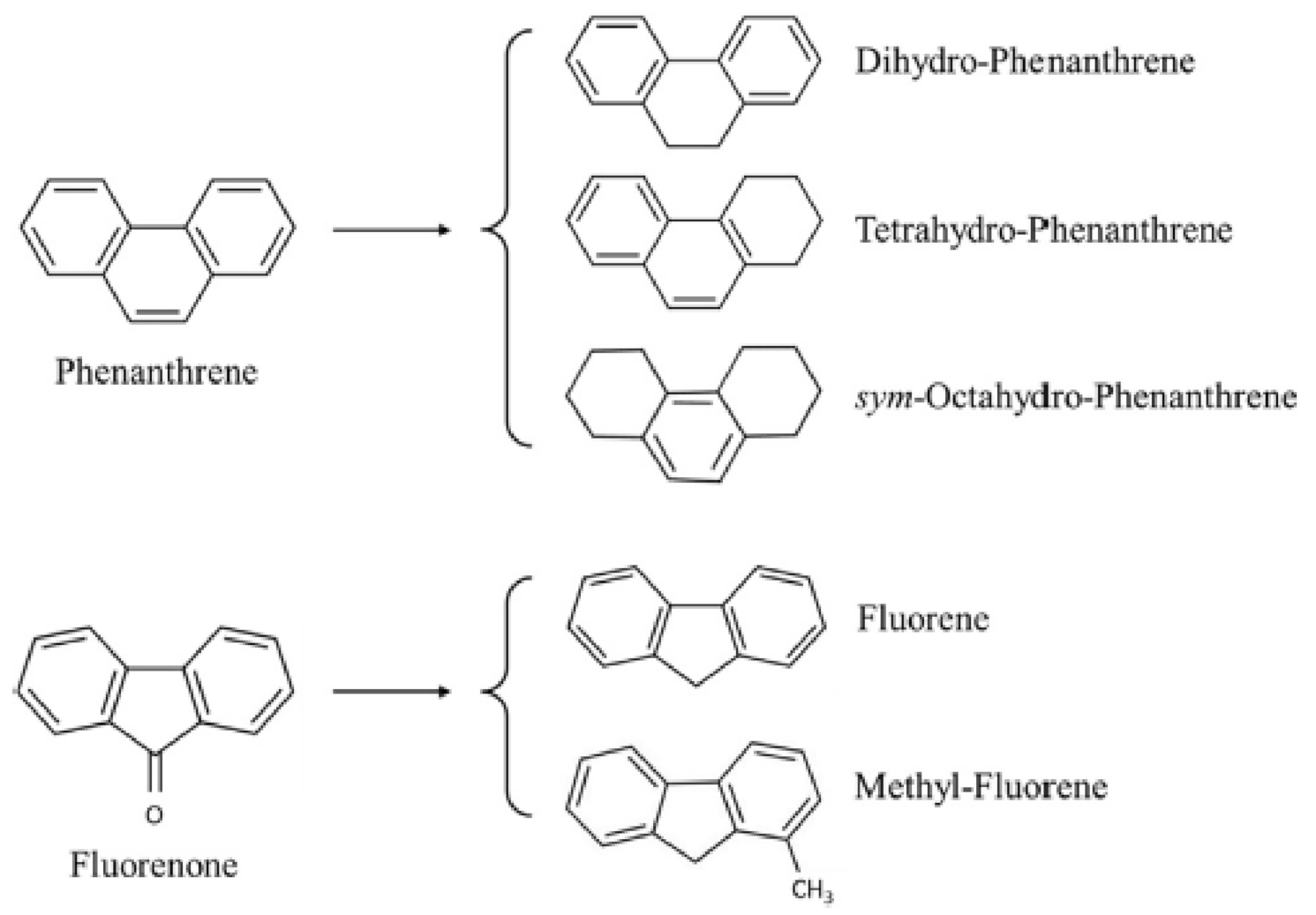
2.2.4. Rock Minerals
2.3. Field Tests of Catalytic Aquathermolysis
3. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yarboboev, T.; Sultanov, S.; Aminov, F.; Navotova, D. Non-traditional oils: Analysis of regional distribution and reserves of heavy oil and natural bitumen. Bull. Sci. Pract. 2020, 6, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hyne, J.B. Aquathermolysis: A Synopsis of Work on the Chemical Reaction between Water (Steam) and Heavy Oil Sands during Simulated Steam Stimulation; Alberta Oil Sands Technology and Research Authority: Edmonton, AB, Canada, 1986; pp. 1–82. [Google Scholar]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquin, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Mullins, O.C.; Betancourt, S.S.; Cribbs, M.E.; Dubost, F.X.; Creek, J.L.; Andrews, A.B.; Venkataramanan, L. The colloidal structure of crude oil and the structure of oil reservoirs. Energy Fuels 2007, 21, 2785–2794. [Google Scholar] [CrossRef]
- Antipenko, V.R.; Golubina, O.A.; Goncharov, I.V.; Nosova, S.V.; Ostroukhov, S.B. Specifics of the composition of monocyclic aromatic hydrocarbons in asphaltite from the Ivanovskoe deposit. Pet. Chem. 2007, 47, 154–161. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Gubaidullin, A.T.; Petrov, S.M.; Romanov, G.V.; Petrukhina, N.N.; Vakhin, A.V. Changes of asphaltenes’ structural phase characteristics in the process of conversion of heavy oil in the hydrothermal catalytic system. Energy Fuels 2016, 30, 773–783. [Google Scholar] [CrossRef]
- Nasyrova, Z.R.; Kayukova, G.P.; Vakhin, A.V.; Djimasbe, R.; Chemodanov, A.E. Heavy oil hydrocarbons and kerogen destruction of carbonate-siliceous domanic shale rock in sub- and supercritical water. Processes 2020, 8, 800. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Barcock, R.A.; Siskin, M.; Olmstead, W.N. Aqueous high-temperature chemistry of carbo- and heterocycles. 23. reactions of pyridine analogs and benzopyrroles in supercritical water at 460 °C. Energy Fuels 1994, 8, 990–1001. [Google Scholar] [CrossRef]
- Korneev, D.S.; Melenevskii, V.N.; Pevneva, G.S.; Golovko, A.K. Group composition of hydrocarbons and hetero compounds in stepwise-thermolysis products of asphaltenes from usa oil. Pet. Chem. 2018, 58, 179–185. [Google Scholar] [CrossRef]
- Hyne, J.B.; Greidanus, J.W.; Tyrer, J.D.; Verona, D.; Clark, P.D.; Clarke, R.A.; Koo, J.H.F. «The Future of Heavy Crude and Tar Sands» Aquathermolysis of heavy oils. In Proceedings of the 2nd International Conference, Caracas, Venezuela, 7–17 February 1982; pp. 404–411. [Google Scholar]
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the molecular structures of asphaltenes by atomic force microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef]
- Korneev, D.S.; Pevneva, G.S.; Golovko, A.K. Thermal transformations of asphaltenes at a temperature of 120 °C. J. Sib. Fed. Univ. Chem. 2019, 12, 101–117. [Google Scholar]
- Lin, R.; Chen, K.; Miao, M.; Zhang, L.; Wang, X.; Jiang, Y.; Zhang, J.; Wang, Y.; Pan, H. Reaction mechanism of H2S generation during tetrahydrothiophene aquathermolysis reaction. Energy Fuels 2020, in press. [Google Scholar] [CrossRef]
- Chemodanov, A.E.; Akhmadullin, R.R.; Sudakov, V.A.; Usmanov, S.A.; Khayrtdinov, R.K. Geochemical modeling with the use of vertical and horizontal relative concentrations of oil compounds for the heavy oil fields. Pet. Sci. Technol. 2018, 36, 1100–1106. [Google Scholar] [CrossRef]
- Montgomery, W.; Court, R.W.; Rees, A.C.; Sephton, M.A. High temperature reactions of water with heavy oil and bitumen: Insights into aquathermolysis chemistry during steam-assisted recovery. Fuel 2013, 113, 426–434. [Google Scholar] [CrossRef]
- Gregoli, A.A.; Rimmer, D.P. Production of Synthetic Crude oil from Heavy Hydrocarbons Recovered by in Situ Hydrovisbreaking. U.S. Patent 6016868A, 25 January 2000. [Google Scholar]
- Ovalles, C.; Rengel-Unda, P.; Bruzual, J.; Salazar, A. Upgrading of extra-heavy crude using hydrogen donor under steam injection conditions. Characterization by pyrolysis GC-MS of the asphaltenes and effects of a radical initiator. Acs Div. Fuel Chem. Prepr. 2003, 48, 59–60. [Google Scholar]
- Lyadov, A.S.; Petrukhina, N.N. Extraction and refining of heavy crude oils: Problems and prospects. Russ. J. Appl. Chem. 2018, 91, 1912–1921. [Google Scholar] [CrossRef]
- Tumanyan, B.P.; Petrukhina, N.N.; Kayukova, G.P.; Nurgaliev, D.K.; Foss, L.E.; Romanov, G.V. Aquathermolysis of crude oils and natural bitumen: Chemistry, catalysts and prospects for industrial implementation. Russ. Chem. Rev. 2015, 84, 1145–1175. [Google Scholar] [CrossRef]
- Ortega, L.C.; Sebakhy, K.O.; Trujillo, M.; Pereira-Almao, P. Proton Nuclear Magnetic Resonance (1H-NMR) Methodology for Monolefin Analysis: Application to Aquaprocessing-Upgraded Bitumen. Energy Fuels 2020, 34, 9252–9261. [Google Scholar] [CrossRef]
- Strauz, O.P.; Lown, E.M. The Chemistry of Alberta oil Sands, Bitumens and Heavy Oils; Alberta Energy Research Institute: Calgary, AB, Canada, 2003; pp. 588–592. [Google Scholar]
- Clark, P.D.; Kirk, M.J. Studies on the upgrading of bituminous oils with water and transition metal catalysts. Energy Fuels 1994, 8, 380–387. [Google Scholar] [CrossRef]
- Pecoraro, T.A.; Chianelli, R.R. Hydrodesulfurization catalysis by transition metal sulfides. J. Catal. 1981, 67, 430–445. [Google Scholar] [CrossRef]
- Lei, J.; Abdulaziz, A.; Ng, F.T.T. Effect of metal ions on light gas oil upgrading over nano dispersed mosx catalysts using in situ H2. ACS Symp. Ser. 2012, 1092, 37–49. [Google Scholar]
- Claydon, R.; Wood, J. A Mechanistic Study of Layered-Double Hydroxide (LDH)-Derived Nickel-Enriched Mixed Oxide (Ni-MMO) in Ultradispersed Catalytic Pyrolysis of Heavy Oil and Related Petroleum Coke Formation. Energy Fuels 2019, 33, 10820–10832. [Google Scholar] [CrossRef] [PubMed]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Voronetskaya, N.G.; Pevneva, G.S.; Korneev, D.S.; Golovko, A.K. Influence of asphaltenes on the direction of thermal transformations of heavy oil hydrocarbons. Pet. Chem. 2020, 60, 166–173. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I.; et al. Hydrogen donating capacity of water in catalytic and non-catalytic aquathermolysis of extra-heavy oil: Deuterium tracing study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Alemán-Vázquez, L.O.; Torres-Mancera, P.; Ancheyta, J.; Ramírez-Salgado, J. Use of hydrogen donors for partial upgrading of heavy petroleum. Energy Fuels 2016, 30, 9050–9060. [Google Scholar] [CrossRef]
- Gyul’maliev, A.M.; Maloletnev, A.S.; Magomadov, E.E.; Kadiev, K.M. Donor capacity of hydroaromatic compounds. Solid Fuel Chem. 2012, 46, 205–211. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ohno, A.; Kunugi, T. Liquid phase hydrogenolysis of thiophene by decaline as hydrogen donor with metal supported active carbon catalysts. Stud. In Surf. Sci. Catal. 1983, 17, 241–249. [Google Scholar]
- Billmers, R.; Griffith, L.L.; Stein, S.E. Hydrogen Transfer between anthracene structures. J. Phys. Chem. 1986, 90, 517–523. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, X.; Wang, Y.; Shi, Y. The catalytic aquathermolysis of heavy oil in the presence of a hydrogen donor under reservoirs conditions. J. Chem. Pharm. Res. 2014, 6, 2037–2041. [Google Scholar]
- Scott, C.E.; Delgado, O.; Bolivar, C. Upgrading of Hamaca crude oil using formic acid as hydrogen precursor under steam injection conditions. Acs Div. Fuel Chem. Prepr. 2003, 48, 52–53. [Google Scholar]
- Hart, A.; Lewis, C.; White, T.; Greaves, M.; Wood, J. Effect of cyclohexane as hydrogen-donor in ultradispersed catalytic upgrading of heavy oil. Fuel Process. Technol. 2015, 138, 724–733. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.; Zhou, C.; Chen, Y. Advances on the transition-metal based catalysts for aquathermolysis upgrading of heavy crude oil. Fuel 2019, 257, 115779. [Google Scholar] [CrossRef]
- Al-Marshed, A.; Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of heavy oil upgrading using dispersed nanoparticulate iron oxide as a catalyst. Energy Fuels 2015, 29, 6306–6316. [Google Scholar] [CrossRef]
- Cheraghian, G.; Rostami, S.; Afrand, M. Nanotechnology in enhanced oil recovery. Processes 2020, 8, 1073. [Google Scholar] [CrossRef]
- Zamani, A.; Maini, B.; Almao, P. Propagation of nanocatalyst particles through Atabasca Sands. In Proceedings of the Canadian Unconventional Resources Conference, Calgary, AB, Canada, 15–17 November 2011. [Google Scholar]
- Pevneva, G.S.; Voronetskaya, N.G.; Sviridenko, N.N.; Golovko, A.K. Effect of WC/Ni–Cr additive on changes in the composition of an atmospheric residue in the course of cracking. Pet. Sci. 2020, 17, 499–508. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Glotov, A.P.; Nikiforova, A.G.; Vutolkina, A.V.; Kardashev, S.V.; Maksimov, A.L.; Lysenko, S.V.; Ivanov, A.O. Catalytic cracking additives based on mesoporous mcm-41 for sulfur removal. Fuel Process. Technol. 2016, 153, 50–57. [Google Scholar] [CrossRef]
- Kadieva, M.K.; Khadzhiev, S.N.; Kadiev, K.M.; GyulMaliev, A.M.; Yakovenko, T.V. The formation of nanosized molybdenum oxide particles in a hydrocarbon medium. Pet. Chem. 2011, 51, 16–23. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Fu, Z.; Zhao, X. Using hydrogen donor with oil-soluble catalysts for upgrading heavy oil. Russ. J. Appl. Chem. 2014, 87, 1498–1506. [Google Scholar] [CrossRef]
- Guo-Rui, L.; Yu, C.; Yong, A.; Yan-Ling, C. Catalytic aquathermolysis of super-heavy oil: Cleavage of CS bonds and separation of light organosulfurs. Fuel Process. Technol. 2016, 153, 94–100. [Google Scholar]
- Liu, X.; Li, Y.; Zhang, Z.; Li, X.; Zhao, M.; Su, C. Synthesis of silica/metatitanic acid nanocomposite and evaluation of its catalytic performance for aquathermolysis reaction of extra-heavy crude oil. J. Energy Chem. 2015, 24, 472–476. [Google Scholar] [CrossRef]
- Avbenake, O.P.; Al-Hajri, R.S.; Jibril, B.Y. Catalytic upgrading of heavy oil using NiCo/Γ-Al2O3 catalyst: Effect of initial atmosphere and water-gas shift reaction. Fuel 2019, 235, 736–743. [Google Scholar] [CrossRef]
- Avbenake, O.P.; Al-Hajri, R.S.; Jibril, B.Y. Saturates and aromatics characterization in heavy crude oil upgrading using Ni–Co/γ-Al2O3 catalysts. Pet. Sci. Technol. 2020, 38, 800–807. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Rakhmatullin, I.Z.; Sitnov, S.A.; Laikov, A.V.; Klochkov, V.V.; Vakhin, A.V. Influence of Co-based catalyst on subfractional composition of heavy oil asphaltenes during aquathermolysis. J. Pet. Sci. Eng. 2020, 186, 106721. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. In-Situ Upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. Spe Reserv. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Yi, S.; Babadagli, T.; Li, H. Stabilization of nickel nanoparticle suspensions with the aid of polymer and surfactant: Static bottle tests and dynamic micromodel flow tests. Pet. Sci. 2020, 17, 1014–1024. [Google Scholar] [CrossRef]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Heavy oil upgrading and enhanced recovery in a steam injection process assisted by NiO- and PdO-functionalized SiO2 nanoparticulated catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Vakhin, A.V.; Ivanova, A.G.; Voronina, E.V. Composition of aquathermolysis catalysts forming in situ from oil-soluble catalyst precursor mixtures. J. Pet. Sci. Eng. 2018, 169, 44–50. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Onishchenko, Y.V.; Feoktistov, D.A. Aquathermolysis of high-viscosity oil in the presence of an oil-soluble iron-based catalyst. Chem. Technol. Fuels Oils 2017, 53, 666–674. [Google Scholar] [CrossRef]
- Chen, M.; Li, C.; Li, G.-R.; Chen, Y.-L.; Zhou, C.-G. In situ preparation of well-dispersed CuO nanocatalysts in heavy oil for catalytic aquathermolysis. Petroleum Science. 2019, 16, 439–446. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Khaidarova, A.R.; Zaripova, R.D.; Mukhamatdinova, R.E.; Sitnov, S.A.; Vakhin, A.V. The composition and structure of ultra-dispersed mixed oxide (II, III) particles and their influence on in-situ conversion of heavy oil. Catalysts 2020, 10, 114. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Shmeleva, E.I.; Aliev, F.A.; Vakhin, A.V. Influence of nanosized iron oxides (II, III) on conversion of biodegradated oil. Pet. Sci. Technol. 2019, 37, 971–976. [Google Scholar] [CrossRef]
- Melnikov, P.; Nascimento, V.A.; Arkhangelsky, I.V.; Zanoni, L.Z.; Consolo, L.; De Oliveira, C.S.S. Thermal decomposition mechanism of iron(III) nitrate and characterization of intermediate products by the technique of computerized modeling. J. Therm. Anal. Calorim. 2014, 115, 145–151. [Google Scholar] [CrossRef]
- Yeletsky, P.M.; Zaikina, O.O.; Sosnin, G.A.; Kukushkin, R.G.; Yakovlev, V.A. Heavy oil cracking in the presence of steam and nanodispersed catalysts based on different metals. Fuel Process. Technol. 2020, 199, 106239. [Google Scholar] [CrossRef]
- Betiha, M.A.; Elmetwally, A.E.; Al-Sabagh, A.M.; Mahmoud, T. Catalytic aquathermolysis for altering the rheology of asphaltic crude oil using ionic liquid modified magnetic MWCNT. Energy Fuels 2020, 34, 11353–11364. [Google Scholar] [CrossRef]
- Khaidarova, A.R.; Pyataev, A.V.; Mukhamatdinov, I.I.; Zaripova, R.D.; Vakhin, A.V. Investigation of structural phase conversions of an iron-containing catalyst by mossbauer spectroscopy (Part 1). J. Appl. Spectrosc. 2020, 87, 680–684. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Sitnov, S.A.; Slavkina, O.V.; Bugaev, K.A.; Laikov, A.V.; Vakhin, A.V. The aquathermolysis of heavy oil from Riphean-Vendian complex with iron-based catalyst: FT-IR spectroscopy data. Pet. Sci. Technol. 2019, 37, 1410–1416. [Google Scholar] [CrossRef]
- Akhmadiyarov, A.A.; Rakipov, I.T.; Khachatrian, A.A.; Petrov, A.A.; Sitnov, S.A.; Gerasimov, A.V.; Osin, Y.N.; Varfolomeev, M.A. Thermocatalytic upgrading of heavy oil by iron oxides nanoparticles synthesized by oil-soluble precursors. J. Pet. Sci. Eng. 2018, 169, 200–204. [Google Scholar] [CrossRef]
- Brown, A.R.; Hart, A.; Coker, V.S.; Lloyd, J.R.; Wood, J. Upgrading of heavy oil by dispersed biogenic magnetite catalysts. Fuel 2016, 185, 442–448. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, H.; Wu, Y.; Lu, T.; Liu, Y.; Chen, X.; Peng, C.; Yang, C.; Feng, X. Morphological insights into the catalytic aquathermolysis of crude oil with an easily prepared high-efficiency Fe3O4-containing catalyst. Fuel 2019, 245, 420–428. [Google Scholar] [CrossRef]
- Lin, D.; Feng, X.; Wu, Y.; Ding, B.; Lu, T.; Liu, Y.; Chen, X.; Chen, D.; Yang, C. Insights into the synergy between recyclable magnetic Fe3O4 and zeolite for catalytic aquathermolysis of heavy crude oil. Appl. Surf. Sci. 2018, 456, 140–146. [Google Scholar] [CrossRef]
- Olvera, J.N.R.; Gutiérrez, G.J.; Serrano, J.A.R.; Ovando, A.M.; Febles, V.G.; Arceo, L.D.B. Use of unsupported, mechanically alloyed NiWMoC nanocatalyst to reduce the viscosity of aquathermolysis reaction of heavy oil. Catal. Commun. 2014, 43, 131–135. [Google Scholar] [CrossRef]
- Almao, P. In situ upgrading of bitumen and heavy oils via nanocatalysis. Can. J. Chem. Eng. 2012, 90, 320–329. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira-Almao, P. Transport behavior of multimetallic ultradispersed nanoparticles in an oil-sands-packed bed column at a high temperature and pressure. Energy Fuels 2012, 26, 1645–1655. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: A study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 2013, 27, 2194–2201. [Google Scholar] [CrossRef]
- Zamani, A.; Maini, B.; Pereira Almao, P. Flow of nanodispersed catalyst particles through porous media: Effect of permeability and temperature. Can. J. Chem. Eng. 2012, 90, 304–314. [Google Scholar] [CrossRef]
- Foroozesh, J.; Kumar, S. Nanoparticles behaviors in porous media: Application to enhanced oil recovery. J. Mol. Liq. 2020, 316, 113876. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B. Chemistry of organosulphur compound types occurring in heavy oil sands: 3. Reaction of thiophene and tetrahydrothiophene with vanadyl and nickel salts. Fuel 1984, 63, 1649–1654. [Google Scholar]
- Fan, H.; Liu, Y.; Zhao, X.; Zhong, L. Studies on effect of metal ions on aquathermolysis reaction of Liaohe heavy oils under steam treatment. J. Fuel Chem. Technol. 2001, 29, 430–433. [Google Scholar]
- Zhong, L.G.; Liu, Y.J.; Fan, H.F.; Jiang, S.J. Liaohe extra-heavy crude oil underground aquathermolytic treatments using catalyst and hydrogen donors under steam injection conditions. In Proceedings of the SPE international improved oil recovery conference in Asia Pacific. Society of Petroleum Engineers, Kuala Lumpur, Malaysia, 20–21 October 2003. [Google Scholar]
- Jiang, S.; Liu, X.; Liu, Y.; Zhong, L. In situ upgrading heavy oil by aquathermolytic treatment under steam injection conditions. In Proceedings of the SPE international improved oil recovery conference in Asia Pacific. Society of Petroleum Engineers, Kuala Lumpur, Malaysia, 5–6 December 2005. [Google Scholar]
- Speight, J.G. The Chemistry and Technology of Petroleum; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Panariti, N.; del Bianco, A.; Del Piero, G.; Marchionna, M. Petroleum residue upgrading with dispersed catalysts. Part 1. Catalysts activity and selectivity. Appl. Catal. A Gen. 2000, 204, 203–213. [Google Scholar] [CrossRef]
- Suwaid, M.A.; Varfolomeev, M.A.; Al-muntaser, A.A.; Yuan, C.; Starshinova, V.L.; Zinnatullin, A.; Vagizov, F.G.; Rakhmatullin, I.Z.; Emelianov, D.A.; Chemodanov, A.E. In-situ catalytic upgrading of heavy oil using oil-soluble transition metal-based catalysts. Fuel 2020, 281, 118753. [Google Scholar] [CrossRef]
- Kadieva, M.K.; Maximov, A.L.; Kadiev, K.M. Ex-situ synthesis and study of nanosized MO-containing catalyst for petroleum residue hydro-conversion. Catalysts 2019, 9, 649. [Google Scholar] [CrossRef]
- Husein, M.M.; Nassar, N.N. Nanoparticle Uptake by (w/o) Microemulsions. CRC Press 2008, 144, 465–479. [Google Scholar]
- Alemán-Vázquez, L.O.; Cano-Domínguez, J.L.; García-Gutiérrez, J.L. Effect of tetralin, decalin and naphthalene as hydrogen donors in the upgrading of heavy oils. Procedia Eng. 2012, 42, 532–539. [Google Scholar] [CrossRef]
- Golovko, A.K.; Kopytov, M.A.; Sharonova, O.M.; Kirik, N.P.; Anshits, A.G. Cracking of heavy oils using catalytic additives based on coal fly ash ferrospheres. Catal. Ind. 2015, 7, 293–300. [Google Scholar] [CrossRef]
- Zou, R.; Xu, J.; Kuffner, S.; Becker, J.; Li, T.; Guan, X.; Zhang, X.; Li, L.; Cohen Stuart, M.A.; Guo, X. Spherical poly(vinyl imidazole) brushes loading nickel cations as nanocatalysts for aquathermolysis of heavy crude oil. Energy Fuels 2019, 33, 998–1006. [Google Scholar] [CrossRef]
- Topolyuk, Y.A.; Maksimov, A.L.; Kolyagin, Y.G. Catalytic activity of in situ synthesized MoWNi sulfides in hydrogenation of aromatic hydrocarbons. Russ. J. Phys. Chem. A 2017, 91, 205–212. [Google Scholar] [CrossRef]
- Petrukhina, N.N.; Sizova, I.A.; Maksimov, A.L. Nickel–molybdenum and cobalt–molybdenum sulfide hydrogenation and hydrodesulphurization catalysts synthesized in situ from bimetallic precursors. Catal. Ind. 2017, 9, 247–256. [Google Scholar] [CrossRef]
- Sizova, I.A.; Kulikov, A.B.; Onishchenko, M.I.; Serdyukov, S.I.; Maksimov, A.L. Synthesis of nickel-tungsten sulfide hydrodearomatization catalysts by the decomposition of oil-soluble precursors. Pet. Chem. 2016, 56, 44–50. [Google Scholar] [CrossRef]
- Sizova, I.A.; Serdyukov, S.I.; Maksimov, A.L.; Sinikova, N.A. Nickel-tungsten sulfide polyaromatic hydrocarbon hydrogenation nanocatalysts prepared in an ionic liquid. Pet. Chem. 2015, 55, 38–44. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part I—Changes in composition of saturated hydrocarbons. Pet. Sci. Technol. 2018, 36, 1829–1836. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Chemodanov, A.E.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part II—Changes in composition of aromatic hydrocarbons. Pet. Sci. Technol. 2018, 36, 1850–1856. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part III—Changes in composition resins and asphaltenes. Pet. Sci. Technol. 2018, 36, 1857–1863. [Google Scholar] [CrossRef]
- Foss, L.; Petrukhina, N.; Kayukova, G.; Amerkhanov, M.; Romanov, G.; Ganeeva, Y. Changes in hydrocarbon content of heavy oil during hydrothermal process with nickel, cobalt, and iron carboxylates. J. Pet. Sci. Eng. 2018, 169, 269–276. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Vakhin, A.V.; Sitnov, S.A.; Akhmadiayrov, A.A.; Varfolomeev, M.A.; Nurgaliev, D.K. Catalytic heavy oil upgrading by steam injection with using of transition metals catalysts. Neftyanoe Khozyaystvo Oil Ind. 2017, 8, 30–34. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic aquathermolysis of Boca de Jaruco heavy oil with nickel-based oil-soluble catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]
- Elahi, S.M.; Ahmadi Khoshooei, M.; Carbognani Ortega, L.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. Chemical insight into nano-catalytic in-situ upgrading and recovery of heavy oil. Fuel 2020, 278, 118270. [Google Scholar] [CrossRef]
- Nassar, N.N.; Husein, M.M. Ultradispersed particles in heavy oil: Part I, preparation and stabilization of iron oxide/hydroxide. Fuel Process. Technol. 2010, 91, 164–168. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, H. The effect of hydrogen donor additive on the viscosity of heavy oil during steam stimulation. Energy Fuels 2002, 16, 842–846. [Google Scholar] [CrossRef]
- Gould, K.A.; Wiehe, I.A. Natural hydrogen donors in petroleum resids. Energy Fuels 2007, 21, 1199–1204. [Google Scholar] [CrossRef]
- Hyne, J.B.; Clark, P.D. Additive for Inclusion in a Heavy Oil Reservoir Undergoing Steam Injection. U.S. Patent 4,506,733, 26 March 1985. [Google Scholar]
- Hyne, J.B.; Tyrer, J.D. Use of Hydrogen-Free Carbon Monoxide with Steam in Recovery of Heavy Oil at Low Temperatures. U.S. Patent 4,487,264, 11 December 1984. [Google Scholar]
- Carbognani Ortega, L.; Rogel, E.; Perez-Zurita, M.J.; Peluso, E.; Carbognani, J.; Ovalles, C.; Lopez-Linares, F.; Vien, J.; Pradhan, A.; Pereira-Almao, P. Bench-Top Thermal and Steam Catalytic Cracking of Athabasca Residual Fractions: Attainable Upgrading Levels Correlated with Fraction Properties. Energy Fuels 2019, 33, 9351–9362. [Google Scholar] [CrossRef]
- Hewgill, G.S.; Kalfayan, L.J. Enhanced oil recovery technique using hydrogen precursors. U.S. Patent 5105887, 21 April 1992. [Google Scholar]
- Morozov, M.A.; Akimov, A.S.; Zhuravkov, S.P.; Zolotukhina, N.Y.; Sviridenko, N.N.; Golovko, A.K.; Vosmerikov, A.V.; Fedushchak, T.A. Catalytic properties of tungsten carbide powders in cracking heavy petroleum feedstock. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2017, 328, 16–23. [Google Scholar]
- Ferdous, D.; Dalai, A.K.; Adjaye, J.; Kotlyar, L. Surface morphology of NiMo/Al 2O3 catalysts incorporated with boron and phosphorus: Experimental and simulation. Appl. Catal. A Gen. 2005, 294, 80–91. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Pham, V.H.; Kim, D.K.; Kim, D.W.; Oh, S.H.; Chung, J.S.; Kim, W.J.; Shin, E.W. Role of macroporosity in macro-mesoporous red mud catalysts for slurry-phase hydrocracking of vacuum residue. Appl. Catal. A Gen. 2013, 468, 305–312. [Google Scholar] [CrossRef]
- Bano, S.; Ahmad, S.W.; Woo, S.I.; Saleem, F. Heavy oil hydroprocessing: Effect of nanostructured morphologies of MoS2 as catalyst. React. Kinet. Mech. Catal. 2015, 114, 473–487. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Kadiev, K.M.; Zekel, L.A.; Kadieva, M.K. Heavy oil hydroconversion in the presence of ultrafine catalyst. Pet. Chem. 2018, 58, 535–541. [Google Scholar] [CrossRef]
- Contreras, C.; Isquierdo, F.; Pereira-Almao, P.; Scott, C.E. Effect of particle size on the HDS activity of molybdenum sulfide. J. Nanotechnol. 2016, 3752484. [Google Scholar] [CrossRef]
- Looi, P.Y.; Mohamed, A.R.; Tye, C.T. Hydrocracking of residual oil using molybdenum supported over mesoporous alumina as a catalyst. Chem. Eng. J. 2012, 181, 717–724. [Google Scholar] [CrossRef]
- Park, C.; Jung, J.; Lee, C.W.; Cho, J. Synthesis of mesoporous α-Fe2O3 nanoparticles by non-ionic soft template and their applications to heavy oil upgrading. Sci. Rep. 2016, 6, 39136. [Google Scholar] [CrossRef]
- Hur, Y.G.; Kim, M.S.; Lee, D.W.; Kim, S.; Eom, H.J.; Jeong, G.; No, M.H.; Nho, N.S.; Lee, K.Y. Hydrocracking of vacuum residue into lighter fuel oils using nanosheet-structured WS 2 catalyst. Fuel 2014, 137, 237–244. [Google Scholar] [CrossRef]
- Dejhosseini, M.; Aida, T.; Watanabe, M.; Takami, S.; Hojo, D.; Aoki, N.; Arita, T.; Kishita, A.; Adschiri, T. Catalytic cracking reaction of heavy oil in the presence of cerium oxide nanoparticles in supercritical water. Energy Fuels 2013, 27, 4624–4631. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Ahmadi, S.J.; Fatemi, S. Successive co-operation of supercritical water and silica-supported iron oxide nanoparticles in upgrading of heavy petroleum residue: Suppression of coke deposition over catalyst. J. Supercrit. Fluids 2015, 100, 70–78. [Google Scholar] [CrossRef]
- Kadiev, K.M.; Zekel’, L.A.; Kadieva, M.K.; Gyul’maliev, A.M.; Batov, A.E.; Visaliev, M.Y.; Dandaev, A.U.; Magomadov, E.E.; Kubrin, N.A. Effect of Hydroconversion conditions on the composition and properties of an ultrafine mo-containing catalyst formed in situ. Pet. Chem. 2020, 60, 1154–1163. [Google Scholar] [CrossRef]
- Sepelak, V.; Duvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Hyne, J.B.; Greidanus, J.W.; Tyrer, J.D.; Verona, D.; Rizek, C.; Clark, P.D.; Koo, J. Aquathermolysis of heavy oils. Rev. Tec. Intevep 1982, 2, 87–94. [Google Scholar]
- Dobrynkin, N.M.; Batygina, M.V.; Noskov, A.S. Catalytic Redox Transformations in Rock Matrices. Catal. Ind. 2018, 10, 91–96. [Google Scholar] [CrossRef]
- Petrov, S.M.; Abdelsalam, Y.I.I.; Vakhin, A.V.; Baibekova, L.R.; Kayukova, G.P.; Karalin, E.A. Study of the rheological properties of heat-treatment products of asphaltic oils in the presence of rock-forming minerals. Chem. Technol. Fuels Oils 2015, 51, 133–139. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.M.; Kosachev, I.P.; Feoktistov, D.A.; Vakhin, A.V. Conversion of heavy oil with different chemical compositions under catalytic aquathermolysis with an amphiphilic Fe-Co-Cu catalyst and kaolin. Energy Fuels 2018, 32, 6488–6497. [Google Scholar] [CrossRef]
- Elahi, S.M.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. In-situ upgrading and enhanced recovery of heavy oil from carbonate reservoirs using nano-catalysts: Upgrading reactions analysis. Fuel 2019, 252, 262–271. [Google Scholar] [CrossRef]
- Yeletsky, P.M.; Reina, T.R.; Bulavchenko, O.A.; Saraev, A.A.; Gerasimov, E.Y.; Zaikina, O.O.; Bermúdez, J.M.; Arcelus-Arrillaga, P.; Yakovlev, V.A.; Millan, M. Phenanthrene catalytic cracking in supercritical water: Effect of the reaction medium on NiMo/SiO2 catalysts. Catal. Today 2019, 329, 197–205. [Google Scholar] [CrossRef]
- Fan, H.F.; Liu, Y.J.; Zhong, L.G. Studies on the synergetic effects of mineral and steam on the composition changes of heavy oils. Energy Fuels 2001, 15, 1475–1479. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Y.; Lin, Y. The catalytic effects of minerals on aquathermolysis of heavy oils. Fuel 2004, 83, 2035–2039. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Onishchenko, Y.V.; Chemodanov, A.E.; Sitnov, S.A.; Mukhamatdinov, I.I.; Nazimov, N.A.; Sharifullin, A.V. The composition of aromatic destruction products of Domanic shale kerogen after aquathermolysis. Pet. Sci. Technol. 2019, 37, 390–395. [Google Scholar] [CrossRef]
- Fan, H.; Liu, Y. Downhole catalyst upgrades heavy oil. Oil Gas J. 2002, 100, 60–62. [Google Scholar]
- Chao, K.; Chen, Y.; Liu, H.; Zhang, X.; Li, J. Laboratory experiments and field test of a difunctional catalyst for catalytic aquathermolysis of heavy oil. Energy Fuels 2012, 26, 1152–1159. [Google Scholar] [CrossRef]
- Russia’s Zarubezhneft Launches First Well in Boca de Jaruco oil Field. Available online: https://www.caribbean-council.org/russias-zarubezhneft-launches-first-well-in-boca-de-jaruco-oil-field/ (accessed on 29 December 2020).
- Chen, Y.; Wang, Y.; Wu, C.; Xia, F. Laboratory experiments and field tests of an amphiphilic metallic chelate for catalytic aquathermolysis of heavy oil. Energy Fuels 2008, 22, 1502–1508. [Google Scholar] [CrossRef]
- Mohammad, A.A.; Mamora, D.D. In situ upgrading of heavy oil under steam injection with tetralin and catalyst. In Proceedings of the International Thermal Operations and Heavy Oil Symposium, Calgary, AB, Canada, 20–23 October 2008. [Google Scholar]
- Wolf-Gladrow, D.A. Lattice-Gas Cellular Automata and Lattice Boltzmann Models—An Introduction; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Succi, S. The Lattice Boltzmann Equation for Fluid Dynamics and Beyond; Oxford University Press: Oxford, UK, 2001. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals. Processes 2021, 9, 127. https://doi.org/10.3390/pr9010127
Aliev FA, Mukhamatdinov II, Sitnov SA, Ziganshina MR, Onishchenko YV, Sharifullin AV, Vakhin AV. In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals. Processes. 2021; 9(1):127. https://doi.org/10.3390/pr9010127
Chicago/Turabian StyleAliev, Firdavs A., Irek I. Mukhamatdinov, Sergey A. Sitnov, Mayya R. Ziganshina, Yaroslav V. Onishchenko, Andrey V. Sharifullin, and Alexey V. Vakhin. 2021. "In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals" Processes 9, no. 1: 127. https://doi.org/10.3390/pr9010127
APA StyleAliev, F. A., Mukhamatdinov, I. I., Sitnov, S. A., Ziganshina, M. R., Onishchenko, Y. V., Sharifullin, A. V., & Vakhin, A. V. (2021). In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals. Processes, 9(1), 127. https://doi.org/10.3390/pr9010127