Biodiesel from Crude Tall Oil and Its NOx and Aldehydes Emissions in a Diesel Engine Fueled by Biodiesel-Diesel Blends with Water Emulsions
Abstract
Highlights
- Biodiesel was produced from crude tall oil by acid esterification reaction.
- Biodiesel blends emulsified with 5% and 10% water and tested for NOx and aldehydes.
- Maximum NOx reduction of about 15% was achieved with 10% water-emulsion.
- Diesel-biodiesel emulsions’ aldehydes were measured, which is the novelty of this study.
- Maximum aldehyde reduction of 90% was achieved with 10% water with biodiesel.
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Tall Oil Fatty Acids (TOFA) from CTO
2.3. Production of Biodiesel from TOFA
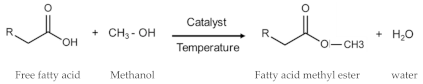
2.4. Biodiesel Blends with Emulsification
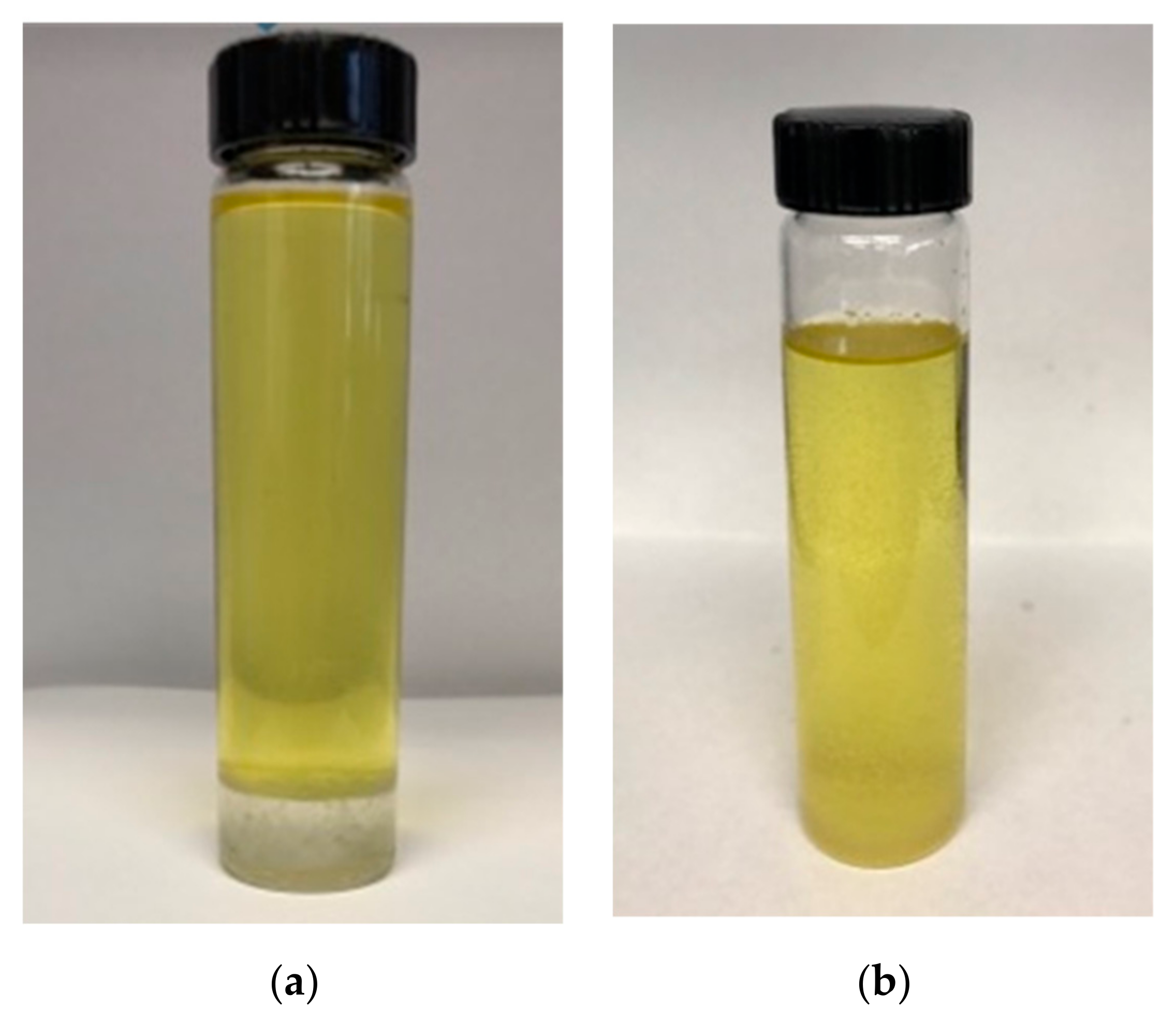
2.5. Emulsion Stability Test
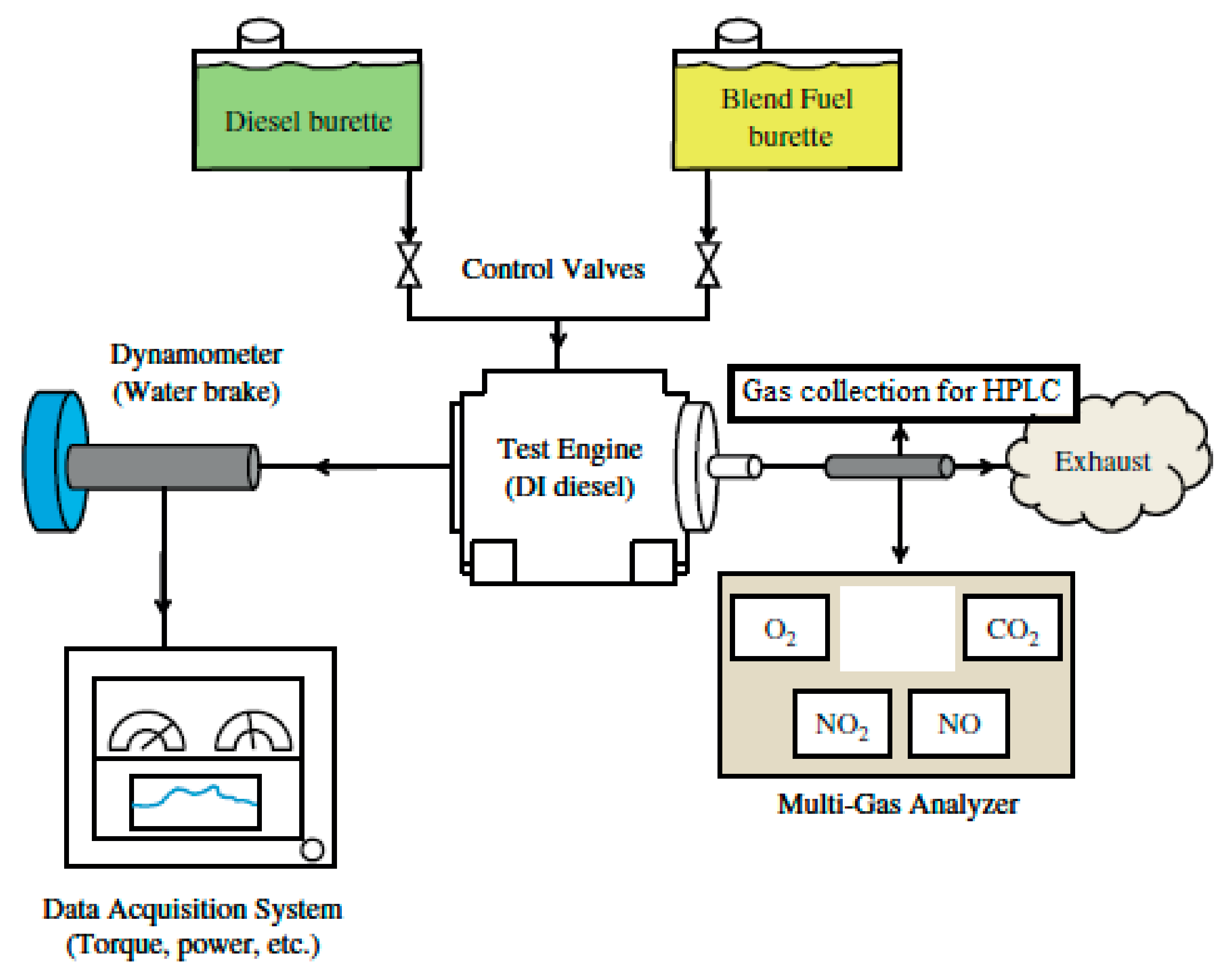
2.6. Engine Specifications and Setup
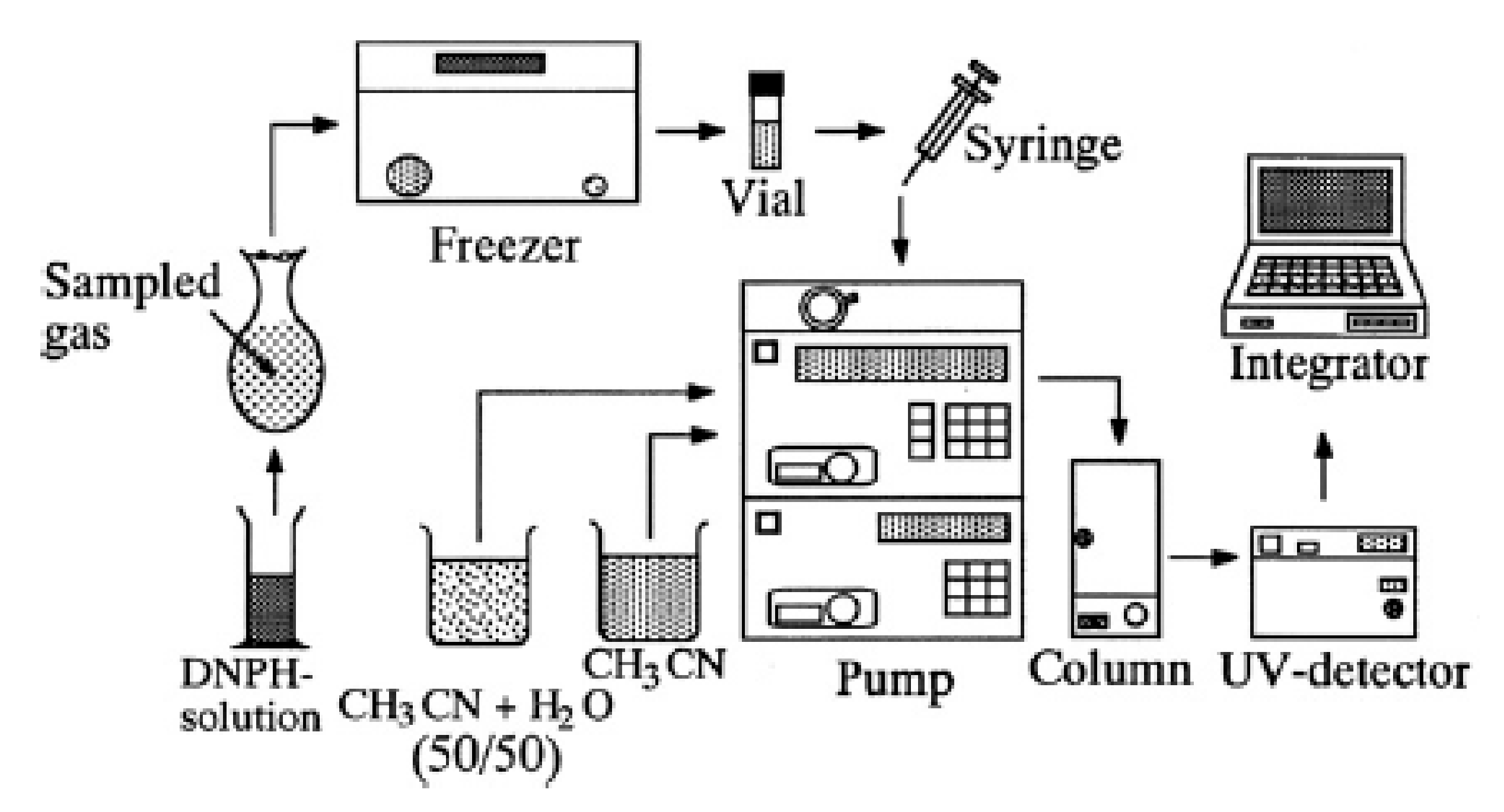
2.7. High Pressure Liquid Chromotagraphy (HPLC) for Aldehyde Analysis
3. Results
3.1. Fuel Properties
3.2. Emissions
3.2.1. NO, NO2 and NOx Emissions
3.2.2. Aldehydes Analysis of Exhaust Gas
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheehan, J.; Camobreco, V.; Duffield, J.; Graboski, M.; Shapouri, H. Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus. Final Report for US Department of Energy’s Office of Fuel Development and the US Department of Agriculture’s Office of Energy by the National Renewable Energy Laboratory. NERL/SR-580-24089. 1988. Available online: https://doi.org/10.2172/658310 (accessed on 1 May 1998).
- US Environmental Protection Agency. A Comprehensive Analysis of Biodiesel Impacts on Exhaust Emissions. EPA420-P-02-001. 2002. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P1001ZA0.PDF?Dockey=P1001ZA0.PDF (accessed on 1 October 2002).
- Kannan, G.R.; Karvembu, R.; Anand, R. Effect of metal-based additive on performance emission and combustion characteristics of diesel engine fueled with biodiesel. Appl. Energy 2011, 88, 3694–3703. [Google Scholar] [CrossRef]
- Di, Y.; Cheung, C.; Huang, Z. Experimental investigation on regulated and unregulated emissions of a diesel engine fueled with ultra-low sulfur diesel fuel blended with biodiesel from waste cooking oil. Sci. Total. Environ. 2009, 407, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Buyukkaya, E. Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2010, 89, 3099–3105. [Google Scholar] [CrossRef]
- Ng, J.-H.; Ng, H.K.; Gan, S. Characterization of engine-out responses from a light duty diesel engine fueled with palm methyl ester (PME). Appl. Energy 2012, 90, 58–67. [Google Scholar] [CrossRef]
- Hazar, H. Cotton methyl ester usage in a diesel engine equipped with insulated combustion chamber. Appl. Energy 2010, 87, 134–140. [Google Scholar] [CrossRef]
- Sun, J.F.; Caton, J.A.; Jacobs, T.J. Oxides of nitrogen emissions from biodiesel-fueled diesel engines. Prog. Energy Combust. Sci. 2010, 36, 677–695. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, S.R.; Goel, V.; Gupta, A.K. Enhancing Diesel Engine Performance and Reducing Emissions Using Binary Biodiesel Fuel Blend. J. Energy Resour. Technol. 2020, 142, 1–28. [Google Scholar] [CrossRef]
- Roy, M.M. Charcoal-adsorption, water-washing, and air-dilution systems to reduce diesel emissions with special emphasis on odor reduction. J. Energy Resour. Technol. 2007, 129, 338–347. [Google Scholar] [CrossRef]
- Roy, M.M.; Parvez, R.; Sarker, R.I. Exhaust odor and smoke rduction of stationary DI diesel engines to acceptable level by water-scrubbing and air-dilution system. Appl. Energy 2011, 88, 2391–2399. [Google Scholar] [CrossRef]
- Kameoka, Y.; Pigford, R.L. Absorption of Nitrogen Dioxide into Water, Sulfuric Acid, Sodium Hydroxide, and Alkaline Sodium Sulfite Aqueous Solutions. Ind. Eng. Chem. Fundam. 1977, 16, 163–169. [Google Scholar] [CrossRef]
- Abdul Karim, Z.A.; Khan, M.Y.; Aziz, A.R.A. Evolution of microexplosion phenomenon in parent–child droplets of water in biodiesel emulsions enhanced by different surfactant dosages and hydrophilic–lipophilic balance values. J. Energy Resour. Technol. 2019, 141, 102204–102217. [Google Scholar] [CrossRef]
- Senthil, R.; Arunan, K.; Silambarasan, R. Experimental investigation of a diesel engine fueled with emulsified biodiesel. Int. J. ChemTech Res. 2015, 8, 190–195. [Google Scholar] [CrossRef]
- Cook, D.H.; Law, C.K. A preliminary study on the utilization of water-in-oil emulsions in diesel engines. Combust. Sci. Technol. 1978, 18, 217–221. [Google Scholar] [CrossRef]
- Law, C.K. A model for the combustion of oil/water emulsion droplets. Combust. Sci. Technol. 1977, 17, 29–38. [Google Scholar] [CrossRef]
- Lawson, A.; Vergeer, E.C.; Mitchell, E.W.; Dainty, E.D. Heavy-Duty Diesel Emission Control: A Review of Technology; CIM: Montreal, QC, Canada, 1986; Volume 36, p. 238. [Google Scholar]
- Srivastava, S.P. AQUAZOLE: Practical Experience Using a Diesel/Water Emulsion. Int. Symp. Fuels Lubr. 2000, 2, 435–444. [Google Scholar] [CrossRef]
- Elsanusi, O.A.; Roy, M.M.; Sidhu, M.S. Experimental investigation on a diesel engine fueled by diesel-biodiesel blends and their emulsions at various engine operating conditions. Appl. Energy 2017, 203, 582–593. [Google Scholar] [CrossRef]
- Rounds, F.G.; Pearsall, H.W. Diesel Exhaust Odor Its Evaluation and Relation to Exhaust Gas Composition. SAE Tech. Pap. Ser. 1957, 65, 608–627. [Google Scholar] [CrossRef]
- Tanaka, T.; Kobashi, K.; Sami, H. Development of a diesel odor measurement method and its application to odor reduction. SAE Tech. Pap. 1992, 920726. [Google Scholar] [CrossRef]
- Roy, M.M.; Tsunemoto, H.; Ishitani, H.; Akiyama, J.; Minami, T.; Noguchi, M. Influence of Aldehyde and Hydrocarbon Components in the Exhaust on Exhaust Odor in DI Diesel Engines. SAE Tech. Pap. Ser. 2000, 4, 2398–2405. [Google Scholar] [CrossRef]
- Roy, M.M.; Tsunemoto, H. Investigation of odorous components and improvement in odor assessment procedure in DI diesel engines. SAE Tech. Pap. 2002, 1, 2875. [Google Scholar] [CrossRef]
- Shiozaki, T. Study of the white smoke and the odor of DI diesel engines in a cold weather environment. Proc. JSAE 1991, 912247, 57–60. [Google Scholar]
- Roy, M.M. Normal heptane-diesel combustion and odorous emissions in direct-injection diesel engines. J. Energy Resour. Technol. 2008, 13, 11101–11108. [Google Scholar] [CrossRef]
- Stephenson, R.M. Mutual solubility of water and aldehydes. J. Chem. Eng. Data 1993, 38, 630–633. [Google Scholar] [CrossRef]
- Roy, M.M. Effect of engine operation parameters, fuel property and exhaust gas treatment on exhaust odor in direct injection diesel engines. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2006, 220, 595–609. [Google Scholar] [CrossRef]
- Roy, M.M. Performance and emissions of a diesel engine fueled by diesel-biodiesel blends with special attention to exhaust odor. Can. J. Mech. Sci. Eng. 2011, 2, 1–10. [Google Scholar] [CrossRef]
- Ruina, L.; Wang, Z.; Xu, G. Study on Carbonyl Emissions of Diesel Engine Fueled with Biodiesel. Int. J. Chem. Eng. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Yang, H.-H.; Lan, C.-H.; Chien, S.-M. Effects of the biodiesel blend fuel on aldehyde emissions from diesel engine exhaust. Atmos. Environ. 2008, 42, 906–915. [Google Scholar] [CrossRef]
- Adewale, P.; Vithanage, L.N.; Christopher, L. Optimization of enzyme-catalyzed biodiesel production from crude tall oil using Taguchi method. Energy Convers. Manag. 2017, 154, 81–91. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M.; Altıparmak, D. Biodiesel production from tall oil with synthesized Mn and Ni based additives: Effects of the additives on fuel consumption and emissions. Fuel 2007, 86, 1139–1143. [Google Scholar] [CrossRef]
- Islam, M.S.; Christopher, L.P.; Alam, M.N. Separation and purification of ω-6 linoleic acid from crude tall oil. Separations 2020, 7, 9. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P. Preparation and characterization of nano-sized phase change emulsions as thermal energy storage and transport media. Appl. Energy 2017, 190, 868–879. [Google Scholar] [CrossRef]
- Roy, M.M.; Wang, W.; Bujold, J. Biodiesel production and comparison of emissions of a DI diesel engine fueled by biodiesel–diesel and canola oil–diesel blends at high idling operations. Appl. Energy 2013, 106, 198–208. [Google Scholar] [CrossRef]
- California Air Resources Board. California Non-Methane Organic Gas Test Procedure. 1993. Available online: https://ww2.arb.ca.gov/sites/default/files/2019-09/nmogtps_2017%2B%20MY_clean%20complete_10-15_accessible.pdf (accessed on 2 September 2015).
- Iwamoto, A.; Sasaki, S.; Tsuruga, F. Sampling and HPLC analysis of aldehydes in automobile exhaust gas. J. Jpn. Automob. Res. Inst. 1996, 18, 22–27. [Google Scholar] [CrossRef]
- Roy, M.M. HPLC analysis of aldehydes in automobile exhaust gas: Comparison of exhaust odor and irritation in different types of gasoline and diesel engines. Energy Convers. Manag. 2008, 49, 1111–1118. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2005, 17, 15–27. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Roy, M.M.; Wang, W. Glycerine emulsions of diesel-biodiesel blends and their performance and emissions in a diesel engine. Appl. Energy 2018, 230, 148–159. [Google Scholar] [CrossRef]








| Engine Parameter | Specifications |
|---|---|
| Engine make and model | HATZ 2G40 |
| Engine type | 4-stroke, air-cooled |
| Number of cylinders | 2 |
| Bore/stroke | 92 mm/75 mm |
| Displacement | 997 cc |
| Compression ratio | 20.5:1 |
| Mean piston speed at 3000 rpm | 7.5 m/s |
| Continuous rated power | 13.2 kW @ 3000 rpm |
| Maximum power | 15.6 kW @ 3600 rpm |
| Maximum torque | 50.1 N.m @ 2100 rpm |
| Analysis Method | HPLC-UV |
|---|---|
| Main column | Zorbax Eclipse XDB-C18 |
| Guard column | Inertsil ODS 1 cm |
| Column temperature | 50 °C |
| Mobile phase | 50%:50% = water:acetonitrile |
| Flow rate of sample | 0.8 mL/min |
| Total time | 20 min |
| Wavelength | 365 nm |
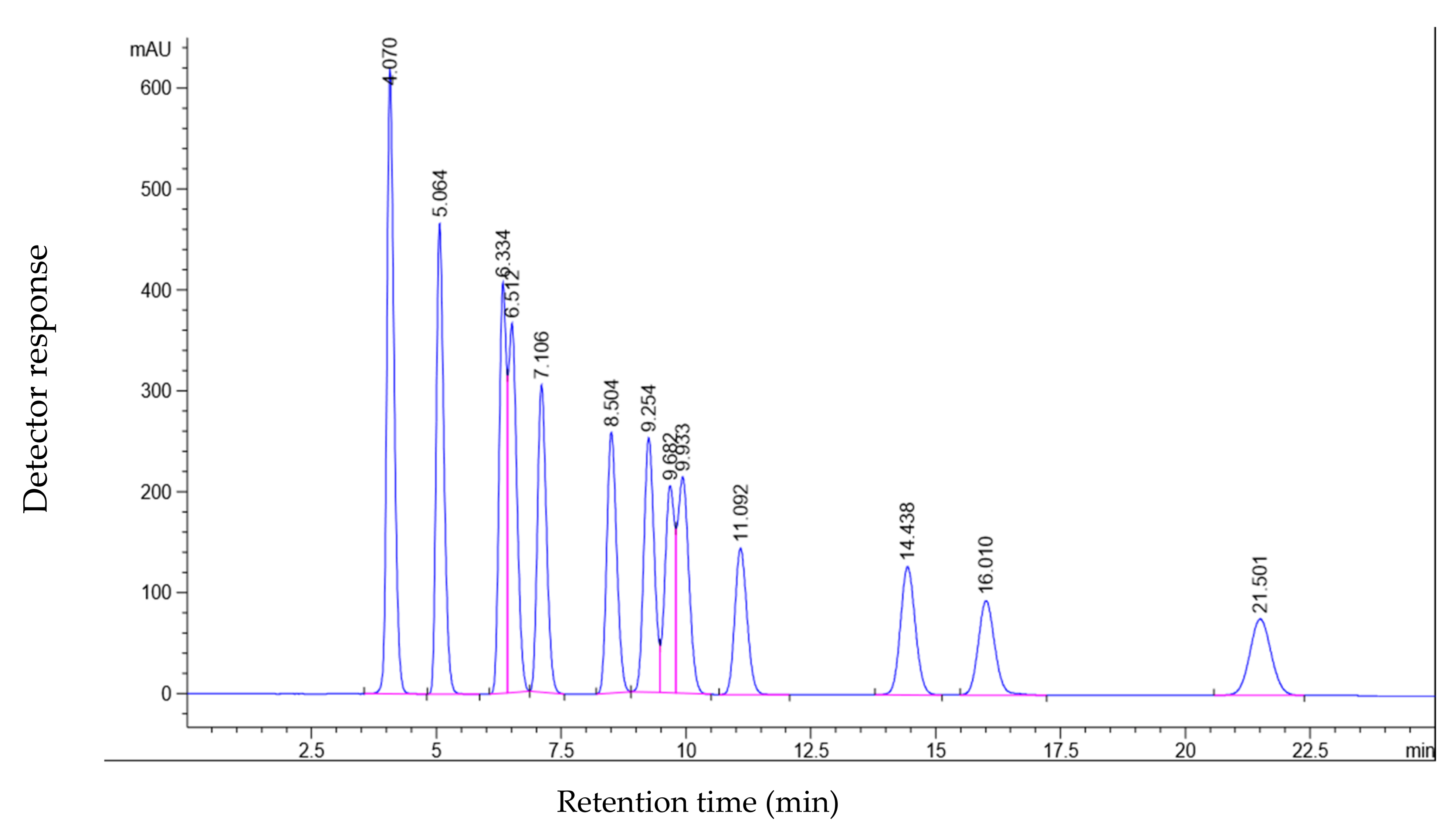
| Peak No. | Identification | Retention Time (min) |
|---|---|---|
| 1 | Formaldehyde | 4.070 |
| 2 | Acetaldehyde | 5.064 |
| 3 | Acetone | 6.334 |
| 4 | Acrolein | 6.512 |
| 5 | Propionaldehyde | 7.106 |
| 6 | Crotanaldehyde | 8.504 |
| 7 | 2-Butanone | 9.254 |
| 8 | Methacrolein | 9.682 |
| 9 | n-Butyraldehyde | 9.933 |
| 10 | Benzaldehyde | 11.092 |
| 11 | Valeraldehyde | 14.438 |
| 12 | m-Tolualdehyde | 16.010 |
| 13 | Hexanaldehyde | 21.501 |
| Name of Properties | Testing Method | Biodiesel |
|---|---|---|
| Cetane index | ASTM D4737 | 50.84 |
| Glycerol content | ASTM D7637 | 0 |
| Cloud point | ASTM D2500 | −5 °C |
| Copper corrosion | ASTM D130 | 1 |
| Acid number | Acid base titration | 0.46 mg KOH/g |
| Kinematic viscosity | ASTM D445 | 5.7 cSt |
| Calorific value | Bomb calorimeter | 41.3 MJ |
| Water and sediment | ASTM D1796 | <0.005% |
| Flash point | ASTM D93 | 87 °C |
| Sulphur content | ASTM D5453 | 1200 ppm |
| Carbon residue | ASTM D189 | 0.01% |
| Sulfated ash | ASTM D874 | 0.01% |
| Name of Fuel | Density (kg/m3) | Heating Value (MJ/kg) | Viscosity (cSt @ 40 °C) |
|---|---|---|---|
| B0 W0% | 840 | 43.2 | 1.74 |
| B0 W5% | 857 | 41.1 | 2.46 |
| B0 W10% | 876 | 39.3 | 2.67 |
| B20 W0% | 847 | 42.82 | 2.24 |
| B20 W5% | 875 | 40.75 | 2.87 |
| B20 W10% | 881 | 38.95 | 2.96 |
| B50 W0% | 856 | 42.25 | 3.02 |
| B50 W5% | 878 | 40.2 | 3.62 |
| B50 W10% | 884 | 38.4 | 3.78 |
| B100 W0% | 867 | 41.3 | 5.70 |
| B100 W5% | 883 | 39.3 | 5.81 |
| B100 W10% | 887 | 37.6 | 6.0 |
| Name of Fuel | Area under Formaldehyde Peak | Formaldehyde Concentration (μg/mL) | Area under Acetaldehyde Peak | Acetaldehyde Concentration (μg/mL) | Formaldehyde + Acetaldehyde in Exhaust Gas (mg/m3) |
|---|---|---|---|---|---|
| B0 W0% | 2584.32 | 6.210 | 97.77 | 0.241 | 12.420 ± 0.482 |
| B0 W5% | 853.16 | 2.005 | 55.78 | 0.114 | 4.010 ± 0.228 |
| B0 W10% | 432 | 0.988 | 44.09 | 0.078 | 1.976 ± 0.156 |
| B20 W0% | 2464.41 | 5.919 | 177.58 | 0.483 | 11.838 ± 0.966 |
| B20 W5% | 486.4 | 1.120 | 32.64 | 0.044 | 2.240 ± 0.088 |
| B20 W10% | 301.43 | 0.671 | 28.20 | 0.030 | 1.342 ± 0.060 |
| B50 W0% | 1866.8 | 4.469 | 238.3 | 0.667 | 8.938 ± 1.334 |
| B50 W5% | 468.5 | 1.076 | 26.09 | 0.024 | 2.152 ± 0.048 |
| B50 W10% | 219.3 | 0.472 | 22.68 | 0.013 | 0.944 ± 0.026 |
| B100 W0% | 1686.61 | 4.032 | 81.07 | 0.190 | 8.064 ± 0.380 |
| B100 W5% | 342.47 | 0.771 | 24.95 | 0.020 | 1.542 ± 0.040 |
| B100 W10% | 187.67 | 0.395 | 18.98 | 0.002 | 0.790 ± 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, M.M.; Islam, M.S.; Alam, M.N. Biodiesel from Crude Tall Oil and Its NOx and Aldehydes Emissions in a Diesel Engine Fueled by Biodiesel-Diesel Blends with Water Emulsions. Processes 2021, 9, 126. https://doi.org/10.3390/pr9010126
Roy MM, Islam MS, Alam MN. Biodiesel from Crude Tall Oil and Its NOx and Aldehydes Emissions in a Diesel Engine Fueled by Biodiesel-Diesel Blends with Water Emulsions. Processes. 2021; 9(1):126. https://doi.org/10.3390/pr9010126
Chicago/Turabian StyleRoy, Murari Mohon, Md Shariful Islam, and Md Nur Alam. 2021. "Biodiesel from Crude Tall Oil and Its NOx and Aldehydes Emissions in a Diesel Engine Fueled by Biodiesel-Diesel Blends with Water Emulsions" Processes 9, no. 1: 126. https://doi.org/10.3390/pr9010126
APA StyleRoy, M. M., Islam, M. S., & Alam, M. N. (2021). Biodiesel from Crude Tall Oil and Its NOx and Aldehydes Emissions in a Diesel Engine Fueled by Biodiesel-Diesel Blends with Water Emulsions. Processes, 9(1), 126. https://doi.org/10.3390/pr9010126




