1. Introduction
Antimony (Sb) has been used extensively worldwide in various commercial applications, including the manufacture of chemicals, plastics, batteries, ceramics, semiconductor materials, and flame retardants [
1,
2]. As a result, inorganic antimony (trivalent antimony (Sb[III]) and pentavalent antimony (Sb[V])) shows elevated concentrations in natural water bodies in different parts of the world. For example, Sb concentrations of 239, 6384, and 157 µg/L have been found in Stampede and Slate Creek watersheds (Alaska, USA), rivers around antimony mines in the Xikuangshan area (Hunan Province, China), and lakes in the South East region (Sindh, Pakistan), respectively [
3,
4,
5]. Oral uptake of water-soluble Sb into the human body imparts toxic health impacts, including abdominal cramps, cardiac toxicity, vomiting, and diarrhea [
6]. Therefore, Sb is considered to be a pollutant of high priority interest by the United States Environmental Protection Agency (USEPA) and the European Union (EU) [
7]. To protect human health and the environment, the USEPA (6 µg/L), EU (10 µg/L), World Health Organization (WHO) (5 µg/L), South Korea (20 µg/L), and Pakistan (5 µg/L) have set regulatory standards for Sb in drinking water [
8,
9,
10].
Antimony is an emerging pollutant. It exist as Sb(III) under anoxic conditions and Sb(V) in aerobic waters, leading to long and persistent contamination when it is released into an aqueous environment [
11]. Several treatment techniques including membrane separation, coagulation, adsorption, ion exchange, phytoremediation, and electrochemical methods have been extensively applied for the removal of Sb from drinking water [
12]. However, coagulation-flocculation-sedimentation (C/F/S) processes are still favored by the drinking water industry. They have been identified as cost-effective and efficient treatment technologies to remove several heavy metals from water [
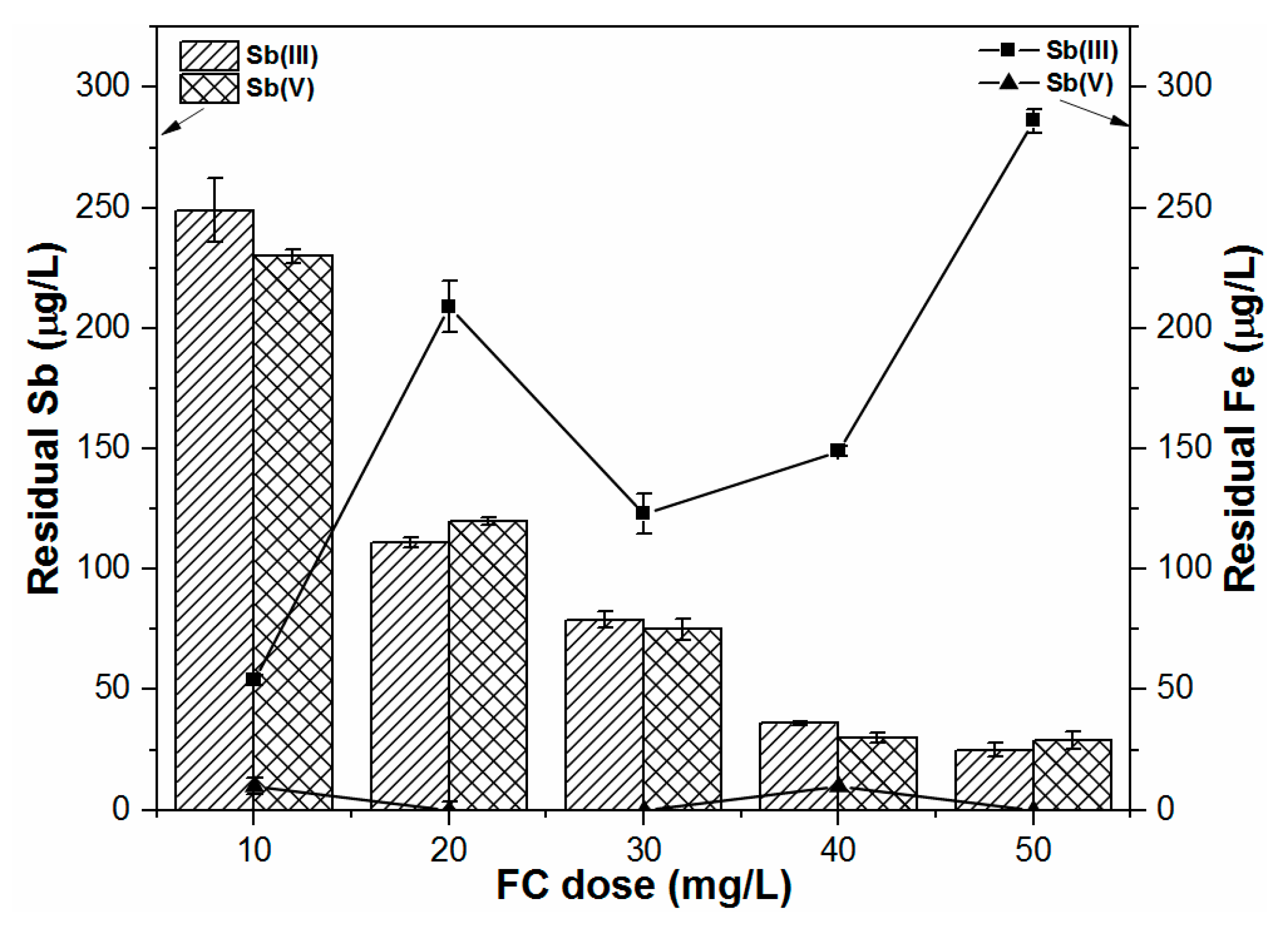
13]. During the removal process, coagulant type and dosage, pH, and initial contaminant loading are key factors in determining the overall C/F/S performance of heavy metals in water. For instance, extensively used commercial coagulants such as ferric chloride (FC) and ferric sulfate (FS) have demonstrated more efficient Sb removal than aluminum-based coagulants [
12,
13]. High Sb(III) removal has been previously reported over a broad pH range (4–10). However, a significant decline in Sb(V) removal at alkaline pH conditions has been observed owing to a significant decrease in Fe solubility [
14,
15,
16]. Higher FC coagulant doses in various aqueous environments lead to greater removal of both Sb(III, V) species [
11,
12,
15]. The initial Sb loading affects the overall C/F/S performance, with higher removal for solutions with higher initial Sb(III, V) concentration than those with lower contaminant loading [
11,
12]. The treatment of Sb-rich water has been addressed in a number of studies [
11,
12,
14,
15,
16,
17] using the C/F/S process. However, previous studies are limited to “one-factor-at-a-time” experiments to determine the coagulation behavior of Sb species in water. Studies that comprehensively determine the interactive effect of major operating parameters on Sb removal involved in the coagulation process are insufficient. Therefore, it is essential to explore an experimental approach for simulating Sb removal by C/F/S.
Toxic Sb species pose substantial threats to human health and the environment. Therefore, their removal from drinking water supplies by C/F/S is of essential importance. Furthermore, it is necessary to optimize the treatment efficiency of the C/F/S process by taking into consideration the concentration and characteristics of Sb species in order to achieve high Sb coagulation efficiency. The jar test procedure has been extensively employed to optimize Sb removal performance by a C/F/S process [
11,
12,
14,
15,
16,
17,
18]. This classical approach is also referred to as the one-factor-at-a-time method, changing the level of one factor while keeping other factors constant. However, it is usually incapable of considering the interactive behavior of various operating factors. Thus, it is an insignificant approach to determine optimum conditions [
19,
20,
21]. To overcome such a problem, numerous statistical and mathematical models have been developed for analyzing and optimizing experimental operating factors. For such a purpose, the Box–Behnken statistical experiment design (BBD) is a classical response surface methodology (RSM) used for modeling and analyzing experimental data [
22]. The RSM is an empirical statistical technique for designing experiments, building models, evaluating the effect of variables, and searching for the optimum conditions of variables to predict targeted responses. It uses regression analysis of experimental data to solve a system of equations to obtain a single function response (the dependent variable examined), which can be graphed as a response surface [
23,
24,
25]. It does not require a large number of runs. It does not require too many levels of independent variables either [
26].
Up to date, a limited number of research studies have been conducted to investigate the coagulation process in drinking water using the RSM technique. Previously, the RSM technique has been applied to optimize the coagulation conditions for arsenic (As) removal using various coagulants [
25,
27]. The interaction of As and natural organic matter has also been investigated using RSM. The model has been validated with real groundwater samples containing both As and organic matter [
28]. To the best of our knowledge, studies that simulate the C/F/S performance of Sb by FC coagulant using RSM have not been reported yet. It is essential to systematically investigate the removal performance of Sb by the C/F/S process using a mathematical modeling approach.
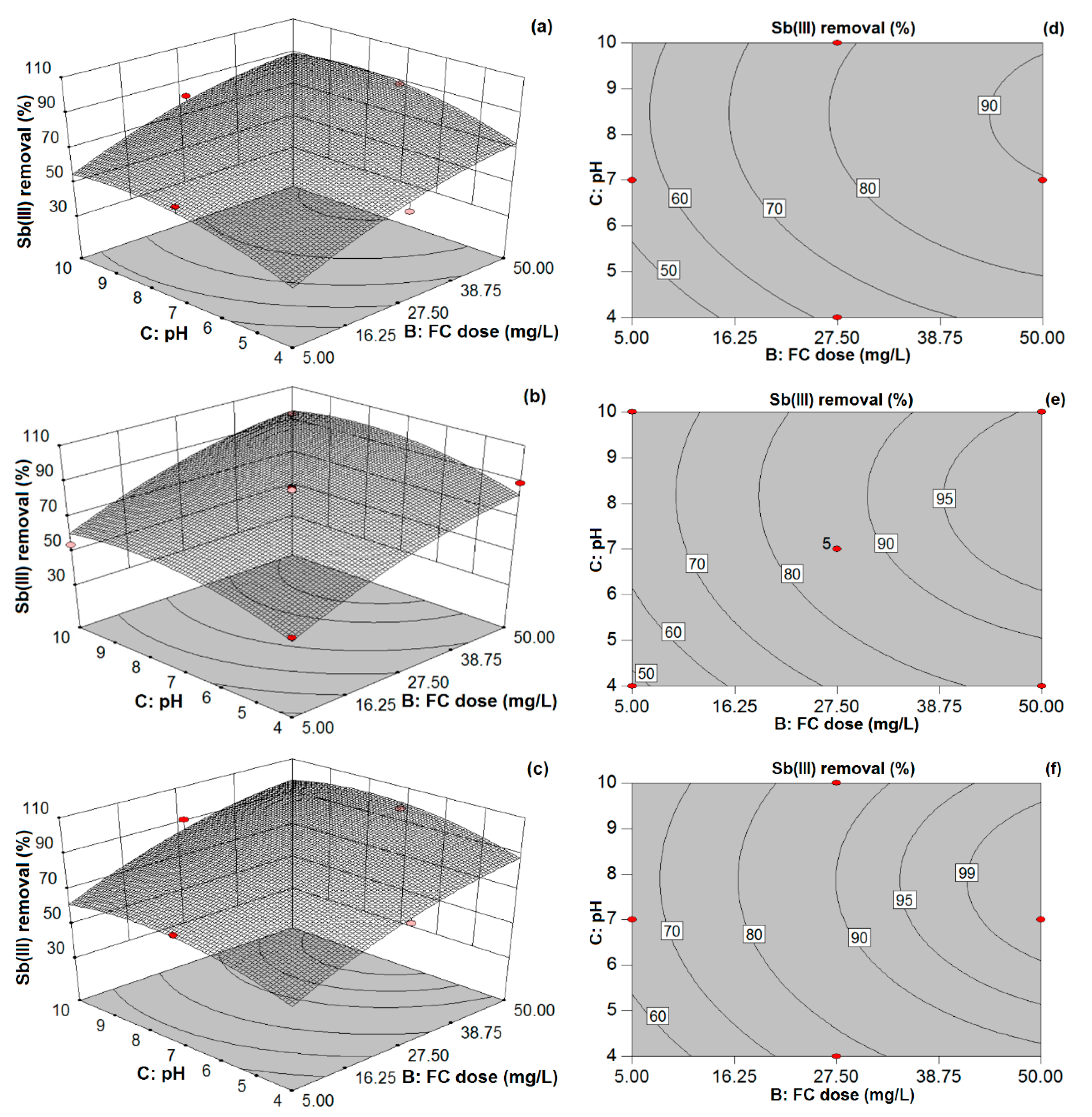
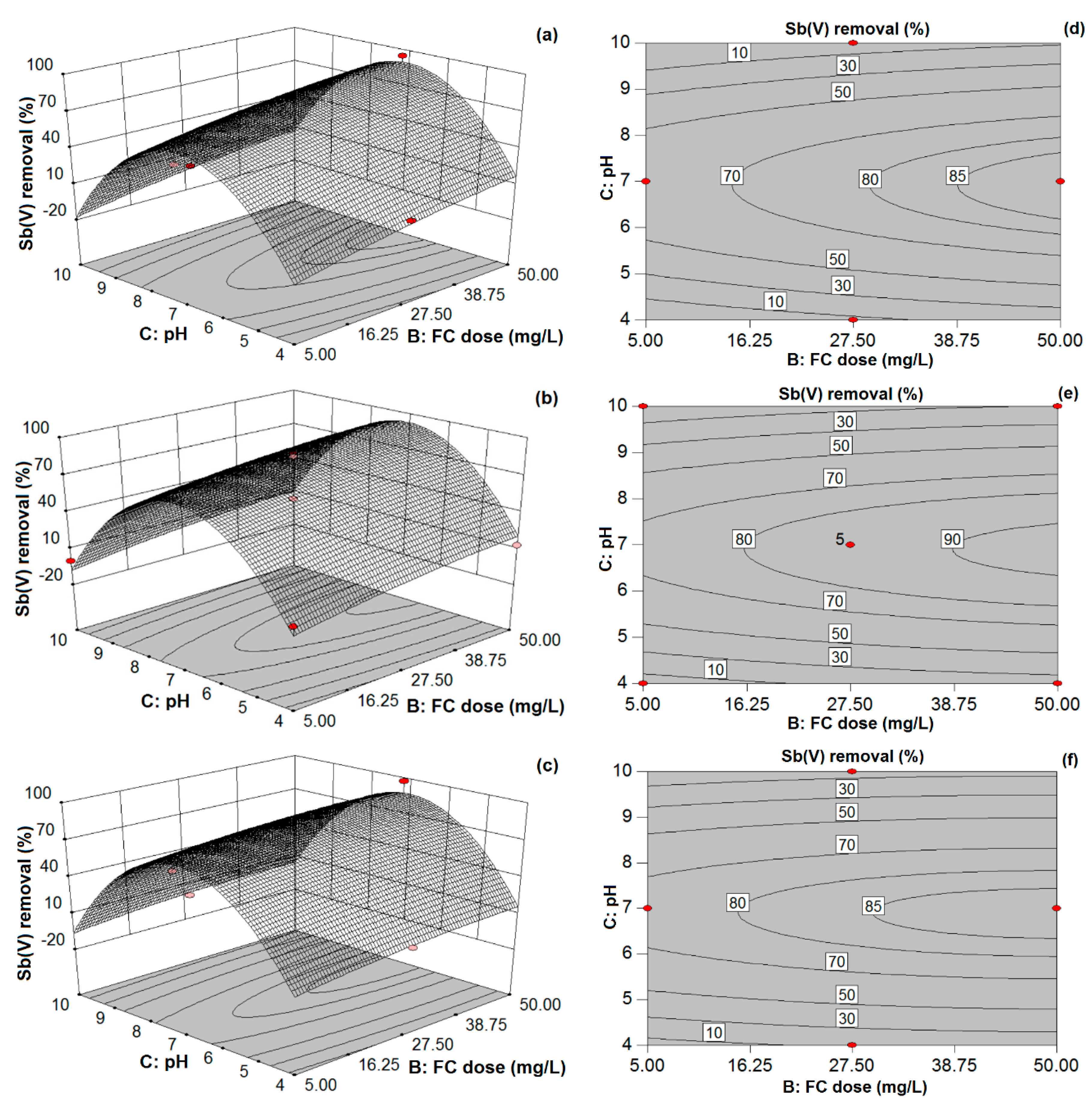
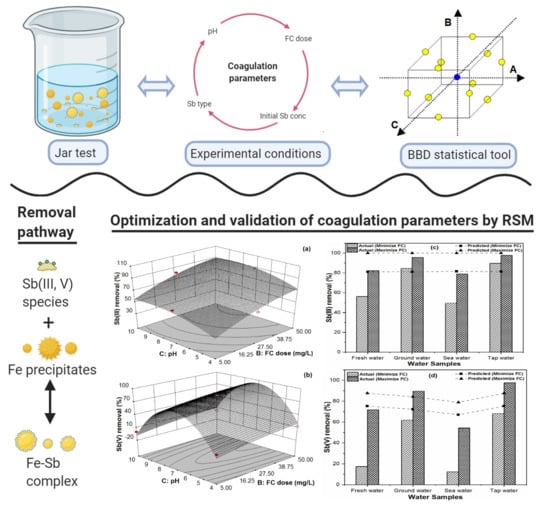
Accordingly, the aim of the present study was to explore the removal capability of redox Sb(III, V) species from aqueous solutions by coprecipitation-adsorption using FC as a coagulant. A Box–Behnken experimental design was used to investigate the effects of various coagulation factors such as pH, FC coagulant doses, and initial Sb(III, V) concentration on the removal efficiency of Sb and to find desirable operating conditions for achieving the maximum Sb removal. Secondly, the adequacy of the model and the reliability of statistical analysis with various experimental data points were determined by comparing the experimental and predicted response values of Sb removal efficiencies. Lastly, observed and modeled removal response values for real water matrices were compared to further illustrate the suitability of the model for Sb removal from drinking water using the C/F/S process.
4. Conclusions
In this study, the coprecipitation-adsorption method was employed to determine the effects of various parameters (i.e., Sb(III, V) concentration, FC dose, and pH) on Sb(III, V) removal to achieve statistically reliable results for both pollutants by FC coagulation. BBD and RSM techniques were applied to determine the optimum C/F/S process conditions for maximizing Sb(III, V) removal from water. The obtained results suggest the potential feasibility of the statistical design approach for Sb(III, V) removal and its usefulness for determining the optimal conditions during Sb removal by FC coagulation with limited experimental trials. Experimental results illustrate that FC is an efficient and effective coagulant for Sb(III, V) removal in respect of the required FC dose, residual Fe and Sb(III, V) concentrations. The optimum pH range obtained for Sb(III, V) removal by the C/F/S process was between 6.5 and 7.5. In order to maximize the Sb(III, V) removal performance (88–98%), model responses suggested that the required FC doses were 50 and 40.39 mg/L for low and high initial Sb(III) loading, respectively, while a 50 mg/L FC dose was required for the studied Sb(V) concentration (100–1000 µg/L). Further, the applicability of the model was assessed for different real water samples, in which ground water and tap water spiked with Sb(III, V) solutions showed good agreement for Sb(III, V) removal. However, discrepancies in Sb(III, V) removal were observed for freshwater and sea water samples owing to other influential parameters not incorporated in the current model. Therefore, future research shall focus on complex environmental waters to evaluate the optimum C/F/S conditions for Sb(III, V) removal using statistical modeling.