Identifying Shared Risk Genes between Nonalcoholic Fatty Liver Disease and Metabolic Traits by Cross-Trait Association Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Linkage Disequilibrium Score Regression Analysis
2.3. Multitrait Association Analysis
2.4. Cross-Trait Meta-Analysis
2.5. Genome-Wide Gene Test
2.6. Transcriptome-Wide Association Study, Pathway Enrichment Analysis, and Protein–Protein Interaction Network Analysis
3. Results
3.1. Genetic Correlation between NAFLD and Metabolic Traits
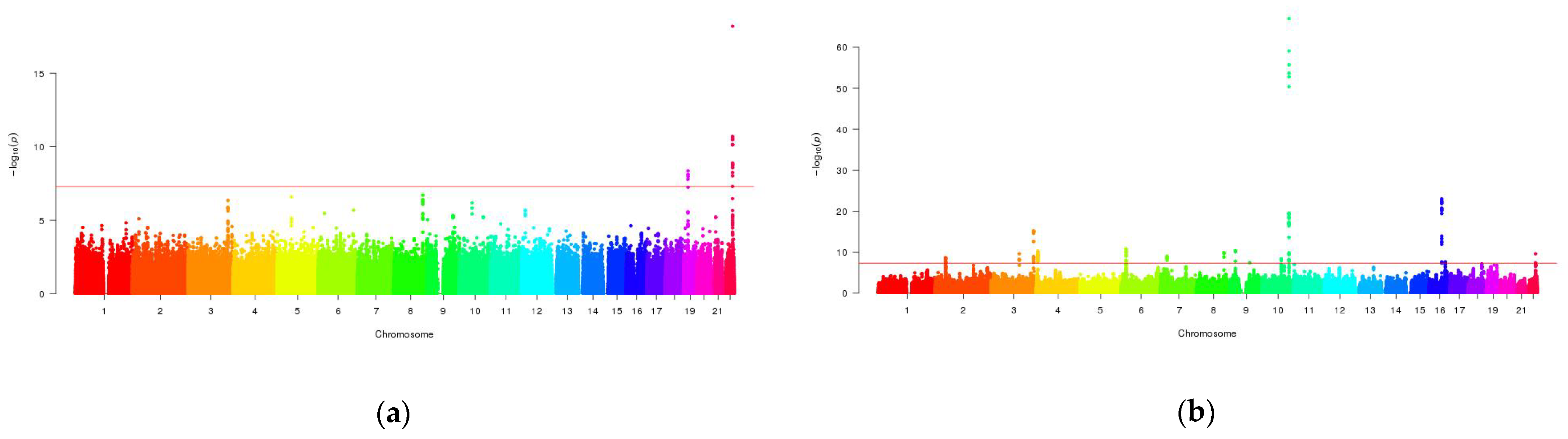
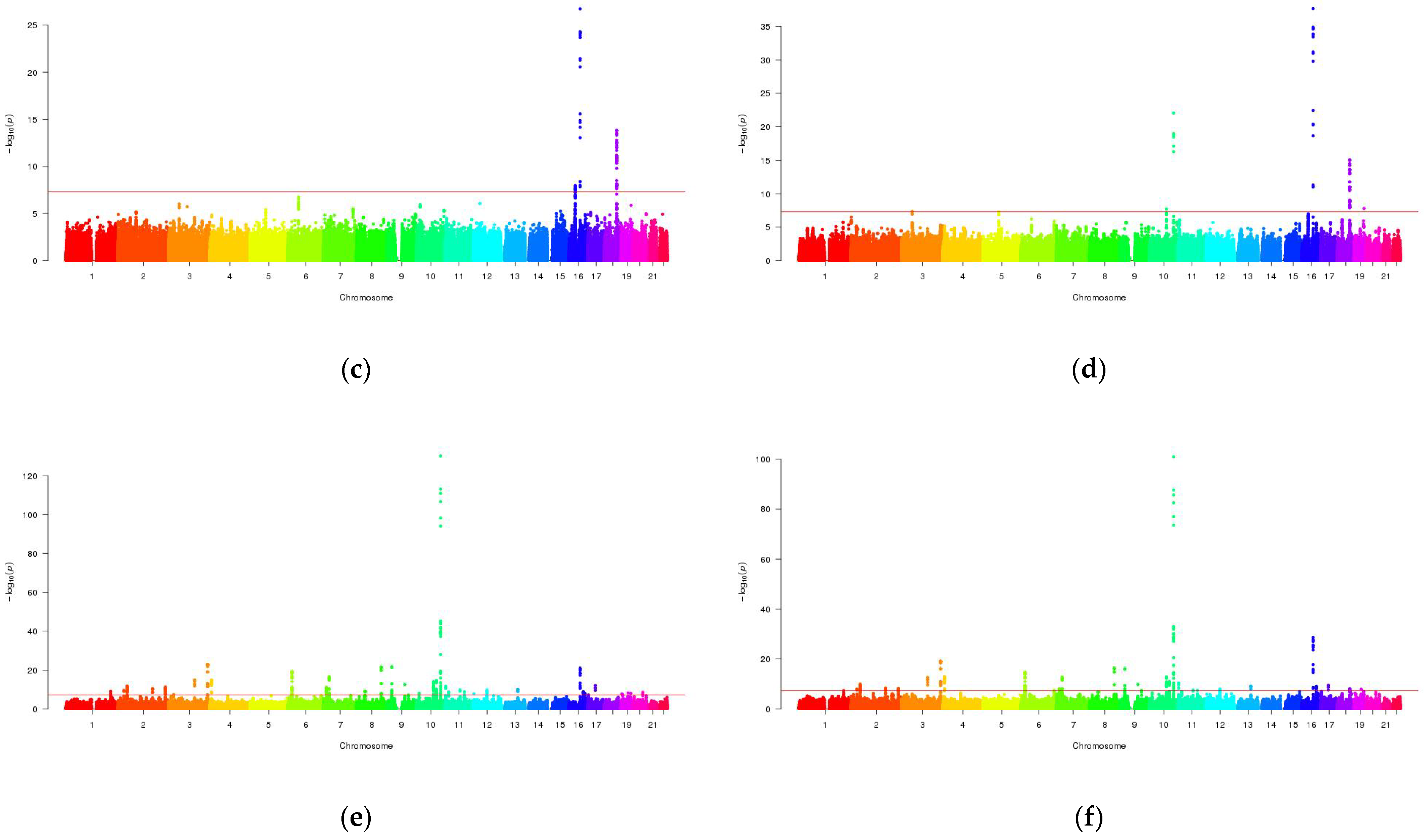
3.2. Mutli-Trait Association Analysis between NAFLD and Metabolic Traits
3.3. Cross-Trait Meta-Analysis between NAFLD and Metabolic Traits
3.4. Overlapping Genes in Genome-Wide Gene Analysis
3.5. Results from the Transcriptome-Wide Association Study, Biological Pathway Analysis and Protein–Protein Interaction Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bettermann, K.; Hohensee, T.; Haybaeck, J. Steatosis and steatohepatitis: Complex disorders. Int. J. Mol. Sci. 2014, 15, 9924–9944. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 40–52. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Joukar, F.; Mobaraki, S.N.; Mavaddati, S.; Hassanipourd, S.; Sepehrimaneshad, M. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: A cross-sectional study in north of Iran. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1591–1596. [Google Scholar] [CrossRef]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9–19. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Celedon, M.A.; Lavine, J.E.; Salem, R.; Campbell, N.; Schork, N.J.; Shiehmorteza, M.; Yokoo, T.; Chavez, A.; Middleton, M.S.; et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology 2009, 136, 1585–1592. [Google Scholar] [CrossRef]
- Cui, J.; Chen, C.H.; Lo, M.T.; Schork, N.; Bettencourt, R.; Gonzalez, M.P.; Bhatt, A.; Hooker, J.; Shaffer, K.; Nelson, K.E.; et al. Shared genetic effects between hepatic steatosis and fibrosis: A prospective twin study. Hepatology 2016, 64, 1547–1558. [Google Scholar] [CrossRef]
- Hannele, Y. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Graham, S.; Wang, X.; Cai, D.; Huang, M.; Pique-Regi, R.; Dong, X.C.; Chen, Y.E.; Willer, C.; et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J. Hepatol. 2020, 73, 263–276. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, Y.; Li, X.; Driver, J.A.; Liang, L. Shared genetic architecture between metabolic traits and Alzheimer’s disease: A large-scale genome-wide cross-trait analysis. Hum. Genet. 2019, 138, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Rao, S.; Xu, Y.; Li, J.; Huang, H.; Zhang, X.; Fu, H.; Wang, Q.; Cao, H.; Baranova, A.; et al. Identifying common genome-wide risk genes for major psychiatric traits. Hum. Genet. 2020, 139, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, X.; Li, X.; Lin, Y.; Shen, S.; Liu, C.L.; Hobbs, B.D.; Hasegawa, K.; Liang, L.; Boezen, H.M.; et al. Genetic overlap of chronic obstructive pulmonary disease and cardiovascular disease-related traits: A large-scale genome-wide cross-trait analysis. Respir. Res. 2019, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; An, J.; Yu, Z. Identifying shared risk genes for asthma, hay fever, and eczema by multi-trait and multiomic association analyses. Front. Genet. 2020, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Namjou, B.; Lingren, T.; Huang, Y.; Parameswaran, S.; Cobb1, B.L.; Stanaway, I.B.; Connolly, J.J.; Mentch, F.D.; Benoit, B.; Niu, X.; et al. GWAS and enrichment analyses of non-alcoholic fatty liver disease identify new trait-associated genes and pathways across eMERGE Network. BMC Med. 2019, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Guo, X.; Loomba, R.; Goodarzi, M.O.; Haritunians, T.; Kwon, S.; Cui, J.; Taylor, K.D.; Wilson, L.; Cummingslow, O.W.; et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 1567–1576. [Google Scholar] [CrossRef]
- Kahali, B.; Halligan, B.; Speliotes, E.K. Insights from genome-wide association analyses of nonalcoholic fatty liver disease. Semin. Liver Dis. 2015, 35, 375–391. [Google Scholar] [CrossRef]
- Fall, T.; Ingelsson, E. Genome-wide association studies of obesity and metabolic syndrome. Mol. Cell. Endocrinol. 2014, 382, 740–757. [Google Scholar] [CrossRef]
- Zhou, W.; Nielsen, J.B.; Fritsche, L.G.; Dey, R.; Gabrielsen, M.E.; Wolford, B.N.; LeFaive, J.; VandeHaar, P.; Gagliano, S.A.; Gifford, A.; et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 2018, 50, 1335–1341. [Google Scholar] [CrossRef]
- Watanabe, K.; Stringer, S.; Frei, O.; Mirkov, M.U.; de Leeuw, C.; Polderman, T.J.C.; vander Sluis, S.; Andreassen, O.A.; Neale, B.M.; Posthuma, D. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 2019, 51, 1339–1348. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Bulik-Sullivan, B.K.; Loh, P.R.; Finucane, H.K.; Ripke, S.; Yang, J.; Schizophrenia Working Group of the Psychiatric Genomics Consortium; Patterson, N.; Daly, M.J.; Price1, A.L.; Neale, B.M. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Turley, P.; Walters, R.K.; Maghzian, O.; Okbay, A.; Lee, J.J.; Fontana, M.A.; Nguyen-Viet, T.A.; Wedow, R.; Zacher, M.; Furlotte, N.A.; et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet. 2018, 50, 229–237. [Google Scholar] [CrossRef]
- Lee, C.H.; Eskin, E.; Han, B. Increasing the power of meta-analysis of genome-wide association studies to detect heterogeneous effects. Bioinformatics 2017, 33, i379–i388. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; Bakker, P.I.W.D.; Daly, M.J. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- De Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized gene-set analysis of GWAS Data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- Mancuso, N.; Freund, M.; Johnson, R.; Shi, H.; Kichaev, G.; Gusev, A.; Pasaniuc, B. Probabilistic fine-mapping of transcriptome-wide association studies. Nat. Genet. 2020, 51, 675–682. [Google Scholar] [CrossRef]
- Kuleshov, M.; Jones, M.; Rouillard, A.; Fernandez, N.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Braun, M.; Rorsman, P. Type 2 Diabetes Susceptibility Gene TCF7L2 and Its Role in β-Cell Function. Diabetes 2009, 58, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Misra, A.; Pandey, R.M. rs7903146 (C/T) polymorphism of Transcription factor 7 like 2 (TCF7L-2) gene is independently associated with non-alcoholic fatty liver disease in Asian Indians. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 175–180. [Google Scholar] [CrossRef]
- Cauchi, S.; Choquet, H.; Gutiérrez-Aguilar, R.; Capel, F.; Grau, K.; Proença, C.; Dina, C.; Duval, A.; Balkau, B.; Marre, M.; et al. Effects of TCF7L2 polymorphisms on obesity in European populations. Obesity 2008, 16, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The bigger picture of FTO--the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014, 10, 51–61. [Google Scholar] [CrossRef]
- Mohlke, K.L.; Boehnke, M. Recent advances in understanding the genetic architecture of type 2 diabetes. Hum. Mol. Genet. 2015, 24, R85–R92. [Google Scholar] [CrossRef]
- Trépo, E.; Valenti, L. Update on NAFLD genetics: From new variants to the clinic. J. Hepatol. 2020, 72, 1196–1209. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Valenti, L. Genetics of nonalcoholic fatty liver disease. Metabolism 2016, 65, 1026–1037. [Google Scholar] [CrossRef]
- Oikonomou, D.; Georgiopoulos, G.; Katsi, V.; Kourek, C.; Tsioufis, C.; Alexopoulou, A.; Koutli, E.; Tousoulis, D. Non-alcoholic fatty liver disease and hypertension: Coprevalent or correlated? Eur. J. Gastroenterol. Hepatol. 2018, 30, 979–985. [Google Scholar] [CrossRef]
- Moeez, S.; Riaz, S.; Masood, N.; Kanwal, N.; Arif, M.A.; Niazi, R.; Khalid, S. Evaluation of the rs3088442 G>A SLC22A3 gene polymorphism and the role of microRNA 147 in groups of adult Pakistani populations with type 2 diabetes in response to metformin. Can. J. Diabetes 2019, 43, 128–135. [Google Scholar] [CrossRef]
- Sonia, G.C.; Alexander, P.; Ville, M.; Vanessa, D.M.; Emma, N.; Jussi, P.; Charlotte, L. Diabetes medication associates with DNA methylation of metformin transporter genes in the human liver. Clin. Epigenet. 2017, 9, 102. [Google Scholar] [CrossRef]
- Bambace, C.; Dahlman, I.; Arner, P.; Kulyté, A. NPC1 in human white adipose tissue and obesity. Bmc Endocr. Disord. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ji, Y.; Qiao, L.; Liu, Y.; Lin, J. Cellular endo-lysosomal dysfunction in the pathogenesis of non-alcoholic fatty liver disease. Liver Int. 2020, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef] [PubMed]


| Phenotype | Data Source | Samples’ Size | # SNPs | ||
|---|---|---|---|---|---|
| Cases | Controls | Total | |||
| NAFLD | UKBB | 1664 | 392,813 | 394,477 | 28,281,760 |
| HTN | UKBB | 99,665 | 189,642 | 289,307 | 10,321,705 |
| Obesity | UKBB | 9805 | 235,085 | 244,890 | 10,154,467 |
| T2D | UKBB | 16,673 | 228,217 | 244,890 | 10,154,467 |
| LDL | GLGC | - | - | 188,577 | 2,437,751 |
| HDL | GLGC | - | - | 188,577 | 2,447,441 |
| TC | GLGC | - | - | 188,577 | 2,439,432 |
| TG | GLGC | - | - | 188,577 | 24,46,981 |
| FG | MAGIC | - | - | 58,074 | 2,628,879 |
| FIN | MAGIC | - | - | 51,750 | 2,627,848 |
| Phenotype 1 | Phenotype 2 | rg | rg _s.e. | p Value |
|---|---|---|---|---|
| NAFLD | HTN | 0.0144 | 0.0509 | 0.7773 |
| Obesity | 0.2276 | 0.1216 | 0.0613 | |
| T2D | 0.2106 | 0.085 | 0.0132 | |
| LDL | 0.0189 | 0.0734 | 0.7964 | |
| HDL | 0.0361 | 0.075 | 0.6303 | |
| TC | 0.0488 | 0.0729 | 0.5029 | |
| TG | −0.058 | 0.073 | 0.4264 | |
| FG | −0.039 | 0.0583 | 0.503 | |
| FIN | 0.0517 | 0.0705 | 0.4635 |
| SNP | Genome Position | A1/A2 | PRE2C | Genes within Clumping Region |
|---|---|---|---|---|
| rs7903146 | chr10:114597109-114914665 | T/C | 6.95 × 10−206 | TCF7L2 |
| rs10811661 | chr9:22130065-22134094 | C/T | 1.50 × 10−34 | - |
| rs1421085 | chr16:53800387-53839135 | C/T | 4.47 × 10−34 | FTO |
| rs7651090 | chr3:185473065-185547917 | G/A | 9.28 × 10−34 | IGF2BP2 |
| rs2206734 | chr6:20529542-20766697 | T/C | 1.61 × 10−32 | CDKAL1 |
| rs13266634 | chr8:118183551-118217307 | T/C | 1.79 × 10−31 | SLC30A8 |
| rs849135 | chr7:28138639-28256240 | G/A | 1.97 × 10−26 | JAZF1 |
| rs7018475 | chr9:22137685-22137685 | G/T | 3.23 × 10−26 | - |
| rs7923837 | chr10:94232247-94499577 | A/G | 3.04 × 10−24 | HHEX, IDE, KIF11 |
| rs2972144 | chr2:226897985-227199263 | A/G | 9.76 × 10−21 | - |
| rs10244051 | chr7:15023729-15065467 | T/G | 1.49 × 10−20 | - |
| rs11708067 | chr3:123065778-123131254 | G/A | 3.18 × 10−20 | ADCY5 |
| rs2796441 | chr9:84304985-84380739 | A/G | 5.11 × 10−20 | - |
| rs738408 | chr22:44324730-44395451 | T/C | 6.84 × 10−20 | PARVB, PNPLA3, SAMM50 |
| rs1128249 | chr2:165501849-165558252 | T/G | 2.05 × 10−19 | COBLL1 |
| rs13411629 | chr2:43450138-43848664 | C/T | 1.06 × 10−18 | THADA, ZFP36L2 |
| rs10885414 | chr10:114835452-114916586 | G/A | 1.29 × 10−18 | TCF7L2 |
| rs1801206 | chr4:6264968-6321396 | C/T | 2.19 × 10−18 | WFS1 |
| rs11651755 | chr17:36098040-36103565 | C/T | 1.43 × 10−16 | HNF1B |
| rs476828 | chr18:57732689-57912785 | C/T | 2.62 × 10−15 | - |
| Gene | Position | # SNPs | PMAGMA | ||
|---|---|---|---|---|---|
| NAFLD | Obesity | T2D | |||
| IGF2BP2 | chr3:185361527-185542827 | 53 | 1.25 × 10−13 | 2.06 × 10−6 | 6.08 × 10−15 |
| SLC22A3 | chr6:160769405-160873613 | 94 | 1.01 × 10−6 | 2.03 × 10−6 | 3.77 × 10−8 |
| ZMIZ1 | chr10:80828751-81076285 | 162 | 2.42 × 10−11 | 1.04 × 10−10 | 7.08 × 10−15 |
| TCF7L2 | chr10:114709978-114927437 | 96 | 1.23 × 10−38 | 3.53 × 10−13 | 2.99 × 10−55 |
| FTO | chr16:53737875-54148379 | 246 | 8.44 × 10−14 | 1.17 × 10−21 | 1.16 × 10−15 |
| NPC1 | chr18:21086148-21166581 | 33 | 2.57 × 10−6 | 2.04 × 10−6 | 2.10 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Yu, Z. Identifying Shared Risk Genes between Nonalcoholic Fatty Liver Disease and Metabolic Traits by Cross-Trait Association Analysis. Processes 2021, 9, 107. https://doi.org/10.3390/pr9010107
Guo H, Yu Z. Identifying Shared Risk Genes between Nonalcoholic Fatty Liver Disease and Metabolic Traits by Cross-Trait Association Analysis. Processes. 2021; 9(1):107. https://doi.org/10.3390/pr9010107
Chicago/Turabian StyleGuo, Hongping, and Zuguo Yu. 2021. "Identifying Shared Risk Genes between Nonalcoholic Fatty Liver Disease and Metabolic Traits by Cross-Trait Association Analysis" Processes 9, no. 1: 107. https://doi.org/10.3390/pr9010107
APA StyleGuo, H., & Yu, Z. (2021). Identifying Shared Risk Genes between Nonalcoholic Fatty Liver Disease and Metabolic Traits by Cross-Trait Association Analysis. Processes, 9(1), 107. https://doi.org/10.3390/pr9010107





