Supported Palladium Nanocatalysts: Recent Findings in Hydrogenation Reactions
Abstract
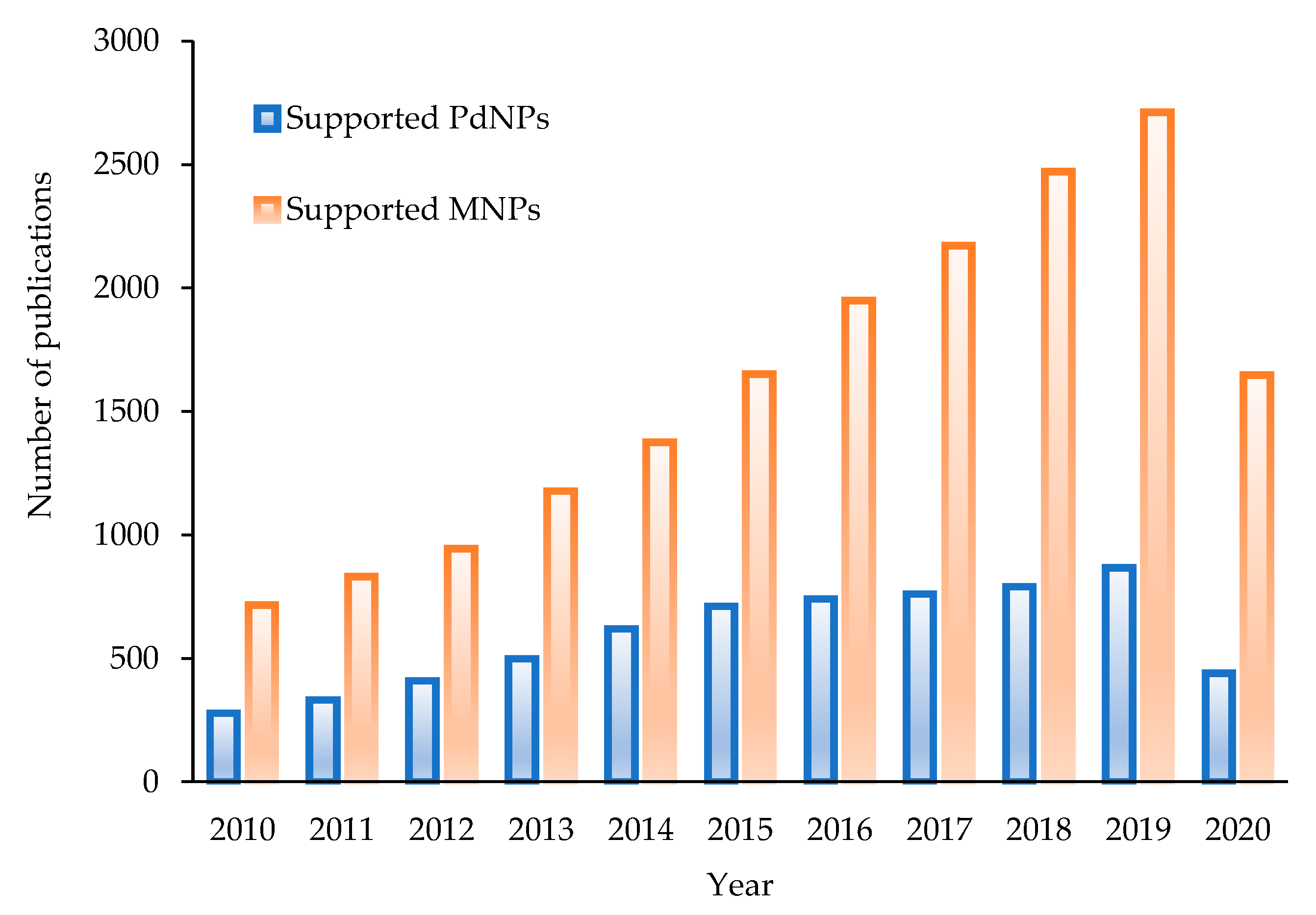
1. Introduction
2. Hydrogenation and Hydrogen Transfer Processes
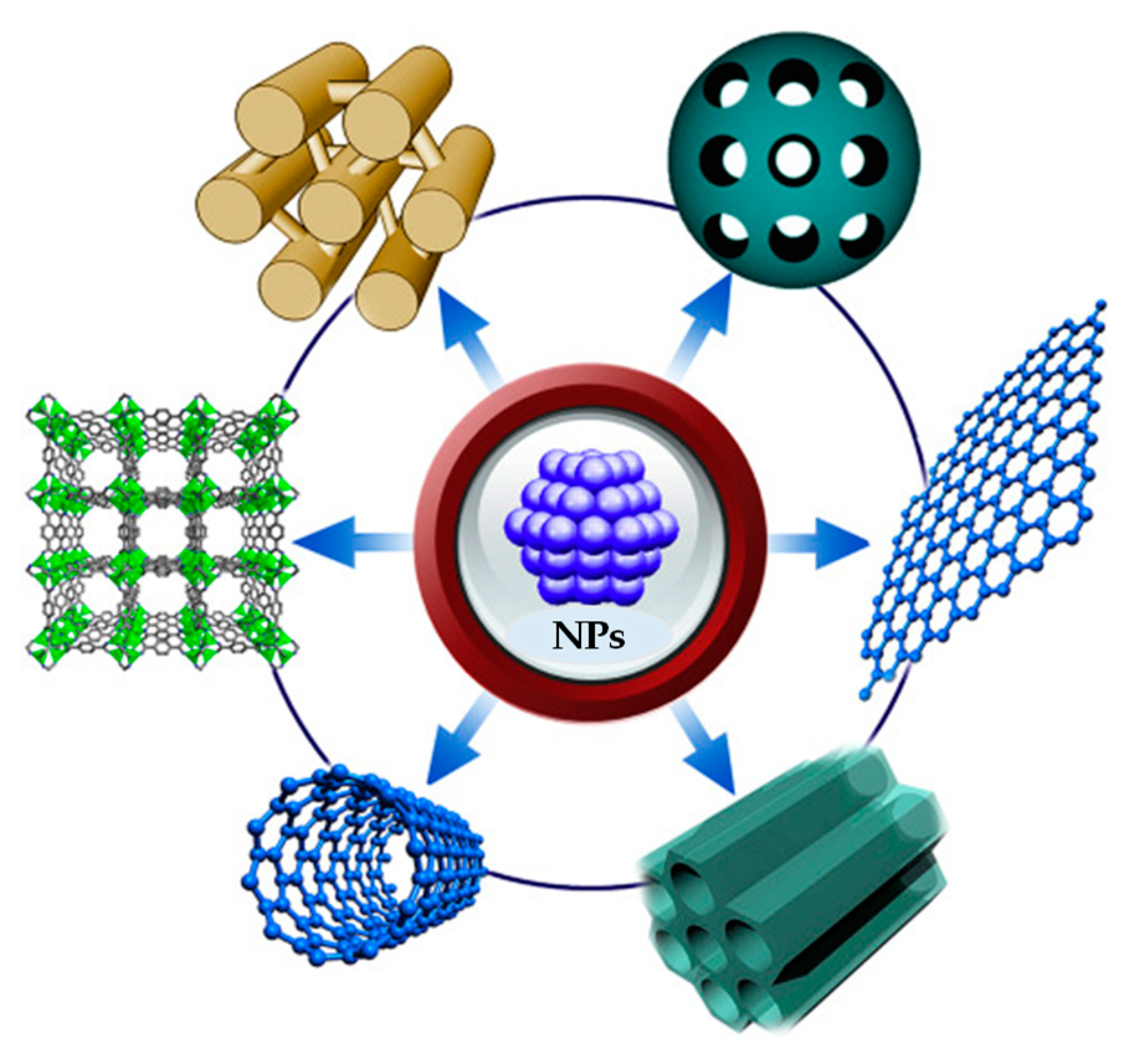
3. PdNPs@supports
4. PdNPs@silicas
5. PdNPs@carbon Materials
6. PdNPs@zeolites
7. PdNPs@MOFs
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zaera, F. Nanostructured materials for applications in heterogeneous catalysis. Chem. Soc. Rev. 2013, 42, 2746–2762. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.P., Jr.; Owens, F.J. Introduction to Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Philippot, K.; Serp, P. Concepts in Nanocatalysis. In Nanomaterials in Catalysis: First Edition; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2013; pp. 1–54. ISBN 9783527331246. [Google Scholar]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal nanoparticles as green catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [PubMed]
- Fedlheim, D.; Foss, C. Metal Nanoparticles: Synthesis, Characterization, and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Catalytic Hydrogenation, Volume 27-1st Edition. Available online: https://www.elsevier.com/books/catalytic-hydrogenation/cerveny/978-0-444-42682-6 (accessed on 25 July 2020).
- Johnstone, R.A.W.; Wilby, A.H.; Entwistle, I.D. Heterogeneous Catalytic Transfer Hydrogenation and Its Relation to Other Methods for Reduction of Organic Compounds. Chem. Rev. 1985, 85, 129–170. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Wu, K.H.; Niu, Y.; Luo, J.; Yang, X.; Zhang, B.; Su, D. Pd@C core-shell nanoparticles on carbon nanotubes as highly stable and selective catalysts for hydrogenation of acetylene to ethylene. Nanoscale 2017, 9, 14317–14321. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Chen, Q.A.; Ye, Z.S.; Duan, Y.; Zhou, Y.G. Homogeneous palladium-catalyzed asymmetric hydrogenation. Chem. Soc. Rev. 2013, 42, 497–511. [Google Scholar] [CrossRef]
- Bulut, S.; Fei, Z.; Siankevich, S.; Zhang, J.; Yan, N.; Dyson, P.J. Aqueous-phase hydrogenation of alkenes and arenes: The growing role of nanoscale catalysts. Catal. Today 2015, 247, 96–103. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Andrade, M.A.; Mestre, A.S.; Carvalho, A.P.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. The role of nanoporous carbon materials in catalytic cyclohexane oxidation. Catal. Today 2019, 1–10. [Google Scholar] [CrossRef]
- Grau-Atienza, A.; Campos, R.; Serrano, E.; Ojeda, M.; Romero, A.A.; Garcia-Martinez, J.; Luque, R. Insights into the active species of Nanoparticle-functionalized hierarchical zeolites in alkylation reactions. ChemCatChem 2014, 6, 3530–3539. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M.D.R.S. Sustainability in catalytic cyclohexane oxidation: The contribution of porous support materials. Catalysts 2020, 10, 2. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, H.; Dai, S. Porous Carbon Supports: Recent Advances with Various Morphologies and Compositions. ChemCatChem 2015, 7, 2788–2805. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, Z.; Dong, S. Carbon-Based Nanostructures for Advanced Catalysis. ChemCatChem 2015, 7, 2806–2815. [Google Scholar] [CrossRef]
- Brunel, D.; Blanc, A.C.; Galarneau, A.; Fajula, F. New trends in the design of supported catalysts on mesoporous silicas and their applications in fine chemicals. Catal. Today 2002, 73, 139–152. [Google Scholar] [CrossRef]
- Thomas, A.M.; Mohan, A.; Rout, L.; Nagappan, S.; Park, S.S.; Ha, C.S. Pd nanoparticle incorporated mesoporous silicas with excellent catalytic activity and dual responsivity. Colloids Surf. Physicochem. Eng. Asp. 2020, 585, 124074. [Google Scholar] [CrossRef]
- Wang, Y.; Biradar, A.V.; Asefa, T. Assembling nanostructures for effective catalysis: Supported palladium nanoparticle multicores coated by a hollow and nanoporous zirconia shell. ChemSusChem 2012, 5, 132–139. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Chen, M.; Tang, L.; Tang, W.; Lai, C.; Cheng, M.; et al. Metal Organic Frameworks as Robust Host of Palladium Nanoparticles in Heterogeneous Catalysis: Synthesis, Application, and Prospect. ACS Appl. Mater. Interfaces 2019, 11, 32579–32598. [Google Scholar] [CrossRef]
- Zanon, A.; Verpoort, F. Metals@ZIFs: Catalytic applications and size selective catalysis. Coord. Chem. Rev. 2017, 353, 201–222. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparation, characterization, and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Navlani-García, M.; Martis, M.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Mori, K.; Yamashita, H. Investigation of Pd nanoparticles supported on zeolites for hydrogen production from formic acid dehydrogenation. Catal. Sci. Technol. 2015, 5, 364–371. [Google Scholar] [CrossRef]
- Dams, M.; Drijkoningen, L.; De Vos, D.E.; Jacobs, P.A.; Pauwels, B.; Van Tendeloo, G. Pd-zeolites as heterogeneous catalysts in Heck chemistry. J. Catal. 2002, 209, 225–236. [Google Scholar] [CrossRef]
- Coq, B. Metal-Support Interaction in Catalysis. In Metal-Ligand Interactions in Chemistry, Physics and Biology; Springer: Dordrecht, The Netherlands, 2000; pp. 49–71. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Immobilization of Ultrafine Metal Nanoparticles to High-Surface-Area Materials and Their Catalytic Applications. Chem 2016, 1, 220–245. [Google Scholar] [CrossRef]
- Pálinkó, I. Heterogeneous Catalysis: A Fundamental Pillar of Sustainable Synthesis; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128095492. [Google Scholar]
- Ravi, S.; Vadukumpully, S. Sustainable carbon nanomaterials: Recent advances and its applications in energy and environmental remediation. J. Environ. Chem. Eng. 2016, 4, 835–856. [Google Scholar] [CrossRef]
- Favier, I.; Madec, D.; Teuma, E.; Gomez, M. Palladium Nanoparticles Applied in Organic Synthesis as Catalytic Precursors. Curr. Org. Chem. 2011, 15, 3127–3174. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Prati, L. Material science for the support design: A powerful challenge for catalysis. Catal. Sci. Technol. 2012, 2, 673–682. [Google Scholar] [CrossRef]
- Gavia, D.J.; Shon, Y.S. Catalytic properties of unsupported palladium nanoparticle surfaces capped with small organic ligands. ChemCatChem 2015, 7, 892–900. [Google Scholar] [CrossRef]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005. [Google Scholar] [CrossRef]
- Yu, W.; Lin, H.W.; Tan, C.S. Direct synthesis of Pd incorporated in mesoporous silica for solvent-free selective hydrogenation of chloronitrobenzenes. Chem. Eng. J. 2017, 325, 124–133. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, H.; Xu, X.; Yan, X. Selective hydrogenation of o-chloronitrobenzene (o-CNB) over supported Pt and Pd catalysts obtained by laser vaporization deposition of bulk metals. J. Mol. Catal. A Chem. 2009, 310, 144–149. [Google Scholar] [CrossRef]
- Liu, R.J.; Crozier, P.A.; Smith, C.M.; Hucul, D.A.; Blackson, J.; Salaita, G. Metal sintering mechanisms and regeneration of palladium/alumina hydrogenation catalysts. Appl. Catal. A Gen. 2005, 282, 111–121. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Gao, X.; Zhu, X.; Ge, Q.; Liu, X.; Han, J. Mesoporous silica-based nanotubes loaded Pd nanoparticles: Effect of framework compositions on the performance in heterogeneous catalysis. Microporous Mesoporous Mater. 2017, 247, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Guan, Z.; Liu, J.; Zhao, J.; Yang, Y.; Yang, Q. Organosilica nanotubes: Large-scale synthesis and encapsulation of metal nanoparticles. Chem. Commun. 2011, 47, 8073–8075. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Lu, S.; Li, C. Enantioselective hydrogenation of α,β-unsaturated carboxylic acid over cinchonidine-modified Pd nanoparticles confined in carbon nanotubes. J. Catal. 2014, 311, 1–5. [Google Scholar] [CrossRef]
- Shabbir, S.; Lee, S.; Lim, M.; Lee, H.; Ko, H.; Lee, Y.; Rhee, H. Pd nanoparticles on reverse phase silica gel as recyclable catalyst for Suzuki-Miyaura cross coupling reaction and hydrogenation in water. J. Organomet. Chem. 2017, 846, 296–304. [Google Scholar] [CrossRef]
- Na Rungsi, A.; Luengnaruemitchai, A.; Wongkasemjit, S.; Chollacoop, N.; Chen, S.Y.; Yoshimura, Y. Influence of silica sources on structural property and activity of Pd-supported on mesoporous MCM-41 synthesized with an aid of microwave heating for partial hydrogenation of soybean methyl esters. Appl. Catal. A Gen. 2018, 563, 80–90. [Google Scholar] [CrossRef]
- Chung, S.H.; Park, Y.M.; Kim, M.S.; Lee, K.Y. The effect of textural properties on the hydrogenation of succinic acid using palladium incorporated mesoporous supports. Catal. Today 2012, 185, 205–210. [Google Scholar] [CrossRef]
- Ganji, S.; Bukya, P.; Liu, Z.W.; Rao, K.S.R.; Burri, D.R. A carboxylic acid functionalized SBA-15 supported Pd nanocatalyst: An efficient catalyst for hydrogenation of nitrobenzene to aniline in water. New J. Chem. 2019, 43, 11871–11875. [Google Scholar] [CrossRef]
- Karimi, B.; Abedi, S.; Clark, J.H.; Budarin, V. Highly efficient aerobic oxidation of alcohols using a recoverable catalyst: The role of mesoporous channels of SBA-15 in stabilizing palladium nanoparticles. Angew. Chem.-Int. Ed. 2006, 45, 4776–4779. [Google Scholar] [CrossRef] [PubMed]
- Saidulu, G.; Anand, N.; Rao, K.S.R.; Burri, A.; Park, S.E.; Burri, D.R. Cu/SBA-15 is an efficient solvent-free and acid-free catalyst for the rearrangement of benzaldoxime into benzamide. Catal. Lett. 2011, 141, 1865–1871. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Saikia, D.; Huang, Y.Y.; Wu, C.E.; Kao, H.M. Size dependence of silver nanoparticles in carboxylic acid functionalized mesoporous silica SBA-15 for catalytic reduction of 4-nitrophenol. RSC Adv. 2016, 6, 35167–35176. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Calvino-Casilda, V.; Soriano, E. Metal-supported carbon-based materials: Opportunities and challenges in the synthesis of valuable products. Catal. Sci. Technol. 2016, 6, 1265–1291. [Google Scholar] [CrossRef]
- Calvino-Casilda, V.; Lopez-Peinado, A.J.; Duran-Valle, C.J.; Martin-Aranda, R.M. Last decade of research on activated carbons as catalytic support in chemical processes. Catal. Rev. 2010, 52, 325–380. [Google Scholar] [CrossRef]
- Mestre, A.S.; Carvalho, A.P. Nanoporous Carbon Synthesis: An Old Story with Exciting New Chapters. In Porosity-Process, Technologies and Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F. Carbon as catalyst. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 177–217. ISBN 9780470178850. [Google Scholar]
- Bandosz, T.J.; Ania, C.O. Surface chemistry of carbon materials. In Activated Carbon Surfaces in Environmental Remediation; Bandosz, T.J., Ed.; Elsevier: New York, NY, USA, 2006; pp. 159–229. [Google Scholar]
- El-Hout, S.I.; El-Sheikh, S.M.; Hassan, H.M.A.; Harraz, F.A.; Ibrahim, I.A.; El-Sharkawy, E.A. A green chemical route for synthesis of graphene supported palladium nanoparticles: A highly active and recyclable catalyst for reduction of nitrobenzene. Appl. Catal. A Gen. 2015, 503, 176–185. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S.; Rostami-Vartooni, A.; Alizadeh, M.; Bagherzadeh, M. Green synthesis of the Pd nanoparticles supported on reduced graphene oxide using barberry fruit extract and its application as a recyclable and heterogeneous catalyst for the reduction of nitroarenes. J. Colloid Interface Sci. 2016, 466, 360–368. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Maham, M.; Rostami-Vartooni, A.; Bagherzadeh, M.; Sajadi, S.M. Barberry fruit extract assisted in situ green synthesis of Cu nanoparticles supported on a reduced graphene oxide-Fe3O4 nanocomposite as a magnetically separable and reusable catalyst for the O-arylation of phenols with aryl halides under ligand-free cond. RSC Adv. 2015, 5, 64769–64780. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Bilgicli, H.G.; Burhan, H.; Diler, F.; Cellat, K.; Kuyuldar, E.; Zengin, M.; Sen, F. Composites of palladium nanoparticles and graphene oxide as a highly active and reusable catalyst for the hydrogenation of nitroarenes. Microporous Mesoporous Mater. 2020, 296, 110014. [Google Scholar] [CrossRef]
- Sadjadi, S.; Akbari, M.; Léger, B.; Monflier, E.; Heravi, M.M. Eggplant-Derived Biochar-Halloysite Nanocomposite as Supports of Pd Nanoparticles for the Catalytic Hydrogenation of Nitroarenes in the Presence of Cyclodextrin. ACS Sustain. Chem. Eng. 2019, 7, 6720–6731. [Google Scholar] [CrossRef]
- Duarte, T.A.G.; Favier, I.; Pradel, C.; Martins, L.M.D.R.S.; Carvalho, A.P.; Pla, D.; Gómez, M. Tetraalkylammonium Functionalized Hydrochars as Efficient Supports for Palladium Nanocatalysts. ChemCatChem 2020, 12, 2295–2303. [Google Scholar] [CrossRef]
- Duarte, T.A.G.; Carvalho, A.P.; Martins, L.M.D.R.S. Styrene oxidation catalyzed by copper(II) C-scorpionates in homogenous medium and immobilized on sucrose derived hydrochars. Catal. Today 2019. [Google Scholar] [CrossRef]
- Mandal, S.; Roy, D.; Chaudhari, R.V.; Sastry, M. Pt and Pd nanoparticles immobilized on amine-functionalized zeolite: Excellent catalysts for hydrogenation and heck reactions. Chem. Mater. 2004, 16, 3714–3724. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Shao, Y.; Wang, Y.; Gates, B.C.; Xiao, F.S. A Pd@Zeolite Catalyst for Nitroarene Hydrogenation with High Product Selectivity by Sterically Controlled Adsorption in the Zeolite Micropores. Angew. Chem.-Int. Ed. 2017, 56, 9747–9751. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, A.; Costa Pessoa, J. Vanadium complexes immobilized on solid supports and their use as catalysts for oxidation and functionalization of alkanes and alkenes. Coord. Chem. Rev. 2011, 255, 2315–2344. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Ojeda, M.; Grau-Atienza, A.; Campos, R.; Romero, A.A.; Serrano, E.; Maria Marinas, J.; García Martínez, J.; Luque, R. Hierarchical zeolites and their catalytic performance in selective oxidative processes. ChemSusChem 2015, 8, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.; Nunes, N.; Martins, A. Hierarchical Zeolites: Preparation, Properties and Catalytic Applications. In Comprehensive Guide for Mesoporous Materials, Vol. 3: Properties and Development; Aliofkhazraei, M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 147–211. ISBN 978-1-63463-318-5. [Google Scholar]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Paixão, V.; Monteiro, R.; Andrade, M.; Fernandes, A.; Rocha, J.; Carvalho, A.P.; Martins, A. Desilication of MOR zeolite: Conventional versus microwave assisted heating. Appl. Catal. A Gen. 2011, 402, 59–68. [Google Scholar] [CrossRef]
- Paixão, V.; Carvalho, A.P.; Rocha, J.; Fernandes, A.; Martins, A. Modification of MOR by desilication treatments: Structural, textural and acidic characterization. Microporous Mesoporous Mater. 2010, 131. [Google Scholar] [CrossRef]
- Zhu, K.; Egeblad, K.; Christensen, C.H. Tailoring the Porosity of Hierarchical Zeolites by Carbon-Templating; Elsevier: Amsterdam, The Netherlands, 2008; Volume 174, ISBN 9780444532961. [Google Scholar]
- Wang, F.; Ren, J.; Cai, Y.; Sun, L.; Chen, C.; Liang, S.; Jiang, X. Palladium nanoparticles confined within ZSM-5 zeolite with enhanced stability for hydrogenation of p-nitrophenol to p-aminophenol. Chem. Eng. J. 2016, 283, 922–928. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, L.; Chen, R.; Xing, W.; Xu, N. Preparation of Pd-B/TiO2 amorphous alloy catalysts and their performance on liquid-phase hydrogenation of p-nitrophenol. Chem. Eng. J. 2008, 138, 517–522. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Z.; Chen, R.; Xing, W. Preparation of Pd/Al2O3 catalysts and its application in synthesis of p-aminophenol. Ind. Catal. 2007, 6, 14. [Google Scholar]
- Cui, T.L.; Ke, W.Y.; Zhang, W.B.; Wang, H.H.; Li, X.H.; Chen, J.S. Encapsulating Palladium Nanoparticles Inside Mesoporous MFI Zeolite Nanocrystals for Shape-Selective Catalysis. Angew. Chem. -Int. Ed. 2016, 55, 9178–9182. [Google Scholar] [CrossRef]
- Cui, T.L.; Li, X.H.; Lv, L.B.; Wang, K.X.; Su, J.; Chen, J.S. Nanoscale Kirkendall growth of silicalite-1 zeolite mesocrystals with controlled mesoporosity and size. Chem. Commun. 2015, 51, 12563–12566. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Z.; Wang, L.; Dong, X.; Zhang, J.; Wang, G.; Han, S.; Meng, X.; Zheng, A.; Xiao, F.S. Importance of Zeolite Wettability for Selective Hydrogenation of Furfural over Pd@Zeolite Catalysts. ACS Catal. 2018, 8, 474–481. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, X.K.; Canlas, C.; Kropf, A.J.; Aich, P.; Greeley, J.P.; Elam, J.W.; Meyers, R.J.; Dumesic, J.A.; Stair, P.C.; et al. Atomic Layer Deposition Overcoating: Tuning Catalyst Selectivity for Biomass Conversion. Angew. Chem. -Int. Ed. 2014, 53, 12132–12136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Feyen, M.; McGuire, R.; Müller, U.; Zhang, W. Pd Nanoparticles Encapsulated in FER Zeolite through a Layer Reassembling Strategy as Shape-selective Hydrogenation Catalyst. ChemCatChem 2018, 10, 2254–2259. [Google Scholar] [CrossRef]
- Zhang, Y.; Fulajtárová, K.; Kubů, M.; Mazur, M.; Hronec, M.; Čejka, J. Electronic/steric effects in hydrogenation of nitroarenes over the heterogeneous Pd@BEA and Pd@MWW catalysts. Catal. Today 2020, 345, 39–47. [Google Scholar] [CrossRef]
- Van Den Broek, A.C.M.; Van Grondelle, J.; Van Santen, R.A. Preparation of highly dispersed platinum particles in HZSM-5 zeolite: A study of the pretreatment process of [Pt(NH3)4]2+. J. Catal. 1997, 167, 417–424. [Google Scholar] [CrossRef]
- Igarashi, H.; Uchida, H.; Suzuki, M.; Sasaki, Y.; Watanabe, M. Removal of carbon monoxide from hydrogen-rich fuels by selective oxidation over platinum catalyst supported on zeolite. Appl. Catal. A Gen. 1997, 159, 159–169. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Fonseca, I.M. New and advanced porous carbon materials in fine chemical synthesis. Emerging precursors of porous carbons. Catalysts 2019, 9, 133. [Google Scholar] [CrossRef]
- Juan-Alcañiz, J.; Gascon, J.; Kapteijn, F. Metal-organic frameworks as scaffolds for the encapsulation of active species: State of the art and future perspectives. J. Mater. Chem. 2012, 22, 10102–10119. [Google Scholar] [CrossRef]
- Zhang, D.; Guan, Y.; Hensen, E.J.M.; Chen, L.; Wang, Y. Porous MOFs supported palladium catalysts for phenol hydrogenation: A comparative study on MIL-101 and MIL-53. Catal. Commun. 2013, 41, 47–51. [Google Scholar] [CrossRef]
- Millange, F.; Serre, C.; Férey, G. Synthesis, structure determination and properties of MIL-53as and MIL-53ht: The first CrIII hybrid inorganic–organic microporous solids: CrIII(OH)·{O2C–C6H4–CO2}·{HO2C–C6H4–CO2H}x†. Chem. Commun. 2002, 822–823. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Jenkins, S.J. Aromatic adsorption on metals via first-principles density functional theory. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 465, 2949–2976. [Google Scholar] [CrossRef]
- Chen, A.; Zhao, G.; Chen, J.; Chen, L.; Yu, Y. Selective hydrogenation of phenol and derivatives over an ionic liquid-like copolymer stabilized palladium catalyst in aqueous media. RSC Adv. 2013, 3, 4171–4175. [Google Scholar] [CrossRef]
- Hermannsdörfer, J.; Kempe, R. Selective palladium-loaded MIL-101 catalysts. Chem.-A Eur. J. 2011, 17, 8071–8077. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, X.; Chen, Z.; Zhang, T.; Li, C. Pd@MIL-100(Fe) composite nanoparticles as efficient catalyst for reduction of 2/3/4-nitrophenol: Synergistic effect between Pd and MIL-100(Fe). Microporous Mesoporous Mater. 2018, 255, 1–6. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Z.; Han, B.; Li, C. Glycol assisted synthesis of MIL-100(Fe) nanospheres for photocatalytic oxidation of benzene to phenol. Catal. Commun. 2017, 98, 112–115. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Luque, R.; Li, Y. Metal−organic framework encapsulated Pd nanoparticles: Towards advanced heterogeneous catalysts. Chem. Sci. 2014, 5, 3708–3714. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef]
- Chen, D.; Yang, W.; Jiao, L.; Li, L.; Yu, S.H.; Jiang, H.L. Boosting Catalysis of Pd Nanoparticles in MOFs by Pore Wall Engineering: The Roles of Electron Transfer and Adsorption Energy. Adv. Mater. 2020, 2000041, 1–6. [Google Scholar] [CrossRef]
- Biswas, S.; Van Der Voort, P. A general strategy for the synthesis of functionalised UiO-66 frameworks: Characterisation, stability and CO2 adsorption properties. Eur. J. Inorg. Chem. 2013, 2154–2160. [Google Scholar] [CrossRef]
- Zhou, W.; Zou, B.; Zhang, W.; Tian, D.; Huang, W.; Huo, F. Synthesis of stable heterogeneous catalysts by supporting carbon-stabilized palladium nanoparticles on MOFs. Nanoscale 2015, 7, 8720–8724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ni, X.; Ye, S.; Gu, Z.G.; Li, Y.; Ngai, T. A Smart Route for Encapsulating Pd Nanoparticles into a ZIF-8 Hollow Microsphere and Their Superior Catalytic Properties. Langmuir 2020, 36, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
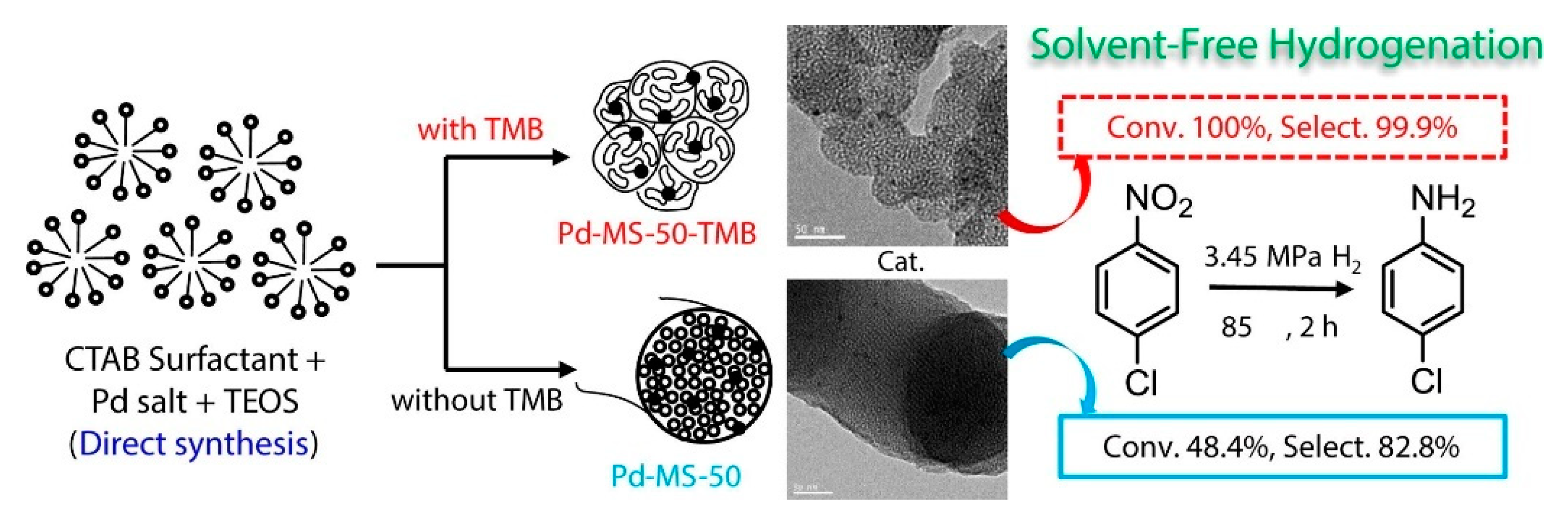





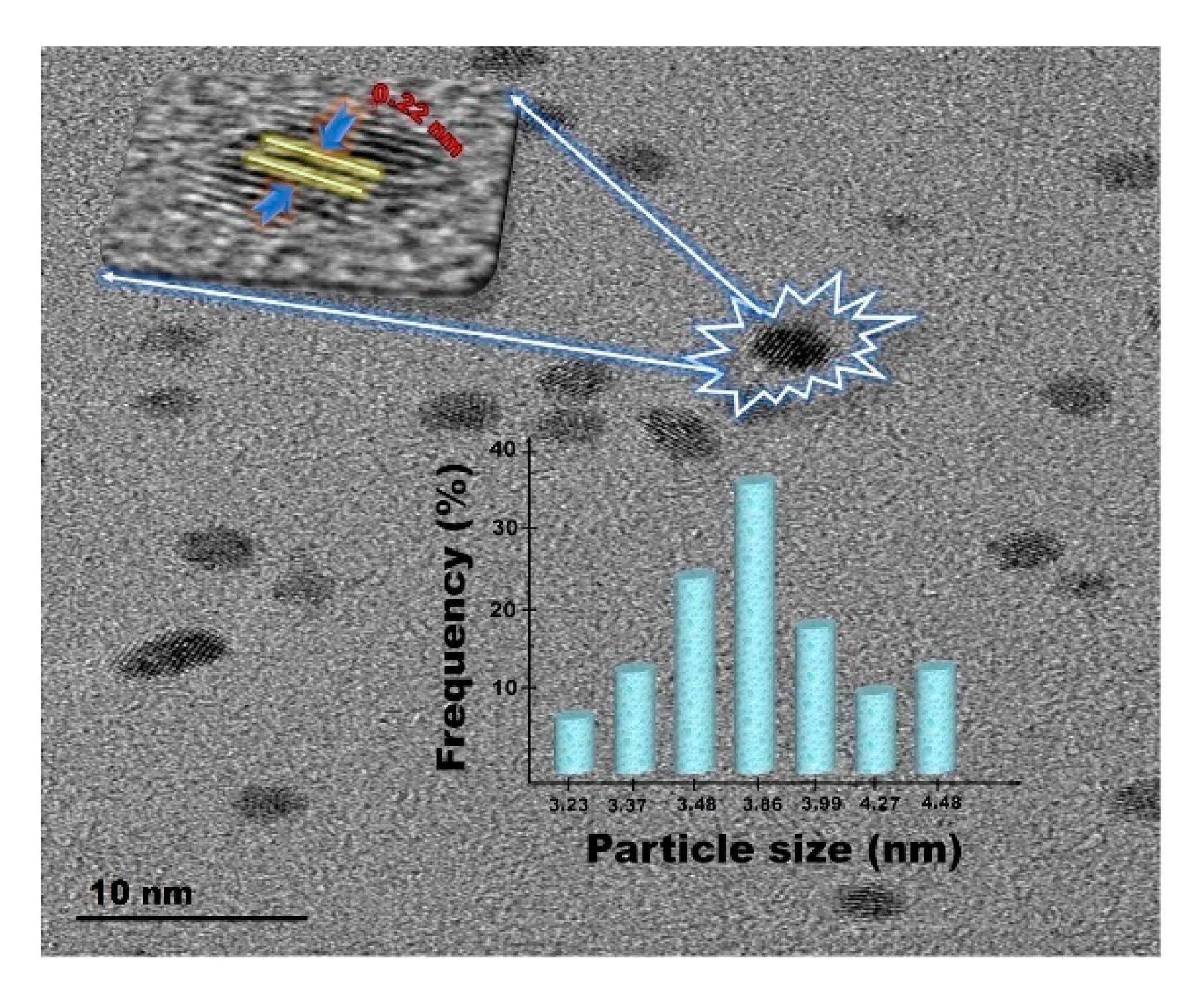
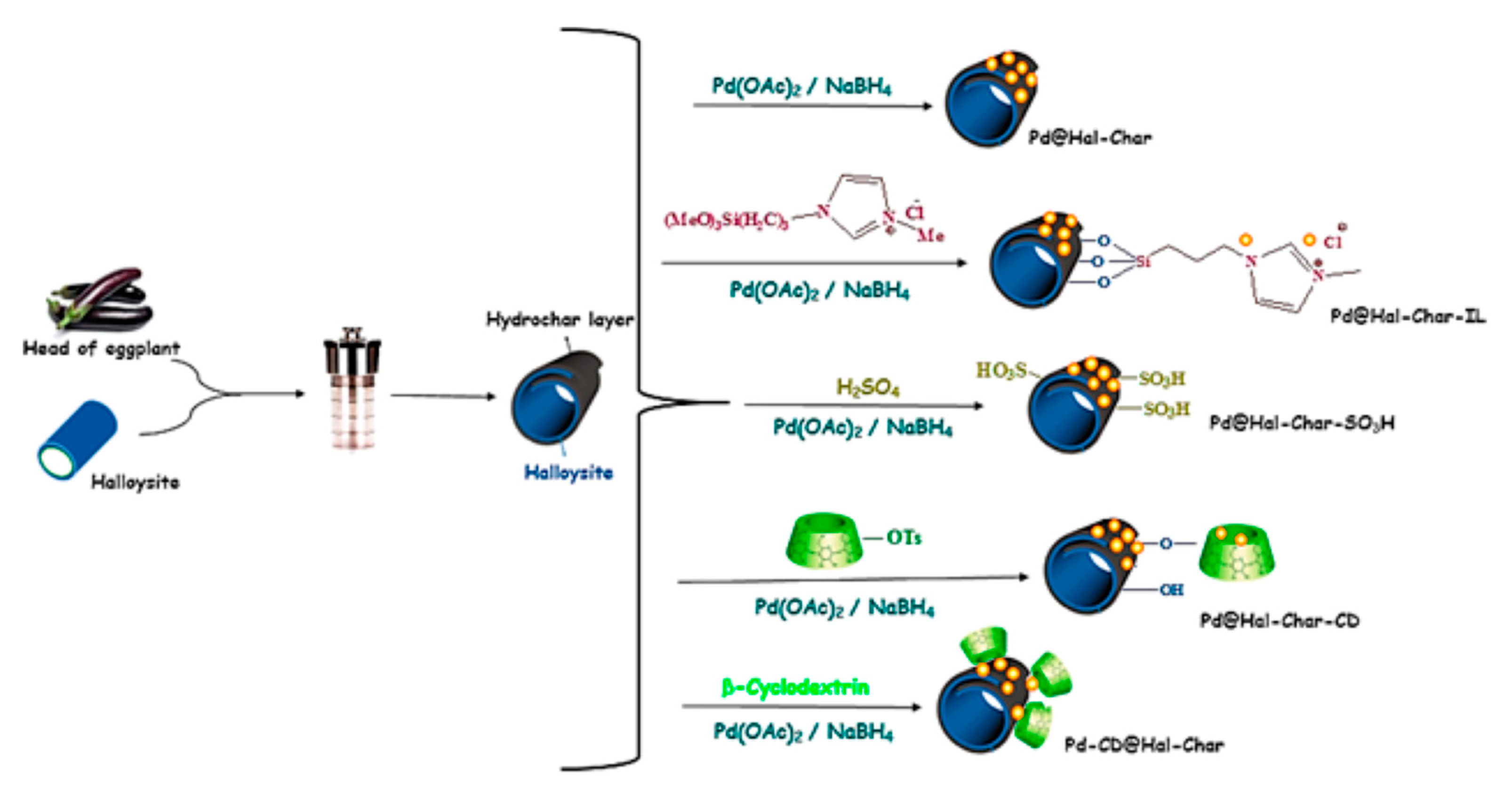

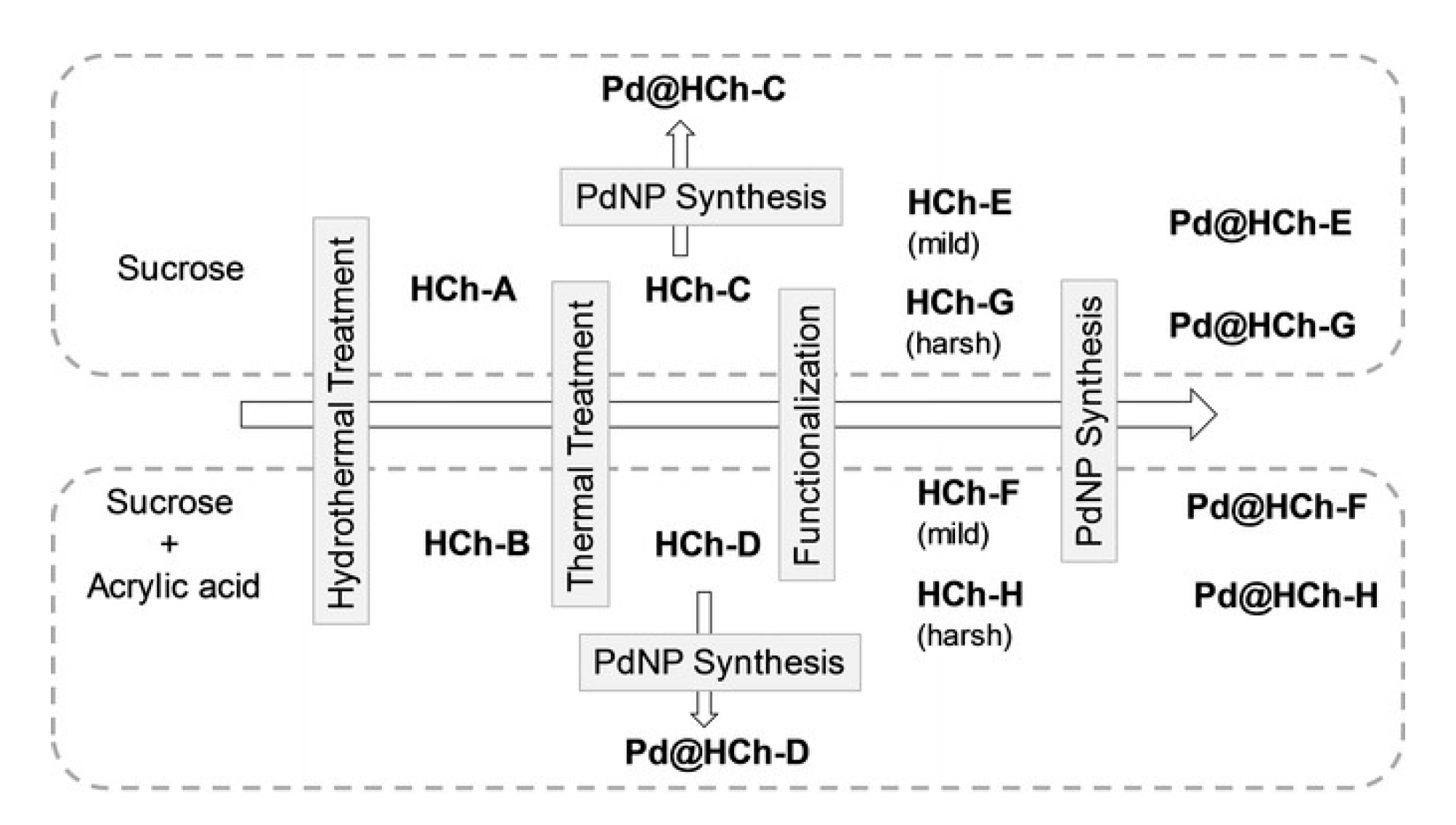
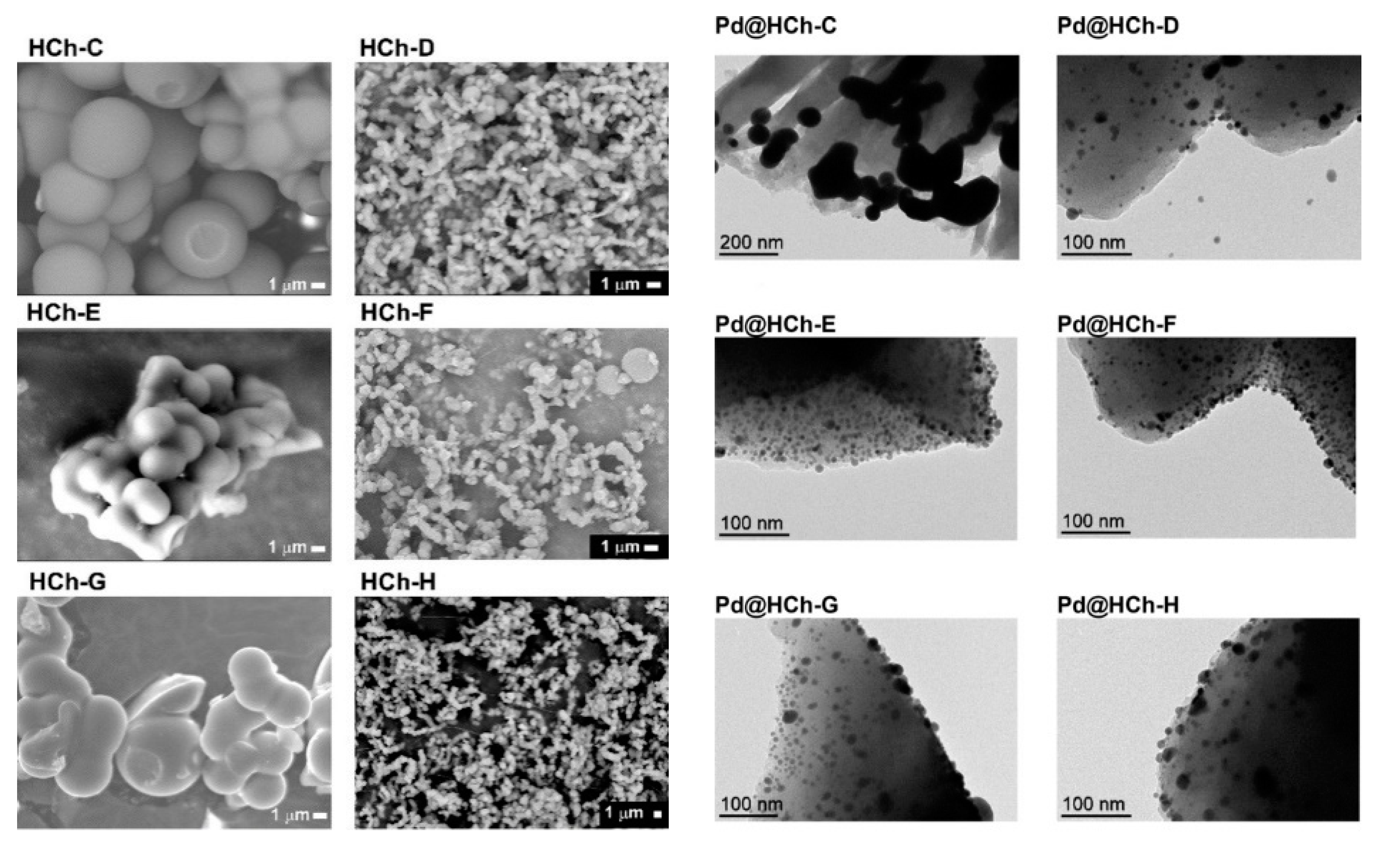

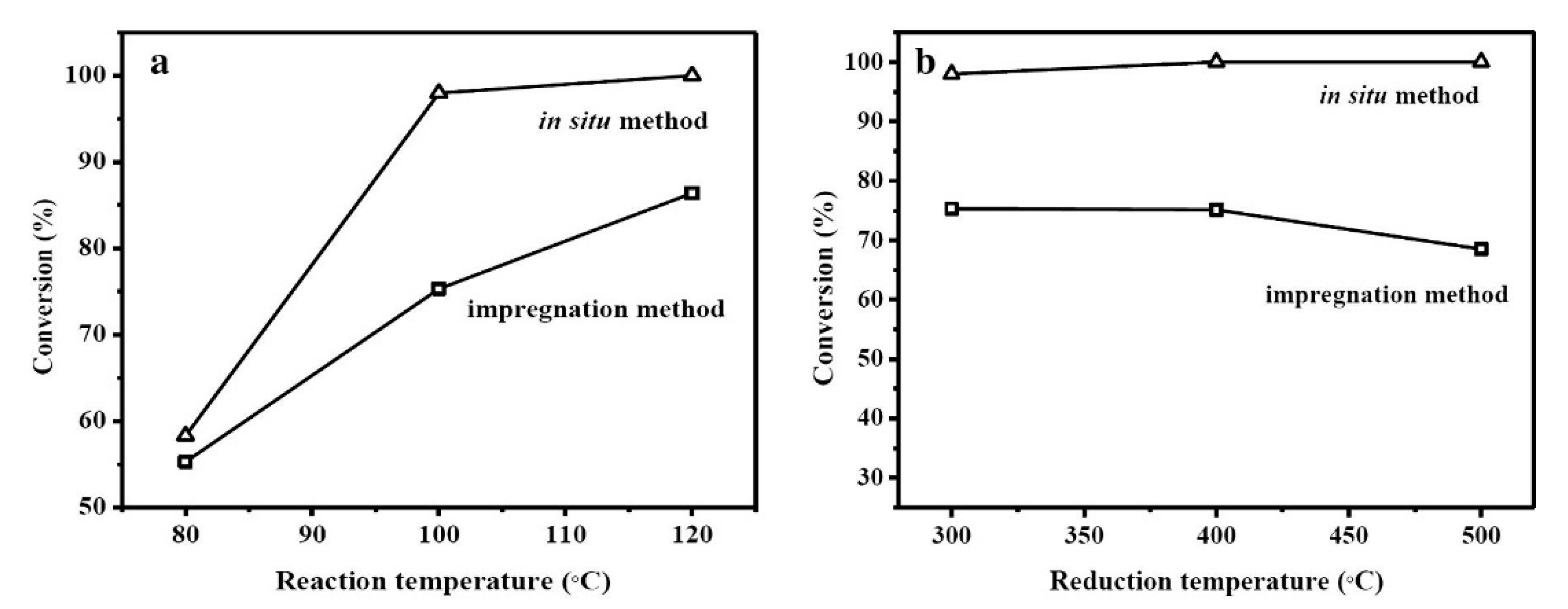

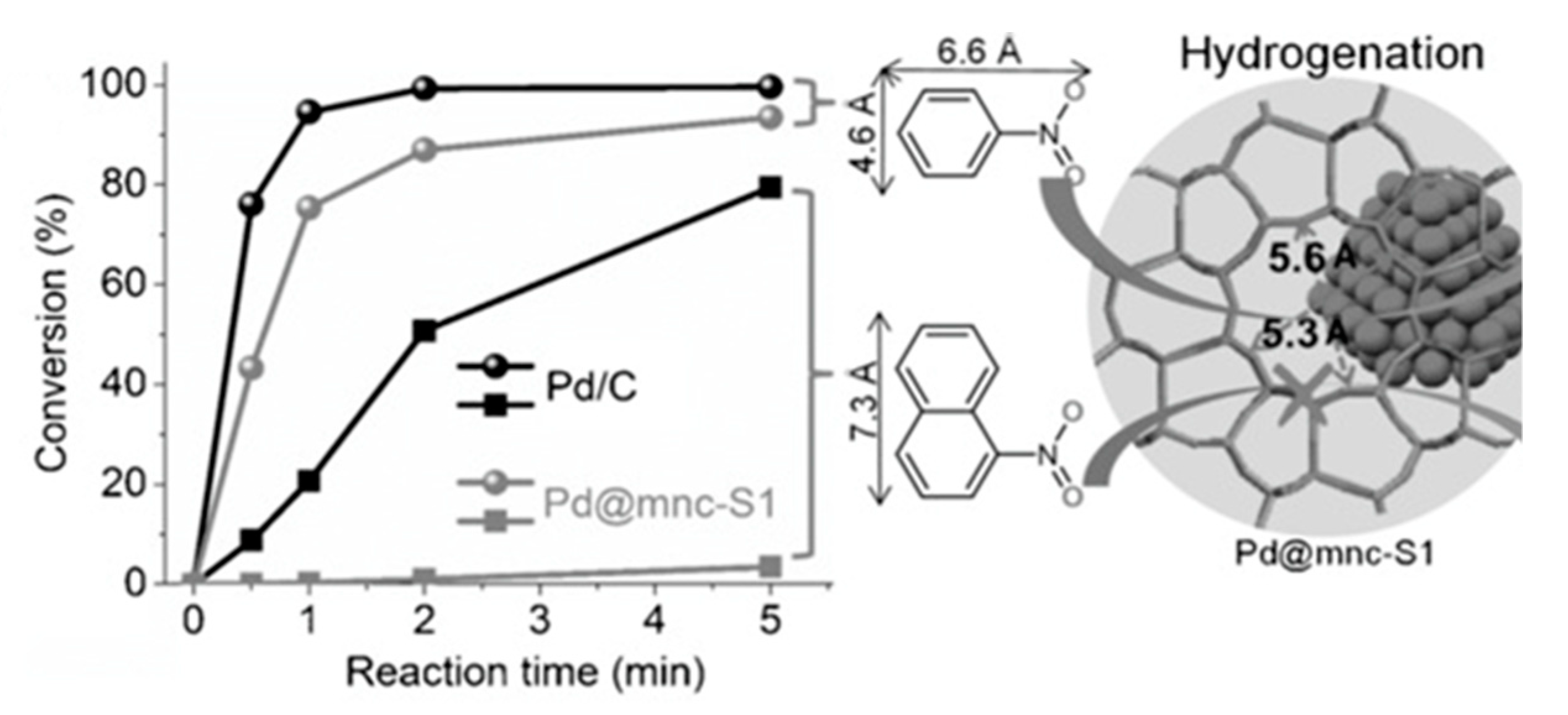




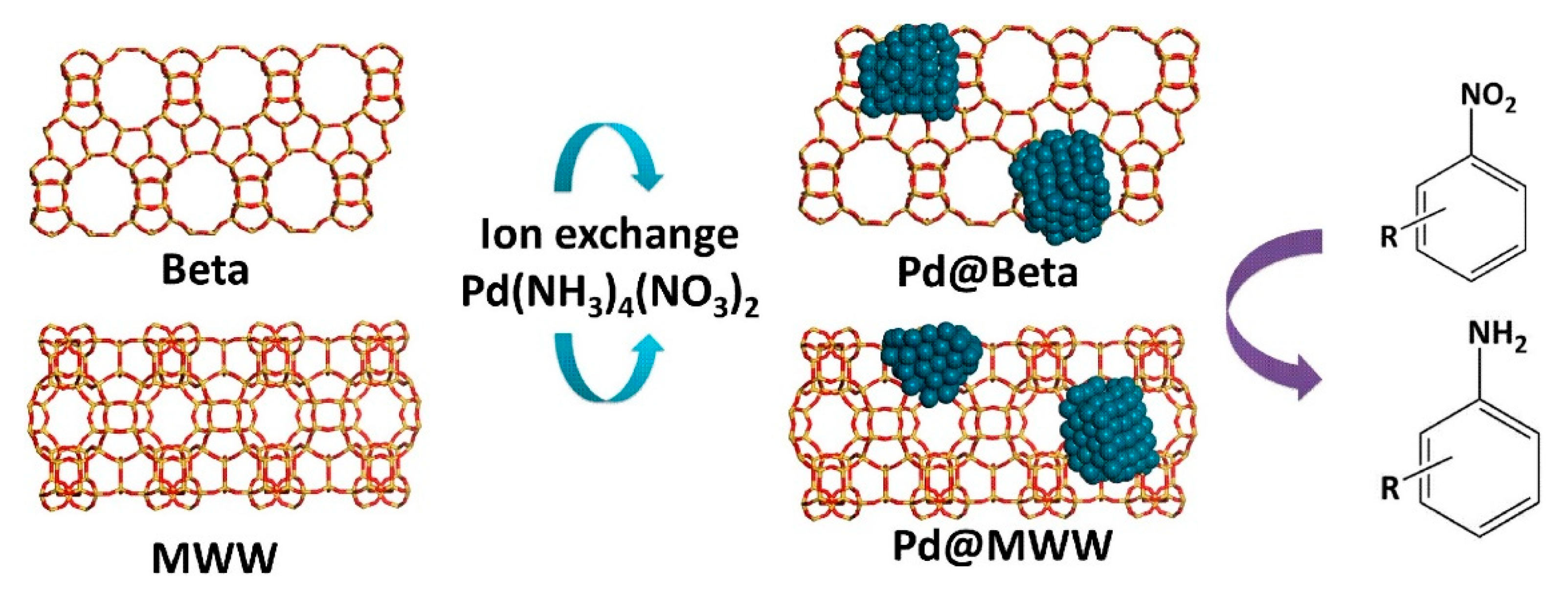



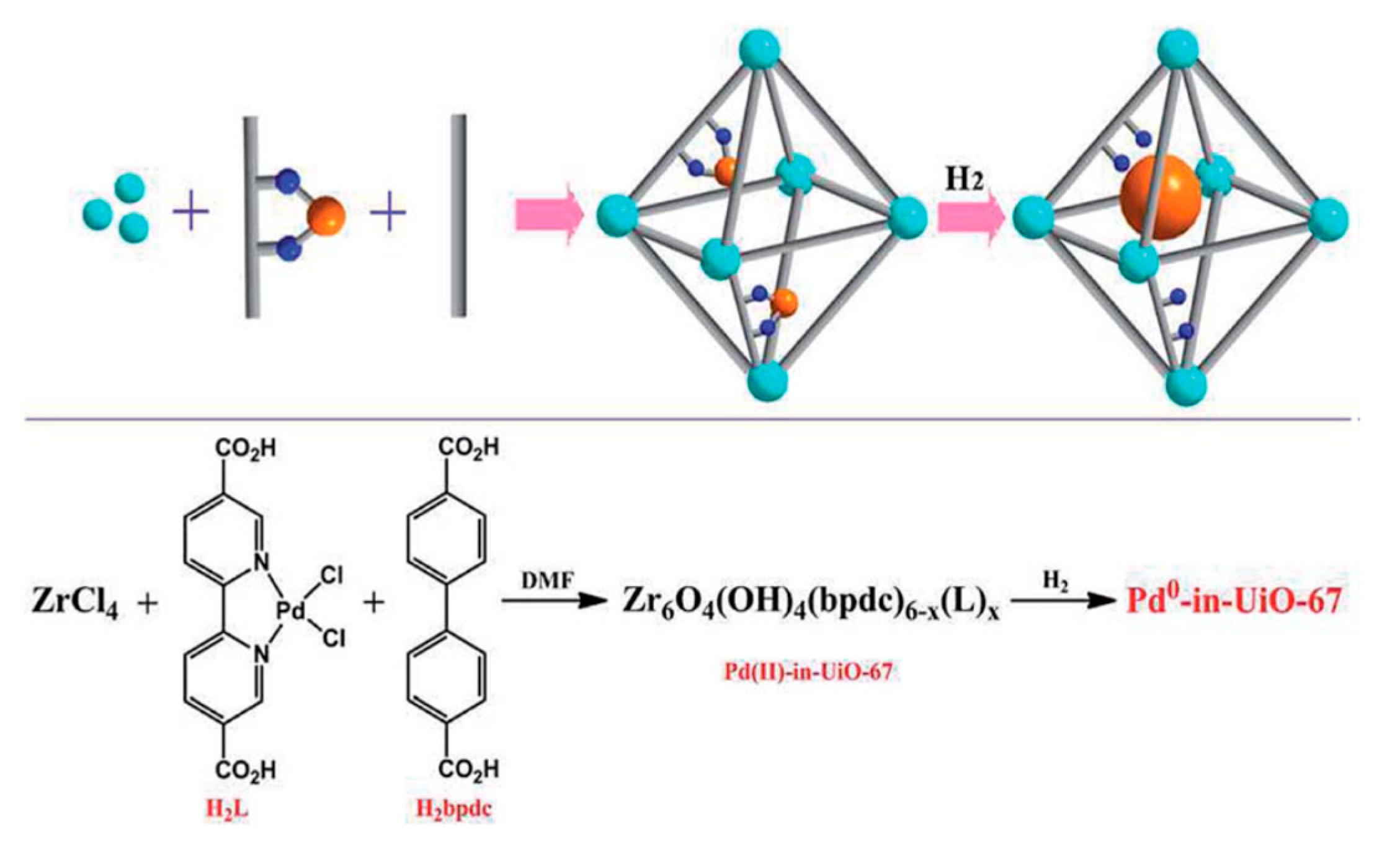






| Catalyst | ABET (m2g−1) | Vp (cm3g−1) | Dp (nm) | Pd Loading (%) | p-CNB Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|---|---|---|
| p-CAN | AN | ||||||
| Pd-MS-05-TMB | 873 | 1.29 | 3.7 | 0.16 | 28.2 | 71.6 | 28.4 |
| Pd-MS-10-TMB | 912 | 1.33 | 3.5 | 0.45 | 41.9 | 94.1 | 5.9 |
| Pd-MS-30-TMB | 980 | 1.43 | 3.3 | 0.54 | 59.7 | 95.3 | 4.7 |
| Pd-MS-50-TMB | 854 | 1.06 | 3.8 | 0.67 | 100 | 99.9 | 0.1 |
| Pd-MS-50 | 919 | 0.0.7 | 2.6 | 0.73 | 48.4 | 82.8 | 17.2 |
| Pd/(MS-50) | 687 | 0.43 | 2.5 | 0.68 | 29.5 | 97.6 | 2.4 |
| Catalyst | Conversion (%) | ee * (%) |
|---|---|---|
| Pd/E-SNT | >99 | 40 |
| Pd/B-SNT | >99 | 48 |
| Pd/E-CS-NT | >99 | 30 |
| Pd/SNT | 46 | 33 |
| Pd/CNT | - | 51 |
 | ||||
|---|---|---|---|---|
| R1, R2, R3 | Catalyst (mol %) | Base | Time (h) | TOF * (h−1) |
| a (-H, -COOH, -H) | BPPd(0)@Si (1) | KOH | 0.5 | 126 |
| a | BPPd(0)@Si (3) | KOH | 0.5 | 58 |
| a | APPd(0)@Si (5) | KOH | 0.5 | 40 |
| a | BPPd(0)@Si (1) | KOH | 0.5 | 40 |
| a | BPPd(0)@Si (5) | K2CO3 | 0.5 | 32.4 |
| b (-H, -COCH3, -H) | BPPd(0)@Si (5) | - | 1 | 20 |
| b | Pd/C (5) | - | 1 | 9 |
| c (-H, -CH2OH, -H) | BPPd(0)@Si (5) | - | 0.5 | 23.2 |
| c | APPd(0)@Si (5) | - | 0.5 | 18.8 |
| c | BPPd(0)@Si (5) | - | 2 | 10 |
| c | APPd(0)@Si (5) | - | 2 | 10 |
| Catalyst | N2H4·H2O (equiv.) | Conversion (%) | Selectivity (%) |
|---|---|---|---|
| SBA-15 | 2 | - | - |
| SBA-COOH | 2 | - | - |
| Pd/SBA-15 | 2 | 68 | 100 |
| Pd/SBA-COOH | 0.5 | 5 | 100 |
| Pd/SBA-COOH | 1 | 44 | 100 |
| Pd/SBA-COOH | 1.5 | 76 | 100 |
| Pd/SBA-COOH | 2 | 100 | 100 |
 | |||
|---|---|---|---|
| Catalyst | Conversion (%) | Selectivity (%) | %Pd |
| RGO-H.H | 0 | 0 | 0 |
| Pd/C-H.H | 11 | 30 | 0.64 |
| Pd/RGO-H.H | 100 | 100 | 0.49 |
| Pd/RGO-salicylic acid | 100 | 100 | 0.75 |
| Pd/RGO-oxalic acid | 100 | 75 | 0.45 |
| Pd/RGO-NaOH | 15 | 44 | 0.55 |
| Pd/RGO-ascorbic acid | 8 | 67 | 0.63 |
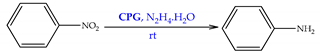 | |||
|---|---|---|---|
| N2H4·H2O (mmol) | Solvent | Time (h) | Conversion (%) |
| 1.0 | EtOH | 8 | 47 |
| 2.0 | EtOH | 7 | 99 |
| 4.0 | EtOH | 4 | >99 |
| 5.0 | EtOH | 4 | >99 |
| 4.0 | EtOH/H2O | 4 | >99 |
| 4.0 | THF | 8 | >99 |
| 4.0 | EtO2 | 8 | 88 |
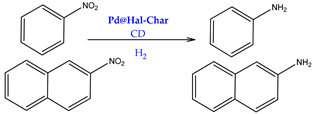 | |||
|---|---|---|---|
| Catalyst | Aniline Yield (%) | Naphthylamine Yield (%) | PdNPs Size (nm) |
| Pd@Hal | 30 | 10 | 4.8 |
| Pd@Hal+β-CD | 65 | 60 | 4.8 |
| Pd@Char | 40 | 30 | 3.6 |
| Pd@Char+β-CD | 75 | 50 | 3.6 |
| Pd@Hal-Char | 75 | 65 | 3.7 |
| Pd@Hal-Char+β-CD | 100 | 95 | 3.7 |
| Pd@Hal-Char-IL | 60 | 30 | 4.5 |
| Pd@Hal-Char-IL+β-CD | 90 | 45 | 4.5 |
| Pd@Hal-Char-SO3H | 45 | 50 | 3.9 |
| Pd@Hal-Char-SO3H+β-CD | 55 | 55 | 3.9 |
| Pd@Hal-Char-CD | 60 | 50 | 4.9 |
| Pd@Hal-Char-CD | 50 | 40 | 3.8 |
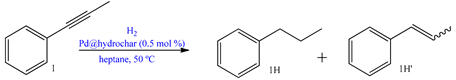 | ||||
|---|---|---|---|---|
| Catalyst | Time (h) | pH2 (bar) | Conversion (%) | Selectivity (%) |
| Pd@HCh-D | 2 | 3 | >99 | >99 |
| Pd@HCh-F | 2 | 3 | >99 | >99 |
| Pd@HCh-D | 1 | 1 | 34 | 91% (cis-1H’) + 9% 1H |
| Pd@HCh-F | 1 | 1 | >99 | 8% trans-1H’ + 72% cis-1H’ +20% 1H |
| Catalyst | Temperature (°C) | Conversion (%) | Selectivity (%)  |
|---|---|---|---|
| Pd@S-1-OH-10 | 150 | 56.7 | 82.5 |
| 175 | 89.5 | 89.7 | |
| 200 | >99.9 | 95.9 | |
| 225 | >99.9 | 98.4 | |
| 250 | >99.9 | >99.9 | |
| 275 | >99.9 | >99.9 | |
| Pd@S-1 | 150 | 48.9 | 85.0 |
| 175 | 62.8 | 91.4 | |
| 200 | 86.6 | 92.8 | |
| 225 | 91.3 | 93.2 | |
| 250 | 92.9 | 97.1 | |
| 275 | 86.6 | 82.6 |
| Substrate | Conversion (%) | |
|---|---|---|
| C@PdZIF-8 | Pd/ZIF-8 | |
 | 95.1 | 98.5 |
 | 75.2 | 77.8 |
 | 47.3 | 44.9 |
 | 24.7 | 21.8 |
 | 19.6 | 17.7 |
| Support | Catalyst | Substrate | Major Outcomes | Ref. |
|---|---|---|---|---|
| Silicas | Pd-MS-xx-TMB | chloronitrobenzenes | The role of TMB as an efficient expanding and structure-directing agent was investigated for the Pd-MS catalysts. Full conversion of p-CNB with a 99.9% selectivity towards p-chloroaniline was observed for Pd-MS-50-TMB. The enhanced activity was attributed to the larger porosity and to the morphology of the support, which enhances mass transfer. TMB catalyst displayed high stability and thermal resistance. | [38] |
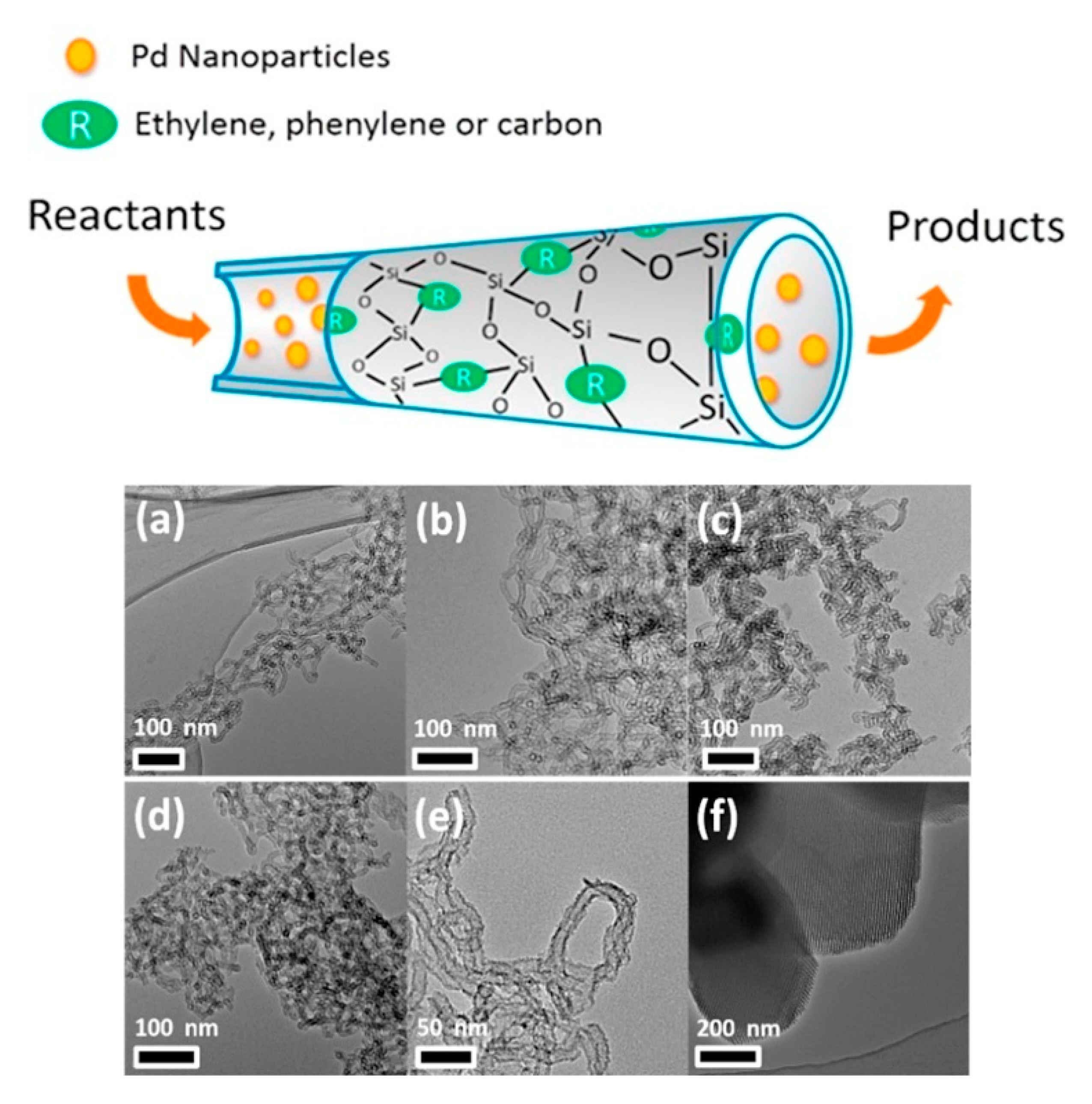
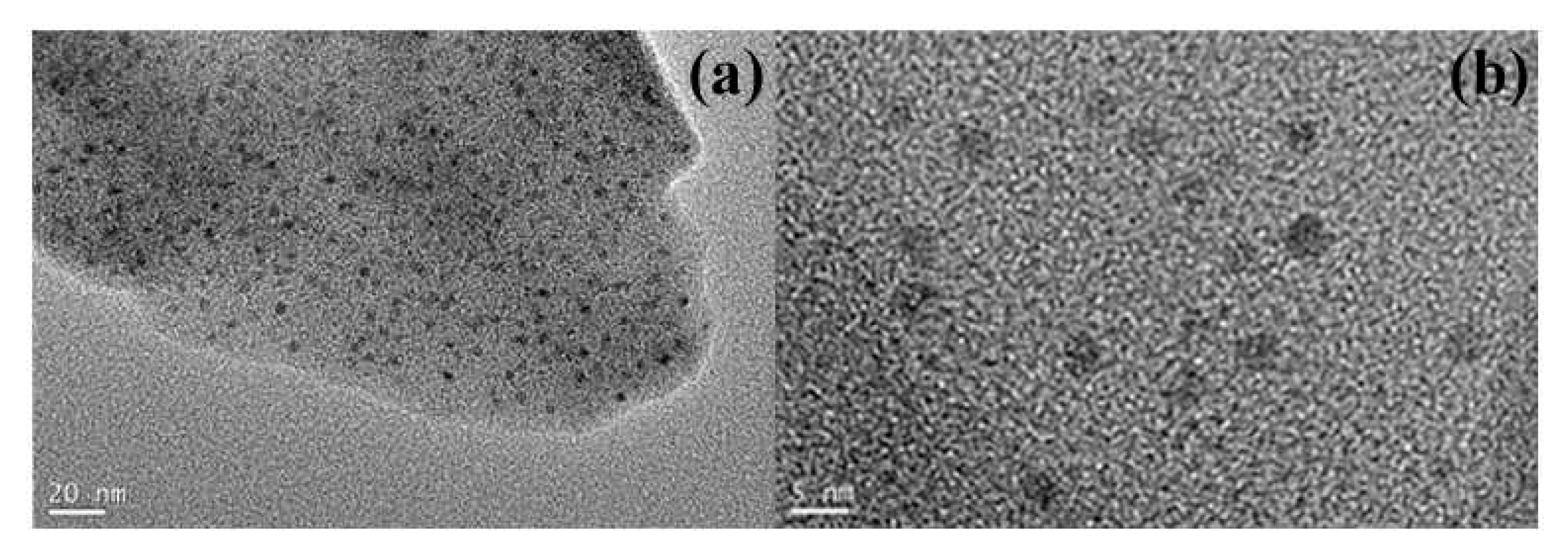
| Silica-based nanotubes supported PdNPs | α,β-unsaturated carboxylic acids | Mesoporous silica-based nanotubes with PdNPs uniformly dispersed were obtained. The catalysts afforded >99% conversion of benzyl alcohol and 89% selectivity to benzaldehyde, as result of the surface hydrophobic/hydrophilic properties, and nanotube morphology. | [41] | |
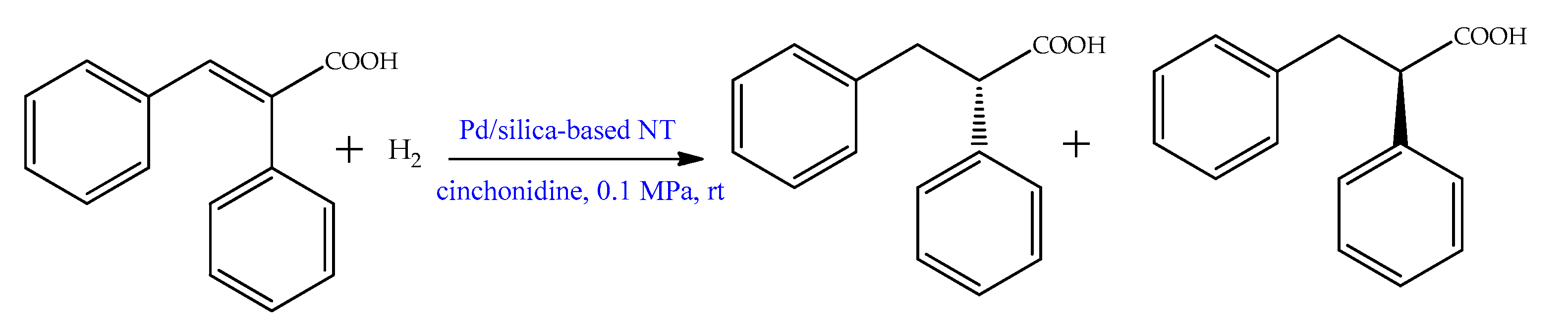
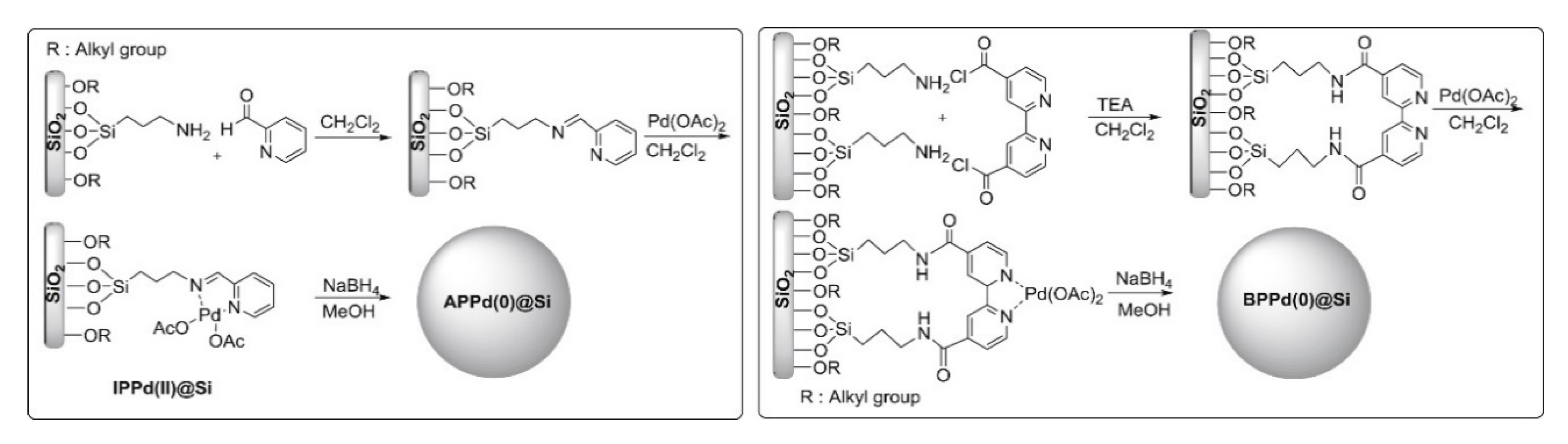
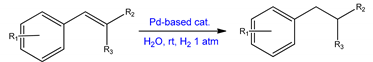
| APPd(0)@Si BPPd(0)@Si | trans-cinnamic acid and related compounds | Green synthesis of Pd(0) catalysts using modified of reverse phase silica, that facilitated metal anchorage. Effective catalysts for hydrogenation of various unsaturated olefins, with excellent recyclability. Product yields above 95% were observed for the hydrogenation of all of the unsaturated esters, ketones, and alcohols in the liquid phase. | [44] | |
| Pd/MCM-41-SiO2 Pd/MCM-41-silatrane | polyunsaturated fatty acid methyl esters | Rapidly and selective conversion. Pd/MCM-41-silatrane has higher activity. The silica source and the Pd amount modified the active sites and size distribution of PdNPs determining the catalytic reactivity and selectivity. | [45] | |
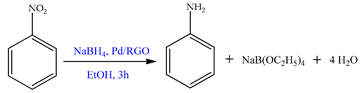
| Pd/SBA-COOH | nitrobenzene | A synergistic effect is observed between the SBA-COOH support and its texture for the particle size control and distribution of PdNPs. Selectivity to nitroaniline reached 100% in all cases. | [47] | |
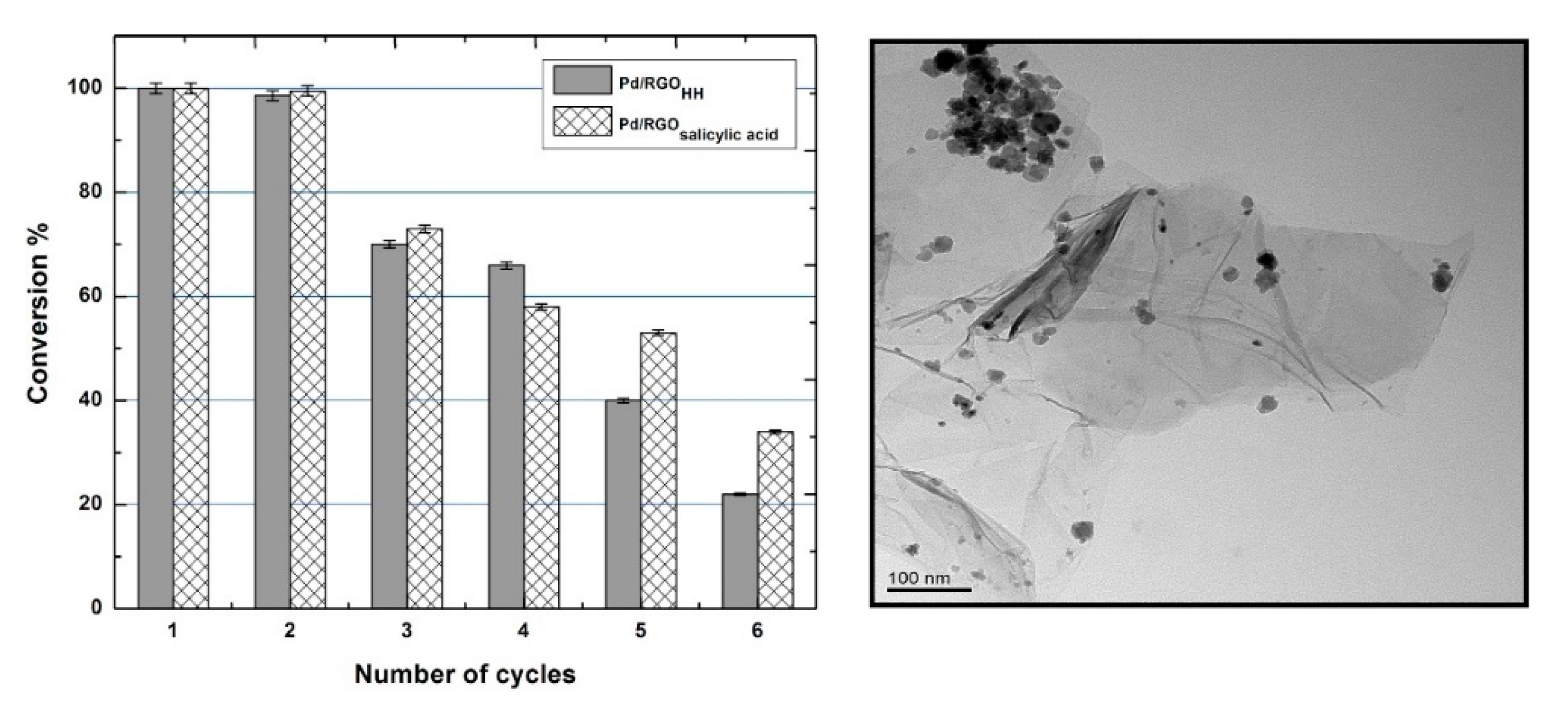
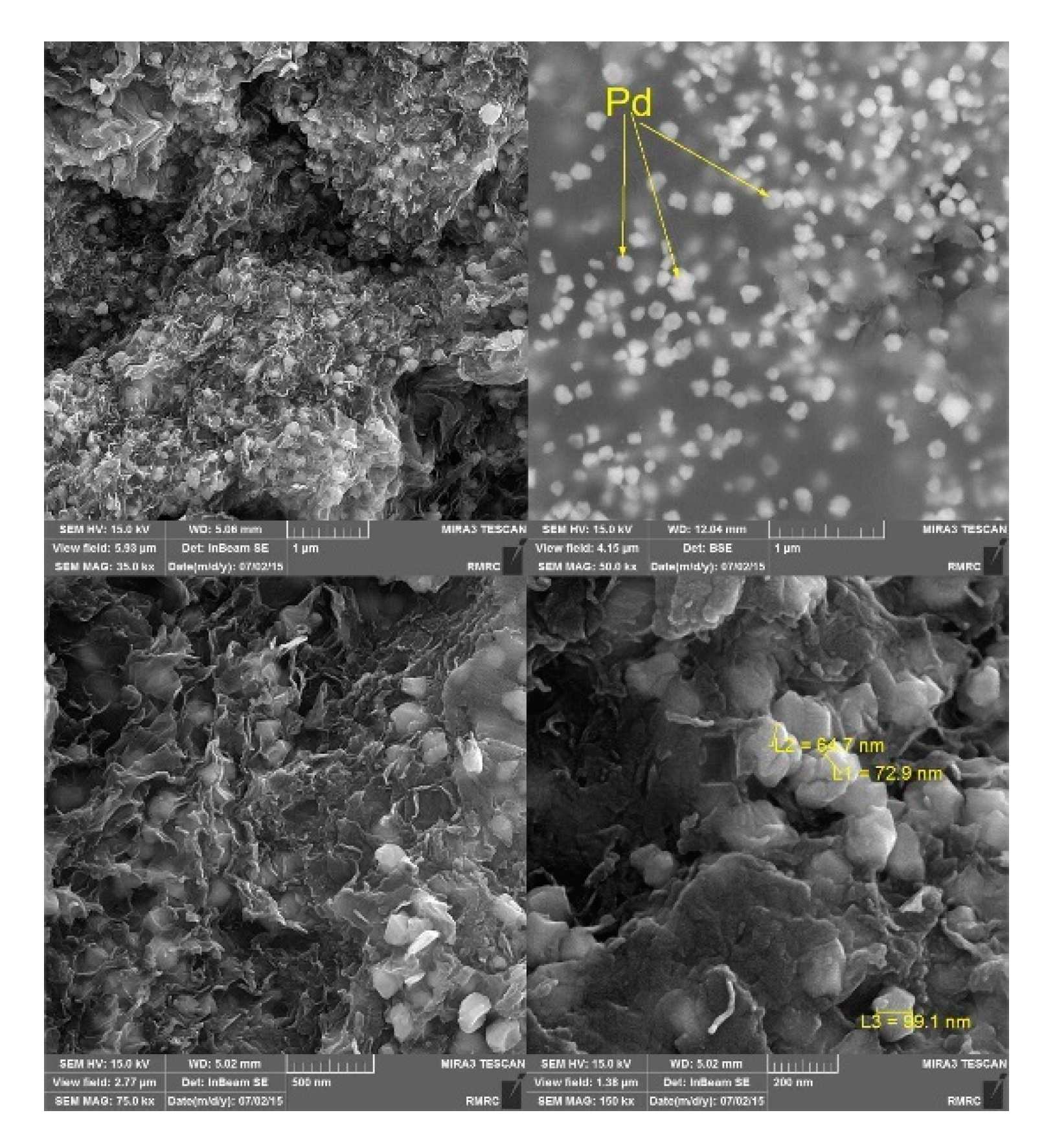
| Carbons | PdNPs/RGO | nitrobenzene | Green in-situ approach using diverse reducing agents. Pd/RGO-salicylic acid exhibited distinct catalytic activity, affording 100% of nitrobenzene conversion, being reused for two times. | [58] |
| PdNPs/RGO | nitroaromatics | Green synthesis through reduction of GO and Pd2+ ions using barberry fruit extract. The catalysts afforded excellent product yield (>95%) for a wide selection of substrates and good catalyst recycling. | [59] | |
| Pd@C/CNT | acetylene | Core–shell structure Pd@C NP CNTs-supported benefited from an N-doped carbon, enhancing the stability of PdNPs, and modifying the electronic structure of PdNP. Pd@C/CNTs presented high selectivity and excellent stability. | [10] | |
| CPG (composite PdNPs graphene) | nitroarenes | Synthesized by a facile route, catalyst presents excellent activity (product yield >99%) and stability for the selective reduction of nitro groups with high yields, ascribed to the synergism between the ultrafine PdNPs and GO support. | [62] | |
| Pd@Hal-Char | nitroarenes | The use of a catalytic amount of β-cyclodextrin significantly improved the reaction yield, reaching 100% in the best case. Hal-Char was more effective as a support than each component individually. PdNPs size and dispersion affected the catalytic activity. | [63] | |
| Pd@HCh | alkynes, alkenes, and carbonyl and nitro derivatives | Bio-sourced PdNPs supported on chars derivatized with ether linkers containing ammonium groups. The latter exhibited high activity in hydrogenations of several substrates, with full hydrogenation of alkyne and alkene substrates. Furthermore, aldehyde and nitro groups were too hydrogenated. The most efficient catalyst was reused ten times outperforming the commercial catalyst. | [64] | |
| Zeolites | Pd/ZSM-5-IS | p-nitrophenol | In-situ synthesis of Pd/ZSM-5-IS, with highly dispersed and small sized PdNPs, showing high catalytic activity, with almost full substrate conversion and remarkable recycle stability. Most of PdNPs were confined within ZSM-5, prevented aggregation and sintering. | [76] |
| Pd@Beta | nitroarenes | The fixation of PdNPs inside Beta zeolite crystals (Pd@Beta) was the key for superior selectivity to the nitro group (for example, 99% selectivity to 4-amino-acetophenone). The outstanding selectivity of Pd@Beta was due to the sterically adsorption on the PdNPs controlled by the zeolite micropores. | [67] | |
| Pd@mnc-S1 | nitrobenzene | A one-pot method was used for PdNPs encapsulation. Excellent stability and activity were observed for the catalyst, due to its unique porosity and nanostructure that provided general shape-selectivity for selective hydrogenation reactions. A conversion of 94% was achieved over Pd@mnc-S1. | [79] | |
| Pd@S-1-OH | furfural | A novel approach for selective hydrogenation using a core-shell Pd@S-1 zeolite catalyst with well-regulated wettability of the zeolite cover. Influence of the hydrophilicity of the microporosity of the zeolite on the diffusion of various molecules was discussed. Catalysts show outstanding activity, selectivity, and stability in furfural hydrogenation, showing furan selectivity as high as 99.9% with a complete conversion of furfural. | [81] | |
| Pd@FER | 1-hexene 1-phenyl-1-cyclohexene | Remarkable shape-selectivity in the catalytic hydrogenation of different molecular sizes olefins, or steric hindrance due to nanostructure and microporosity of the catalyst. Full conversion was achieved for 1-hexene. | [83] | |
| Pd@Beta Pd@MWW | nitroarenes | Electronic/steric effects were accountable for the catalytic activity, with catalyst being less active for nitroarenes containing an electron-withdrawing substituent. The ortho isomers are more difficult to reduce due to steric hindrance effect. Full conversion was observed for 3-nitrotoluene and 3-nitroanisole. | [84] | |
| MOFs | Pd@MIL-101 Pd@MIL-53 | phenol | The effect of framework structure of Pd@MOFs was studied. MIL-101 was a better support, due to its mesoporosity and hydrophilicity. Larger PdNPs on the external surface of MIL-53. Both catalysts provided high selectivity (>98%) to cyclohexanone. The catalysts were recycled 5 times. | [89] |
| Pd@MIL-100(Fe) | nitrophenols | Facile synthesis of Pd@MIL-100(Fe) with uniform morphology and size. The confinement effect leads to high stability within the MIL-100(Fe). High catalytic activity (conversion > 95%) and reusability due to the synergism effect amongst PdNPs and MIL-100(Fe). | [95] | |
| Pd(0)-in-UiO_67 | styrene and tetraphenylethylene | Facile and efficient strategy for Pd@MOF catalysts via controllable introduction of Pd precursors prior to MOF assembly. Catalysts were highly active due to electron donation and confinement effects. High stability and reusability. Full conversion of styrene was reported. | [97] | |
| Pd@UiO-66-X | benzoic acid | Modulation of the surface microenvironment of the MOF via group functionalization and metal substitution. The activity is assigned to charge transfer processes, and to the adsorption energy of the substrate. Pd@UiO-66-2OH, which features a moderate adsorption energy and a high Pd electronic state, has shown the highest activity (>99%). Selectivity to benzyl alcohol was >99% in all cases. | [100] | |
| C@Pd/ZIF-8 | olefins | Preparation of ZIF-8 supported C-stabilized PdNPs through an effective strategy. Due to efficient stabilization, the controlled sized PdNPs can be well dispersed onto the ZIF-8. High activity in the hydrogenation of olefins (95%) and a satisfactory reuse in 5 cycles. | [102] | |
| Pd@ZIF-8 hollow microspheres | alkenes | A green approach for Pd@ZIF-8 hollow microspheres preparation, uniform in size, with good structural stability, high catalytic activity and size selectivity towards the hydrogenation of alkenes (full conversion for 1-hexene), and good recycling stability. | [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, M.A.; Martins, L.M.D.R.S. Supported Palladium Nanocatalysts: Recent Findings in Hydrogenation Reactions. Processes 2020, 8, 1172. https://doi.org/10.3390/pr8091172
Andrade MA, Martins LMDRS. Supported Palladium Nanocatalysts: Recent Findings in Hydrogenation Reactions. Processes. 2020; 8(9):1172. https://doi.org/10.3390/pr8091172
Chicago/Turabian StyleAndrade, Marta A., and Luísa M. D. R. S. Martins. 2020. "Supported Palladium Nanocatalysts: Recent Findings in Hydrogenation Reactions" Processes 8, no. 9: 1172. https://doi.org/10.3390/pr8091172
APA StyleAndrade, M. A., & Martins, L. M. D. R. S. (2020). Supported Palladium Nanocatalysts: Recent Findings in Hydrogenation Reactions. Processes, 8(9), 1172. https://doi.org/10.3390/pr8091172






