Validating the Efficiency of the FeS2 Method for Elucidating the Mechanisms of Contaminant Removal Using Fe0/H2O Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions
2.1.1. Dyes
2.1.2. Iron
2.2. Solid Materials
2.2.1. Metallic Iron (Fe0)
2.2.2. Sand
2.2.3. Pyrite (FeS2)
2.3. Dye Discoloration
2.4. Analytical Methods
2.5. Presentation of Experimental Results
3. Results and Discussion
3.1. Evidence for the pH-Shifting Nature of FeS2
3.2. MB and MO Discoloration in Fe0/FeS2/Sand Systems
3.3. RR120 Discoloration Compared to MB and MO in Fe0/FeS2/Sand Systems
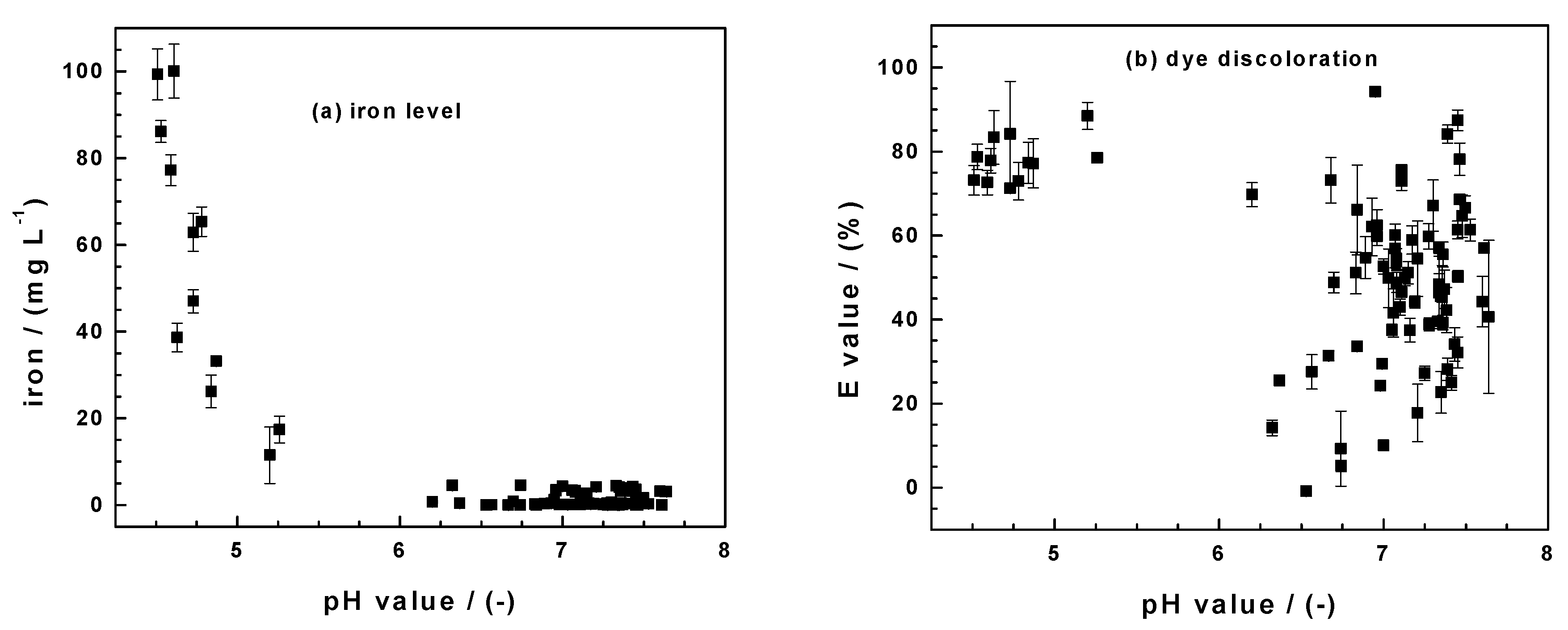
3.3.1. Changes of pH Value and Iron Concentration
3.3.2. Dye Discoloration
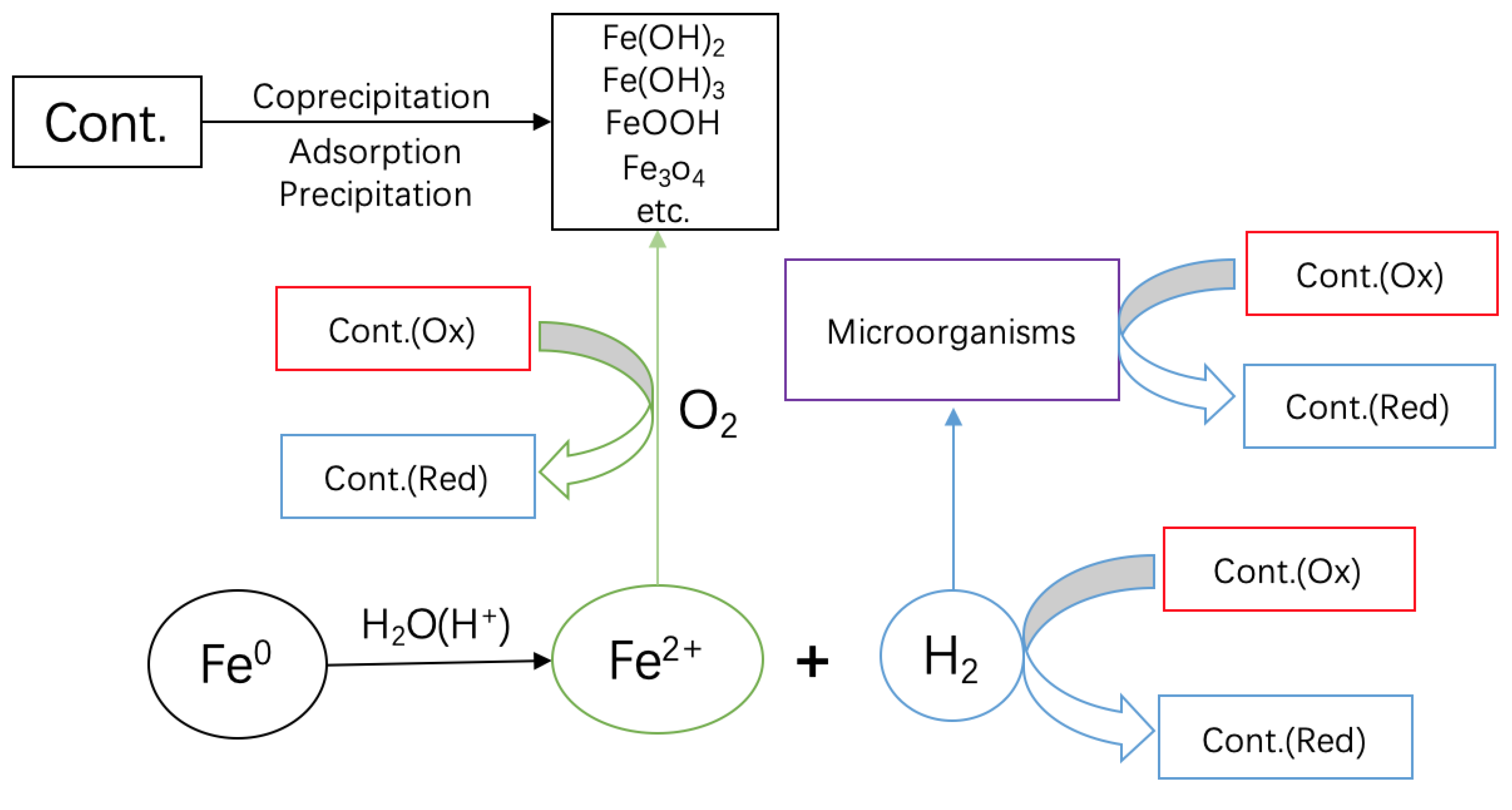
3.4. Implications on Contaminant Removal Mechanisms
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bischof, G. The Purification of Water: Embracing the Action of Spongy Iron on Impure Water; Bell and Bain: Glasgow, Scotland, 1873; p. 19. [Google Scholar]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Works Ass. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for environmental remediation: Prospects and limitations. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; Chapter 36; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2019; pp. 531–544. [Google Scholar]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an In Situ “iron wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Richardson, J.P.; Nicklow, J.W. In Situ permeable reactive barriers for groundwater contamination. Soil Sediment Contam. 2002, 11, 241–268. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freib. Online Geosci. 2015, 32, 1–80. [Google Scholar]
- Antia, D.D.J. ZVI (Fe0) Desalination: Stability of product water. Resources 2016, 5, 15. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, R.; Cui, X.; Gwenzi, W.; Noubactep, C. Understanding the operating mode of Fe0/Fe-sulfide/H2O systems for water treatment. Processes 2020, 8, 409. [Google Scholar] [CrossRef]
- Chen, K.; Han, L.; Li, J.; Lü, Y.; Yao, C.; Dong, H.; Wang, L.; Li, Y. Pyrite enhanced the reactivity of zero valent iron for reductive removal of dyes. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef]
- Jiao, Y.; Qiu, C.; Huang, L.; Wu, K.; Ma, H.; Chen, S.; Ma, L.; Wu, L. Reductive dechlorination of carbon tetrachloride by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2009, 91, 434–440. [Google Scholar] [CrossRef]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited. The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef]
- Ghauch, A.; Abou, A.H.; Bdeir, S. Aqueous removal of diclofenac by plated elemental iron: Bimetallic systems. J. Hazard. Mater. 2010, 182, 64–74. [Google Scholar] [CrossRef]
- Ghauch, A.; Abou Assi, H.; Baydoun, H.; Tuqan, A.M.; Bejjani, A. Fe0-based trimetallic systems for the removal of aqueous diclofenac: Mechanism and kinetics. Chem. Eng. J. 2011, 172, 1033–1044. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 2011, 196, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Sauter, M. Significance of oxide-film in discussing the mechanism of contaminant removal by elemental iron materials. In Photo-Electrochemistry and Photo-Biology for the Sustainability; Union Press: Osaka, Japan, 2012; pp. 97–122. [Google Scholar]
- Huang, Y.H.; Peddi, P.K.; Zeng, H.; Tang, C.-L.; Teng, X. Pilot-scale demonstration of the hybrid zero-valent iron process for treating flue-gas-desulfurization wastewater: Part, I. Water Sci. Technol. 2013, 67, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Tang, C.; Zeng, H. Removing molybdate from water using a hybridized zero-valent iron/magnetite/Fe(II) treatment system. Chem. Eng. J. 2012, 200–202, 257–263. [Google Scholar] [CrossRef]
- Lü, Y.; Li, J.F.; Li, Y.M.; Liang, L.P.; Dong, H.P.; Chen, K.; Yao, C.; Li, Z.; Li, J.; Guan, X. The roles of pyrite for enhancing reductive removal of nitrobenzene by zero-valent iron. Appl. Catal. B Environ. 2019, 242, 9–18. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Characterizing the impact of MnO2 on the efficiency of Fe0-based filtration systems. Chem. Eng. J. 2014, 250, 416–422. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Sustaining the efficiency of the Fe(0)/H2O system for Cr(VI) removal by MnO2 amendment. Chemosphere 2019, 214, 389–398. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Song, D.-I.; Kim, Y.H.; Shin, W.S. A simple mathematical analysis on the effect of sand in Cr(VI) reduction using zero valent iron. Korean J. Chem. Eng. 2005, 22, 67–69. [Google Scholar] [CrossRef]
- Bi, E.; Devlin, J.F.; Huang, B. Effects of mixing granular iron with sand on the kinetics of trichloroethylene reduction. Ground Water Monit. Remed. 2009, 29, 56–62. [Google Scholar] [CrossRef]
- Hu, R.; Yang, H.; Tao, R.; Cui, X.; Xiao, M.; Konadu-Amoah, B.; Cao, V.; Lufingo, M.; Soppa-Sangue, N.P.; Ndé-Tchoupé, A.I.; et al. Metallic iron for environmental remediation: Starting an overdue progress in knowledge. Water 2020, 12, 641. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water treatment using metallic iron: A tutorial review. Processes 2019, 7, 622. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Effects of mixing granular iron with sand on the efficiency of methylene blue discoloration. Chem. Eng. J. 2012, 200–202, 433–438. [Google Scholar] [CrossRef]
- Kim, H.; Yang, H.; Kim, J. Standardization of the reducing power of zerovalent iron using iodine. Environ. Lett. 2014, 49, 514–523. [Google Scholar]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe(0) content and reactivity of zero valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 1–60. [Google Scholar]
- Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Designing metallic iron based water filters: Light from methylene blue discoloration. J. Environ. Manag. 2016, 166, 567–573. [Google Scholar] [CrossRef]
- Mitchell, G.; Poole, P.; Segrove, H.D. Adsorption of methylene blue by high-silica sands. Nature 1955, 176, 1025–1026. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in groundwater: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Meinrath, G.; Merkel, J.B. Investigating the mechanism of uranium removal by zerovalent iron materials. Environ. Chem. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Gatcha-Bandjun, N.; Noubactep, C.; Loura, B.B. Mitigation of contamination in effluents by metallic iron: The role of iron corrosion products. Environ. Technol. Innov. 2017, 8, 71–83. [Google Scholar] [CrossRef]
- Groysman, A. Corrosion for Everybody; Springer: Dordrecht, The Netherlands, 2010; p. 368. [Google Scholar]
- Chopard, A.; Benzaazoua, M.; Bouzahzah, H.; Plante, B.; Marion, P. A contribution to improve the calculation of the acid generating potential of mining wastes. Chemosphere 2017, 175, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Chopard, A.; Marion, P.; Mermillod-Blondin, R.; Plante, B.; Benzaazoua, M. Environmental impact of mine exploitation: An early predictive methodology based on ore mineralogy and contaminant speciation. Minerals 2019, 9, 397. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Kojima, M.; Hiroyoshi, N.; Ito, M. Galvanic microencapsulation (GME) using zero-valent aluminum and zero-valent iron to suppress pyrite oxidation. Mater. Trans. 2019, 60, 277–286. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Li, X.; Seng, S.; Villacorte-Tabelin, M.; Igarashi, T.; Ito, M.; Hiroyoshi, N. Development of advanced pyrite passivation strategies towards sustainable management of acid mine drainage. IOP Conf. Ser. Earth Environ. Sci. 2019, 351. [Google Scholar] [CrossRef]
- Rodríguez, A.; García, J.; Ovejero, G.; Mestanza, M. Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: Equilibrium and kinetics. J. Hazard. Mater. 2009, 172, 1311–1320. [Google Scholar] [CrossRef]
- Attia, A.A.; Girgis, B.S.; Fathy, N.A. Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: Batch and column studies. Dyes Pigment. 2008, 76, 282–289. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef]
- Huang, J.H.; Huang, K.L.; Liu, S.Q.; Wang, A.T.; Yan, C. Adsorption of Rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 55–61. [Google Scholar] [CrossRef]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Xiao, M.; Qiu, P.; Lufingo, M.; Gwenzi, W.; Noubactep, C. Characterizing the suitability of granular Fe0 for the water treatment industry. Processes 2019, 7, 652. [Google Scholar] [CrossRef]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Characterizing the impact of sand addition on the efficiency of granular iron for water treatment. Chem. Eng. J. 2015, 262, 891–896. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Varlikli, C.; Bekiari, V.; Kus, M.; Boduroglu, N.; Oner, I.; Lianos, P.; Lyberatos, G.; Icli, S. Adsorption of dyes on Sahara desert sand. J. Hazard. Mater. 2009, 170, 27–34. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489. [Google Scholar] [CrossRef]
- Shiba, M.; Uddin, M.A.; Kato, Y.; Ono, T. Degradation of chlorinated organic compounds by mixed particles of iron/iron sulfide or iron/iron disulfide. Mater. Trans. 2014, 55, 708–712. [Google Scholar] [CrossRef]
- Kantar, C.; Ari, C.; Keskin, S.; Dogaroglu, Z.G.; Karadeniz, A.; Alten, A. Cr(VI) removal from aqueous systems using pyrite as the reducing agent: Batch, spectroscopic and column experiments. J. Contam. Hydrol. 2015, 174, 28–38. [Google Scholar] [CrossRef]
- Kantar, C.; Bulbul, M.S. Effect of pH-buffering on Cr(VI) reduction with pyrite in the presence of various organic acids: Continuous-flow experiments. Chem. Eng. J. 2016, 287, 173–180. [Google Scholar] [CrossRef]
- Saywell, L.G.; Cunningham, B.B. Determination of iron: Colorimetric o-phenanthroline method. Ind. Eng. Chem. Anal. Ed. 1937, 9, 67–69. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Hussain, I.; Du, X.; Huang, S.; Wen, W. Effect of pyrite on enhancement of zero-valent iron corrosion for arsenic removal in water: A mechanistic study. Chemosphere 2019, 233, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S.; Crane, R.A. Nanoscale metallic iron for environmental remediation: Prospects and limitations. Water Air Soil Pollut. 2012, 223, 1363–1382. [Google Scholar] [CrossRef]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using three azo dyes in batch systems. J. Environ. Chem. Eng. 2016, 4, 65–72. [Google Scholar] [CrossRef]
- Phukan, M. Characterizing the Fe0/sand system by the extent of dye discoloration. Freiberg Online Geosci. 2015, 40, 1–70. [Google Scholar]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef]
- Nanseu-Njiki, C.P.; Gwenzi, W.; Pengou, M.; Rahman, M.A.; Noubactep, C. Fe0/H2O filtration systems for decentralized safe drinking water: Where to from here? Water 2019, 11, 429. [Google Scholar] [CrossRef]
- Ebelle, T.C.; Makota, S.; Tepong-Tsindé, R.; Nassi, A.; Noubactep, C. Metallic iron and the dialogue of the deaf. Fresenius Environ. Bull. 2019, 28, 8331–8340. [Google Scholar]
- Lü, Y.; Li, Z.F.; Li, J.F.; Chen, K.; Dong, H.P.; Shou, J.X.; Li, Y. Synergetic effect of pyrite on Cr(VI) removal by zero valent iron in column experiments: An investigation of mechanisms. Chem. Eng. J. 2018, 349, 522–529. [Google Scholar] [CrossRef]
- Khudenko, B.M. Feasibility evaluation of a novel method for destruction of organics. Water Sci. Technol. 1991, 23, 1873–1881. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Volke, P.; Peter, H.-J.; Dietrich, P.; Merkel, B. Understanding the Mechanism of the Uranium Mitigation by Zero Valent Iron in Effluents; Wiss. Mitt. Institut für Geologie der TU Bergakademie Freiberg: Freiberg, Germany, 2001; pp. 37–44. [Google Scholar]
- Noubactep, C.; Meinrath, G.; Volke, P.; Dietrich, P.; Merkel, B.J. Mechanisms of uranium fixation by zero valent iron: The importance of co-precipitation. In Uranium in the Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 2002; pp. 577–586. [Google Scholar]
- Howe, K.J.; Hand, D.W.; Crittenden, J.C.; Trussell, R.R.; Tchobanoglous, G. Principles of Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 647. [Google Scholar]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3050–3056. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of multiple heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3057–3062. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Meinrath, G. Mechanism of uranium removal from the aqueous solution by elemental iron. J. Hazard. Mater. 2006, 132, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Antia, D.D.J. Water treatment and desalination using the eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz[mH2O], and n-Fex[Cation]nOyHz[Anion]m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Cao, V.; Yang, H.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. Tracing the Scientific History of Fe0-Based Environmental Remediation Prior to the Advent of Permeable Reactive Barriers. Processes 2020, 8, 977. [Google Scholar] [CrossRef]
- He, F.; Gong, L.; Fan, D.; Tratnyek, P.G.; Lowry, G.V. Quantifying the efficiency and selectivity of organohalide dechlorination by zerovalent iron. Environ. Sci. Process. Impacts 2020, 22, 528. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Ruppert, H.; Noubactep, C. Designing the next generation of Fe0-based filters for decentralized safe drinking water treatment: A conceptual framework. Processes 2020, 8, 745. [Google Scholar] [CrossRef]
- Cui, X. Study on the Material Selection of Zero Valent Iron Permeable Reactive Barrier and the Effect of Pyrite on Its Treatment Efficiency. Master’s Thesis, Hohai University, Nanjing, China, 2020. (In Chinese). [Google Scholar]
- Bojic, A.L.; Bojic, D.; Andjelkovic, T. Removal of Cu2+ and Zn2+ from model wastewaters by spontaneous reduction–coagulation process in flow conditions. J. Hazard. Mater. 2009, 168, 813–819. [Google Scholar] [CrossRef]
- Sato, N. Surface oxides affecting metallic corrosion. Corros. Rev. 2001, 19, 253–272. [Google Scholar] [CrossRef]
- Ullah, S.; Guo, X.; Luo, X.; Zhang, X.; Li, Y.; Liang, Z. The coupling of sand with ZVI/oxidants achieved proportional and highly efficient removal of arsenic. Front. Environ. Sci. Eng. 2020, 14, 94. [Google Scholar]
- Heimann, S.; Ndé-Tchoupé, A.I.; Hu, R.; Licha, T.; Noubactep, C. Investigating the suitability of Fe0 packed-beds for water defluoridation. Chemosphere 2018, 209, 578–587. [Google Scholar] [CrossRef]
- Roberts, A.L.; Totten, L.A.; Arnold, W.A.; Burris, D.R.; Campbell, T.J. Reductive elimination of chlorinated ethylenes by zero-valent metals. Environ. Sci. Technol. 1996, 30, 2654–2659. [Google Scholar] [CrossRef]
- Miehr, R.; Tratnyek, P.G.; Bandstra, Z.J.; Scherer, M.M.; Alowitz, J.M.; Bylaska, J.E. Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 2004, 38, 139–147. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Wang, C.; Li, X.-z. Electron efficiency of zero-valent iron for groundwater remediation and wastewater treatment. Chem. Eng. J. 2013, 215–216, 90–95. [Google Scholar] [CrossRef]
- Wu, C.-Q.; Fu, D.-T.; Chen, X. Nitrobenzene degradation by micro-sized iron and electron efficiency evaluation. Chem. Pap. 2014, 68, 1350–1357. [Google Scholar] [CrossRef]
- Gillham, R.W. Development of the granular iron permeable reactive barrier technology (good science or good fortune). In Advances in Environmental Geotechnics, Proceedings of the International Symposium on Geoenvironmental Engineering in Hangzhou, China, 8–10 September 2007; Chen, Y., Tang, X., Zhan, L., Eds.; Springer: Berlin, Germany, 2008; pp. 5–15. [Google Scholar]
- Duan, R.; Dong, Y.; Zhang, Q. Characteristics of aggregate size distribution of nanoscale zero-valent iron in aqueous suspensions and its effect on transport process in porous media. Water 2018, 10, 670. [Google Scholar] [CrossRef]
- Shao, Q.; Xu, C.; Wang, Y.; Huang, S.; Zhang, B.; Huang, L.; Fan, D.; Tratnyek, P.G. Dynamic interactions between sulfidated zerovalent iron and dissolved oxygen: Mechanistic insights for enhanced chromate removal. Water Res. 2018, 135, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Mondino, F.; Piscitello, A.; Bianco, C.; Gallo, A.; de Folly D’Auris, A.; Tosco, T.; Tagliabue, M.; Sethi, R. Injection of zerovalent iron gels for aquifer nanoremediation: Lab experiments and modeling. Water 2020, 12, 826. [Google Scholar] [CrossRef]
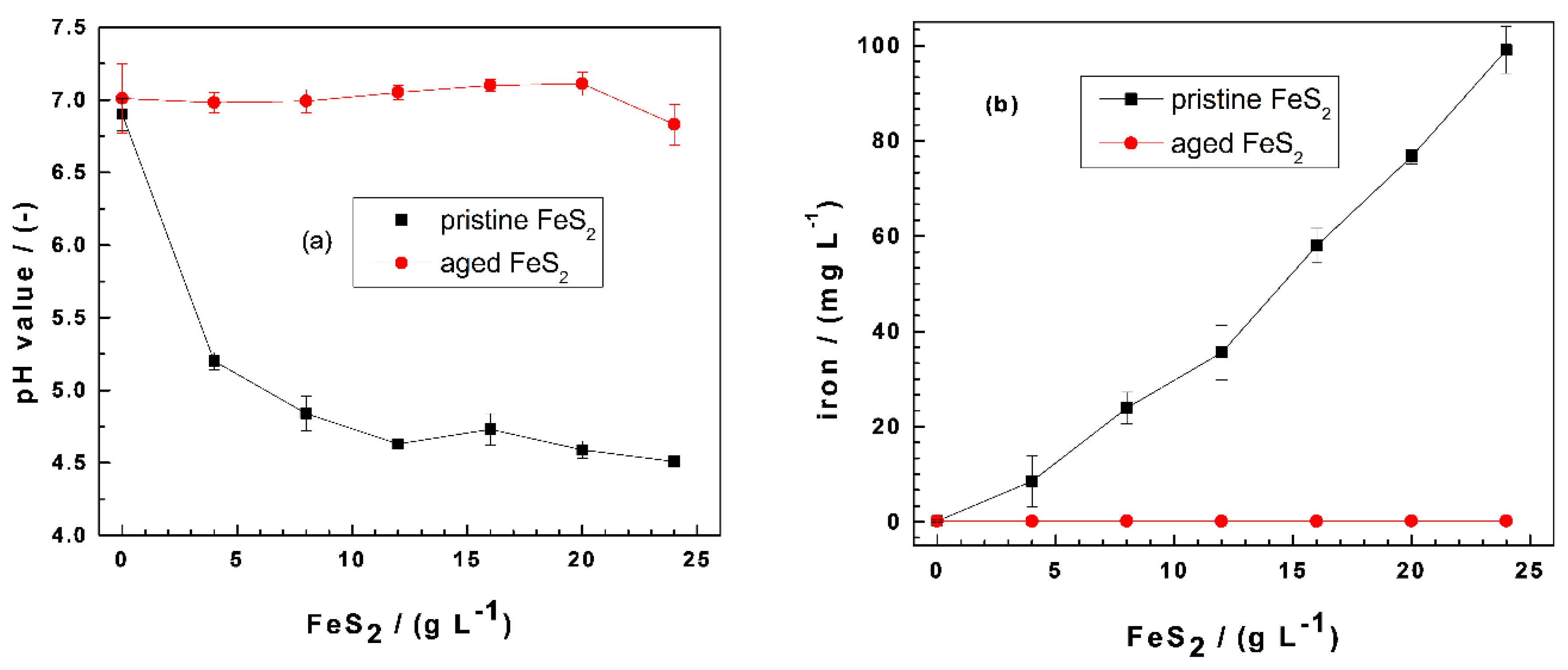
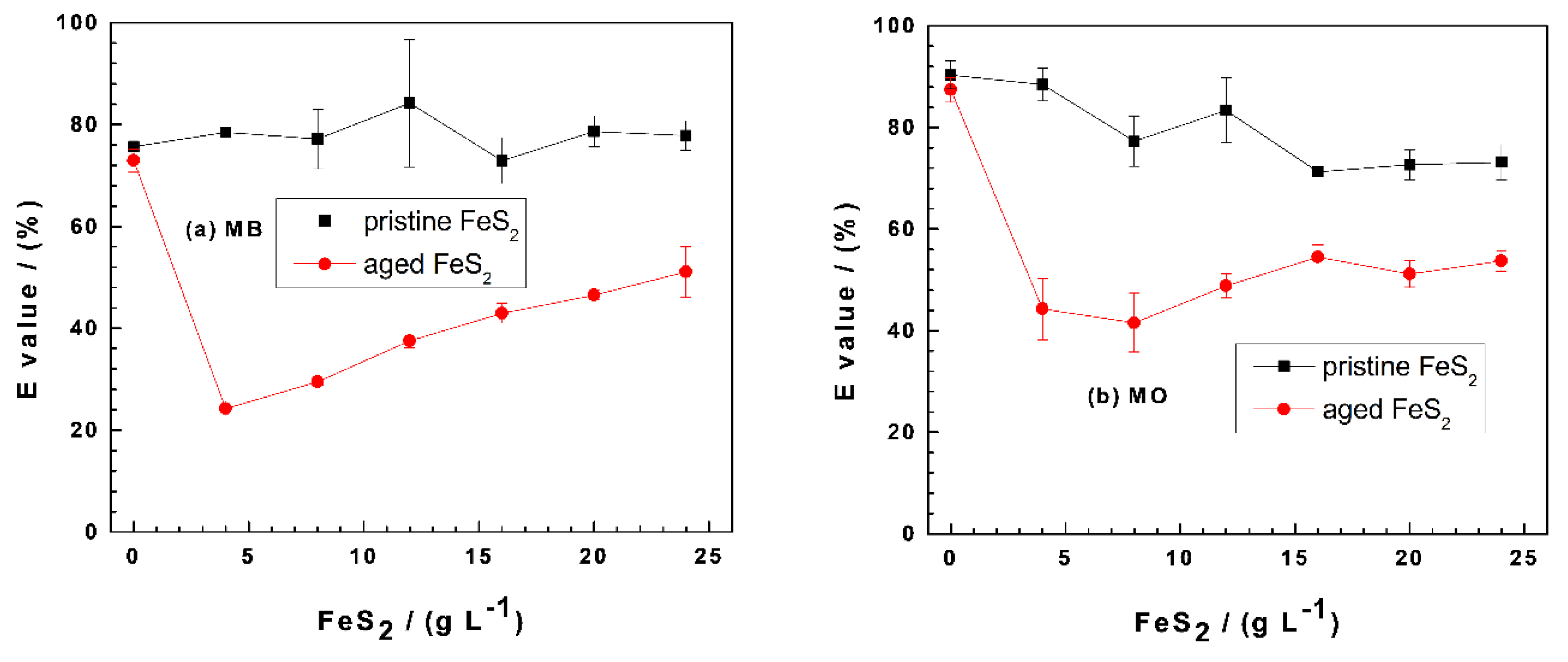
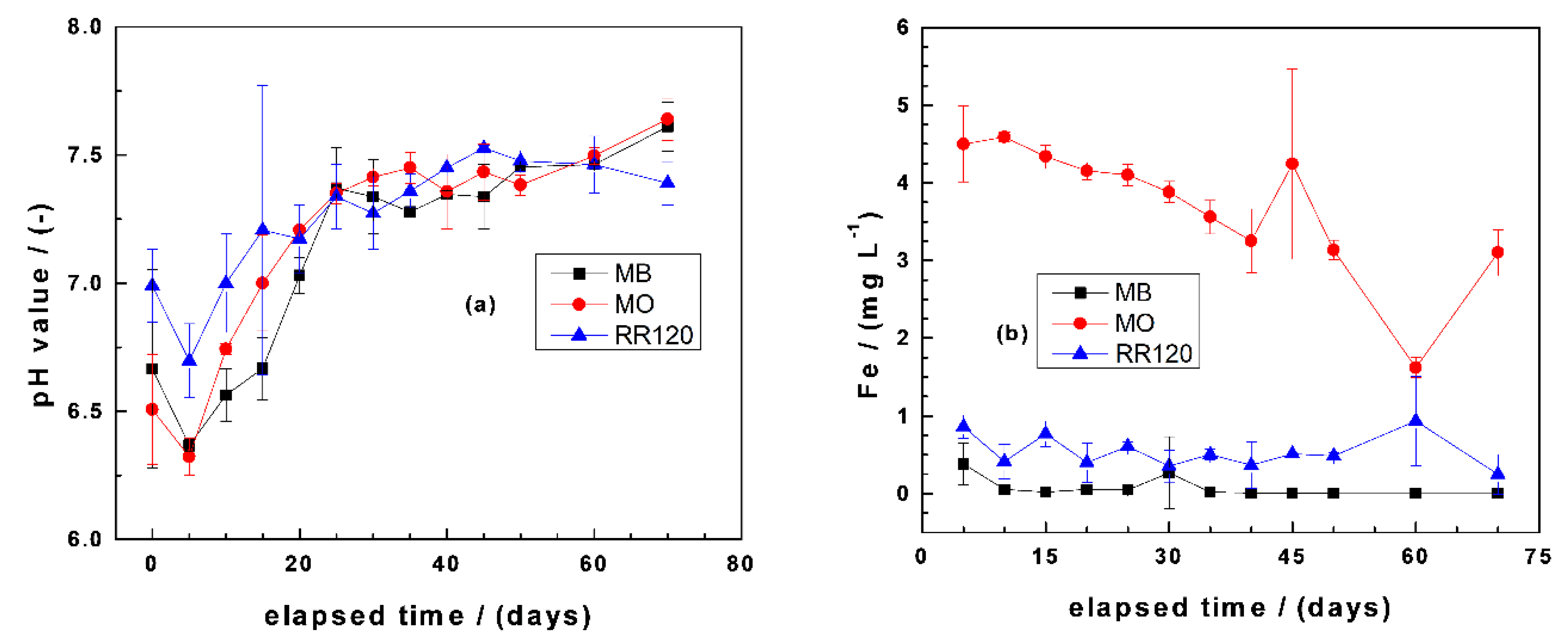
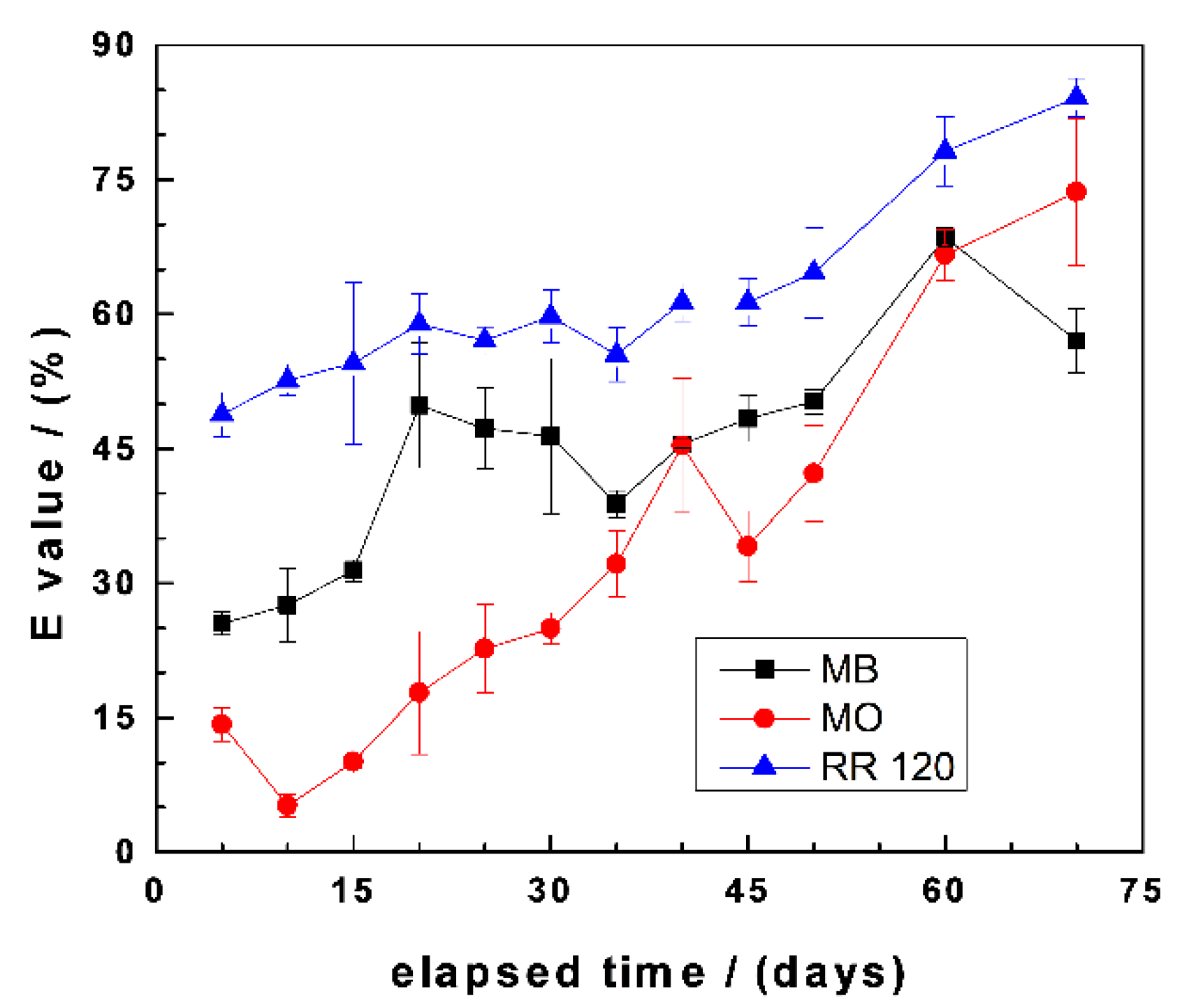
| Dye | Symbol | Formula | MW (g mol−1) | Nature | λmax (nm) |
|---|---|---|---|---|---|
| Methylene blue | MB | C16H18ClN3S·3H2O | 319.00 | cationic | 664.5 |
| Methyl orange | MO | C14H12N3O3NaS | 327.34 | anionic | 464.0 |
| Reactive red 120 | RR120 | C44H30Cl2N14O20S6 | 1469.98 | anionic | 515.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Cui, X.; Hu, R.; Gwenzi, W.; Noubactep, C. Validating the Efficiency of the FeS2 Method for Elucidating the Mechanisms of Contaminant Removal Using Fe0/H2O Systems. Processes 2020, 8, 1162. https://doi.org/10.3390/pr8091162
Xiao M, Cui X, Hu R, Gwenzi W, Noubactep C. Validating the Efficiency of the FeS2 Method for Elucidating the Mechanisms of Contaminant Removal Using Fe0/H2O Systems. Processes. 2020; 8(9):1162. https://doi.org/10.3390/pr8091162
Chicago/Turabian StyleXiao, Minhui, Xuesong Cui, Rui Hu, Willis Gwenzi, and Chicgoua Noubactep. 2020. "Validating the Efficiency of the FeS2 Method for Elucidating the Mechanisms of Contaminant Removal Using Fe0/H2O Systems" Processes 8, no. 9: 1162. https://doi.org/10.3390/pr8091162
APA StyleXiao, M., Cui, X., Hu, R., Gwenzi, W., & Noubactep, C. (2020). Validating the Efficiency of the FeS2 Method for Elucidating the Mechanisms of Contaminant Removal Using Fe0/H2O Systems. Processes, 8(9), 1162. https://doi.org/10.3390/pr8091162







