Abstract
Recently reported acetosolv soft- and hardwood lignins as well as ionosolv soft- and hardwood lignins were transformed into monomeric aromatic compounds using either a vanadate or a molybdate-based catalyst system. Monomers were generated with remarkable, catalyst-dependent selectivity and high depolymerisation yields via oxidative exo- and endo-depolymerisation processes. Using the vanadate–hydrogen peroxide system on acetosolv pine lignin, vanillin and isovanillin were produced as main products with depolymerisation yields of 31%. Using the molybdate system on acetosolv and ionosolv lignin, vanillic acid was the practically exclusive product, with depolymerisation yields of up to 72%. Similar selectivities, albeit with lower depolymerisation yields of around 50% under standardised conditions, were obtained for eucalyptus acetosolv lignin, producing vanillin and syringaldehyde or vanillic acid as products, by using the vanadate- or the molybdate-based systems respectively.
1. Introduction
Lignocellulosic biomass received great interest as a sustainable and renewable source of fuel and platform chemicals in recent years [1,2]. Numerous studies target the conversion of cellulose and hemicelluloses into ethanol and other biofuels as well as platform chemicals for the chemical industries [3,4,5]. In sharp contrast, research on the conversion of lignin has often been limited to its removal from the other two principal biomass components either to enhance their chemical and/or enzymatic valorisation. Conversion of lignin—representing after all 30% of the weight and 40% of the energy content of lignocellulosic biomass and being isolated as a by-product in form of various technical lignins with different characteristics by cellulose-focused processes—is still a challenge [6,7]. Enzymatic and chemical reactions have been proposed for oxidative lignin valorisation. Several biocatalysts, mimics of biocatalysts, and inorganic catalysts have been studied regarding formation of aromatic monomers for the chemical industries. Nevertheless, both reductive and oxidative degradation methods have been studied and presented [8,9,10,11,12]. Mechanistic insights obtained using lignin model compounds to simulate the most abundant bonding motifs within the backbones of various technical lignins were only scarcely applicable to the complexity of a lignin oligomeric and/or polymeric material. In case of oxidative degradation, a series of oxidation products is obtained even in case of simple models, pointing at a lack of selectivity of the reactions triggered by the various catalytic sstems. As a noteworthy exception, only the methylrheniumoxide catalyst system has been reported [13,14].
Vanadium-based catalysts have been reported for lignin valorisation, interestingly both for oxidative and reductive depolymerisation approaches [8,9,11,15,16,17,18,19,20]. Molybdenum catalysts have been widely reported for the oxidative valorisation of technical lignins, especially in form of polyoxometalates (POMs) [21,22]. More recently, mixed, i.e., bifunctional catalyst systems such as copper-vanadium [23] and molybdenum-vanadium [24] systems have been reported for lignin valorisation.
In case of enzymatically mediated reactions, obtaining a large panel of different oxidation products—due to the natural evolution of phenoxy radical intermediates—is furthermore associated to a limited biocatalyst lifetime, which constitutes an additional problem for lignin valorisation. Potential ways to tackle both issues consist of supporting and/or encapsulating the enzymes or to mimic the catalytic centre of lignolytic enzymes using stable organometallic complexes that would eventually exhibit tuning possibilities towards a higher product selectivity [25,26,27,28].
In an effort to combine such an active centre-mimicking approach with our interest in evaluating the use of non-lignolytic enzymes in lignin valorisation [29], we tested a vanadate (V)-based and a molybdate (Mo)-based catalytic system [V] and [Mo], respectively, as mimics of the reactive vanadate and molybdate centres in bromide peroxidases [EC 1.11.1.18] and xanthine oxidase [EC 1.17.3.2], respectively. The vanadate and molybdate catalyst systems can, based on their reactivity, be eventually considered suitable non-lignolytic biocatalyst mimics for lignin degradation. The reactivity of the catalyst systems holds the promise that they are capable of oxidising lignin in a more selective way than the typical lignolytic enzymes, i.e., laccases or manganese peroxidases due to the different range of electric potentials [30]. The present study deals with the investigation of the oxidation potential of vanadate and molybdate catalysts toward lignin oxidation.
Two hardwood and two softwood lignins, isolated from Eucalyptus nitens and Pinus pinaster, in form of both organosolv and ionosolv lignins have been selected. These lignins have been reported and characterised before [31,32,33] and were chosen as starting materials for this study also because the structural characterization had revealed noteworthy structural differences between the acetosolv and ionosolv lignin of each biomass. These lignins were used in this study without any further refinements or fractional purifications. We refrained in this study from following the wide-spread approach in which the catalyst activity is demonstrated using monomeric, dimeric, or sometimes trimeric lignin models. While this approach allows relatively facile mechanistic understanding of product formation, applicability of the results to whole lignin degradation is often scarce, due to the high density of reactive sites along the lignin backbone prone to undergo inter- and intramolecular reactions not delineable using lignin models.
2. Materials and Methods
General information: Reagents and solvents were purchased from Sigma-Aldrich/Merck KGaA, Darmstadt, Germany and Carlo Erba, Milano, Italy, and used without further purification, if not stated otherwise. Acetosolv pine lignin (AP) and acetosolv eucalyptus lignin (AE), as well as ionosolv pine lignin (IPB) and ionosolv eucalyptus lignin IEB were produced and characterised as described elsewhere [31,32,33]. Ammonium vanadate (V), NH4VO3, and ammonium molybdate tetrahydrate (Mo), (NH4)6Mo7O24·4H2O were purchased from Sigma Aldrich/Merck KGaA and used without further purification.
Oxidation of lignins: 50 mg of lignin were weighted in a screw cap vial and suspended in 1 mL of a pre-made stock solution containing the catalyst. Stock solutions comprised: (i) 30 µL perchloric acid; (ii) 450 µL hydrogen peroxide 30% (w/v); (iii) 2520 µL distilled water; (iv) the vanadate or molybdate catalyst at concentrations of 5%, 7.5%, or 10% (w/w) of liquid phase. Reactions were heated to 80 °C for 9 h while being continuously stirred and allowed to cool down to room temperature overnight.
Degradation products were isolated in an extraction process. The reaction mixture was centrifuged to separate any solids, and the liquid phase was taken and mixed with an equal volume of ethyl acetate. As internal standard, 50 µL of a solution of 4-ethoxy-3-methoxy benzaldehyde in ethyl acetate at a concentration of typically 50 μm were mixed into the solution. The biphasic system was vigorously shaken for 30 s. The organic phase was separated, dried over MgSO4, and centrifuged. An aliquot was sampled for analysis by gas chromatography coupled to mass spectrometry (GC-MS) as described below.
Solid lignin residues were isolated by centrifugation and washed four times using a 1 m aqueous solution of sulphuric acid. Lignin residues were dried to constant weight for 31P NMR analysis as described below. Experiments were run in duplicate by default, and selected experiments were additionally repeated for verification of results. An error margin for solid mass returns/depolymerisation yields of max ±6% was encountered.
Quantitative 31P NMR spectroscopy: In general, a procedure similar to the one originally published and previously applied was used [34]: approx. 30 mg of the lignin were accurately weighed for analysis after phosphitylation using an excess of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxa-phospholane (Cl-TMDP). 31P NMR spectra were recorded on a Bruker, (Billerica, MA, USA, 300 MHz or Bruker 700 MHz NMR spectrometer controlled by TopSpin software, using an inverse gated decoupling technique with the probe temperature set to 20 °C. The maximum standard deviation of the reported data is 0.02 mmol g−1, while the maximum standard error is 0.01 mmol g−1 [35,36]. NMR data were processed with MestreNova (Version 8.1.1, Mestrelab Research, Santiago di Compostella, Spain). Technical loadings are determined by comparing the abundancies of total aromatic hydroxyl groups of the product lignin with the starting lignin.
Gel permeation chromatography (GPC): For GPC measurements, approx. 2–3 mg of solid were dissolved in HPLC-grade dimethylsulphoxide (DMSO) (Chromasolv®, Sigma-Aldrich/Merck KGaA) containing 0.1% (m/v) lithium chloride (LiCl). A Shimadzu, Kyoto, Japan, instrument was used consisting of a controller unit (CBM-20A), a pumping unit (LC 20AT), a degasser (DGU-20A3), a column oven (CTO-20AC), a diode array detector (SPD-M20A), and a refractive index detector (RID-10A) and was controlled by Shimadzu LabSolutions (Version 5.42 SP3). For separation, a PLgel 5 μm MiniMIX-C column (Agilent, Santa Clara, CA, USA, 250 × 4.6 mm) was eluted at 70 °C at 0.25 mL min−1 flow rate with DMSO containing 0.1% lithium chloride for 20 min. Standard calibration is performed with polystyrene sulfonate standards (Sigma Aldrich/Merck KGaA, MW range 0.43–2.60 × 106 g mol−1) in acid form; lower calibration limits are verified by the use of monomeric and dimeric lignin models. Final analyses of each sample were performed using the intensities of the UV signal at λ = 280 nm employing the Shimadzu analysis software.
Gas chromatography coupled to mass spectrometry (GC-MS): A prepared sample of extractives of each reaction was analysed by gas chromatography coupled with mass spectrometry, using a Shimadzu, Kyoto, Japan, GCMS QP2010 Ultra equipped with an AOi20 autosampler unit. A SLB®-5ms Capillary GC Column (L × I.D. 30 m × 0.32 mm, df 0.50 μm) was used as the stationary phase, ultrapure helium as the mobile phase. The Shimadzu LabSolutions GCMS Solution software (Version 2.61) was used. The various components were identified by comparison against the NIST11 library. For control of sensitivity of analysis, selected samples were analysed after silylating the OH-groups present in the analytes: after this first analysis, 100 µL dry pyridine and 100 µL of N,O-bis(trimethylsilyl)-trifluoroacetamide were added to the aliquot, in order to repeat the analysis, after gently stirring the mixture for 30 min at room temperature, using identical GC-MS conditions. Reproducibility of single sample measurements was encountered with an error of maximum ±0.5%. Reproducibility across duplicated experiments was approx. ±5%.
3. Results and Discussion
3.1. Catalytic Systems
Taking inspiration from natural enzymes, several V- and Mo-based systems have been proposed in the literature, to perform sustainable oxidations on various substrates [37,38,39]. Basically, the mechanism of action of the [V]- and [Mo]-catalysts here adopted tracks the one of vanadium-dependent haloperoxidase enzymes [38,40]. Dissolving a [V]-catalyst precursor, such as NH4VO3, in acidic aqueous solution (pH = 1), in the presence of H2O2, a vanadium-monoperoxido complex forms, which is the effective catalytic species in solution. Conversely, at higher pH values, the formation of the diperoxido vanadium species occurs, which is less effective than the mono-peroxido in oxidation and oxybromination reactions. Therefore, in this study, pH = 1 was chosen to perform the oxidative lignin degradation.
Similarly, dissolution of ammonium molybdate at pH = 1 in water, with H2O2, leads to the formation of the diperoxido–molybdenum complex, which is much more stable than the molybdenum monoperoxo derivative [38].
Importantly, the reactivity of vanadium-peroxo and molybdenum-diperoxo complexes is definitely superior than that of H2O2 in oxidation reactions, therefore the oxidation of different alkanes, alkenes, alcohols, aromatic substrates, sulphides, as well as oxybromination reactions were successfully achieved with such catalytic systems [41,42,43,44]. Literature reports suggest that vanadate/peroxide oxidation system acts following substrate specifics in radical or ionic modes [38,41,45,46].
3.2. Lignin Starting Materials
The lignins chosen for this study are representative samples of a series of lignins isolated in course of a process optimisation and have recently been presented and characterised [31,32,33]. More specifically, they comprised two softwood lignins isolated from Pinus pinaster (acetosolv, AP, and ionosolv isolated using [bmim] HSO4, IPB), and two hardwood lignins isolated from Eucalyptus nitens (acetosolv, AE, and ionosolv isolated using [bmim] HSO4, IEB).
With respect to the acetosolv lignins that exhibited structural features typical for this type of lignin and close to those attributed to pristine lignin, ionosolv species showed clear signs of structural degradation when compared to the milder isolation process, i.e., the organosolv treatments. IPB and IEB presented lower molecular weight distributions and comparatively lower contents of aliphatic OH-groups (Table 1), a fact ascribed to a partial degradation of the backbone [31]. The use of these lignin samples was envisaged to allow for delineating the effect of a ‘pre-degradation’ to the oxidative valorisation process.

Table 1.
Distribution of OH-groups and molecular weight key data of acetosolv and ionosolv lignins tested for oxidative valorisation.
The catalytic activity of the chosen oxidative systems was expected to take effect mainly via the OH-groups present in the lignin structures; changes in relative abundances of hydroxyl groups based on consumption upon degradation and/or polymerisation is indicative of catalyst activity and allows mechanistic insight. Table 1 details again the OH-group contents as determined by 31P NMR spectroscopy.
3.3. Catalytic Degradation of Softwood Lignins
Degradation studies started using the softwood samples reported in Table 1, i.e., AP and IPB, and the Vanadate system discussed above. In order to test for a potential direct downstream application of the degrading catalyst in an industrial set-up, lignins were used as obtained in the initial isolation process. Effectiveness of treatments was tested via the analysis of newly generated low molecular weight components extractable in ethyl acetate. Results are summarised in Table 2.

Table 2.
Results obtained in the oxidative degradation of aceto- and ionosolv pine lignins, AP and IP, respectively, using various loadings of vanadate- and molybdate catalyst systems.
Using the softwood acetosolv lignin AP, an initial screening of conditions revealed that optimum conversions are obtained employing catalyst loadings of 7.5% or 10% (w/w) at elevated temperatures of 80 °C for reaction times of 9 h. Lower catalyst loadings of 5% (w/w) resulted in lower conversions under otherwise unchanged conditions.
Under the chosen conditions, the vanadate catalyst system [V] generated a series of oxidation products on the basis of AP that are comparable to products observed in the archival literature (Table 2). The molybdate catalyst system [Mo], on the other hand, led to detectable product formation on the basis of AP when applied in loadings of 5–10% (w/w). In any case, the molybdate system revealed a higher selectivity as fewer products were formed in comparatively higher amounts (Table 2). In both cases, catalytic activity of the present metal species is evident from the significantly increased depolymerisation yields compared to the blank sample.
In case of both [V]- and [Mo]-catalysed degradation, lignin polymerisation takes place as a background reaction, as indicated by the increase in both the number average molecular weight (Mn) and the polydispersity as compared to the starting material. This polymerisation can be explained by the activation of the lignin by the catalyst system by formation of radicals (vide infra, mechanistic discussion). Importantly, also in case a metal species is absent, polymerisation is observed (Table 2, blank sample), pointing towards an expected activation of the lignin by the perchloric acid alone. Control of this intrinsic activation is obtained, however, only when a metal catalyst is present as well. Polydispersities in case of [V]- and [Mo]-mediated reactions are significantly lower than in the case of the blank sample. Overall, both depolymerisation yields as well as the characteristics of the reisolated lignins thus indicate a beneficial role of the metal catalysts in the investigated valorisation system.
When subjecting ionosolv pine lignin IPB to different loadings of vanadate and molybdate systems under otherwise unchanged conditions, product formation is actually overall enhanced (Table 2). Both catalyst systems show an overall comparable activity, under various loadings, but a rather remarkable selectivity in product formation is observed. The [V]-system, at a loading of 10% (w/w), delivers as dominant product of more than 90% relative abundance vanillin. The [Mo]-system, on the other hand, delivers isovanillic acid as the most abundant species under otherwise unchanged conditions.
When estimating the absolute amount of these products against an internal standard during work-up and GC-analysis, a depolymerisation yield can be calculated based on the amount of total extractives generated upon the reaction. This depolymerisation yield was found to be 34% in case of the vanadate system and, remarkably, 62% in case of molybdate-based catalyst applied each at a loading of 10% (w/w). Also, in case of IPB, the presence of the metal catalysts led to a significant increase in depolymerisation yields compared to the blank.
Residues of the IPB lignin successfully depolymerised using 10% of vanadate-based catalyst or molybdate-based catalyst were subsequently isolated and analysed for structural changes using quantitative 31P NMR. Results are given in Table 1; Figure 1 shows a comparison of the 31P NMR spectra of starting IPB and residues isolated after treatment with the [V]- and [Mo]-based catalysts systems.
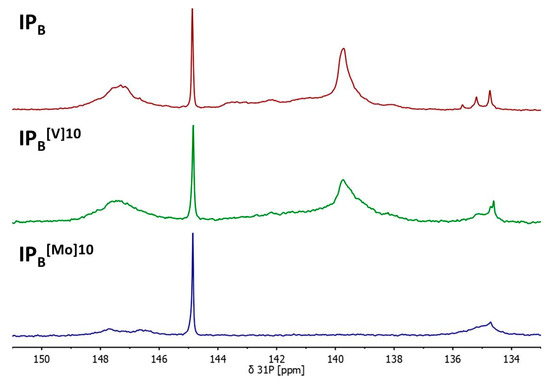
Figure 1.
Comparison of the 31P NMR spectra of starting IPB and residues isolated after treatment with the [V]-based catalyst system and the [Mo]-based catalyst system at 10% (w/w).
Results show a drastic decease of OH-groups, independent of the catalyst type. This suggests significant oxidation along the lignin backbone. An increase in carboxylic acid group content as seen in the 31P NMR of IPB subjected to 10% (w/w) supports this analysis (Figure 1). Analysis of the number and the weight average molecular weights of reisolated lignins sustain the picture that emerged during the analysis of the AP system: the lignins are mainly activated towards polymerisation by perchloric acid, while in presence of the metal species, depolymerisation is favoured and re-polymerisation is present as a background reaction (compare mechanistic discussion below).
3.4. Catalytic Degradation of Hardwood Lignins
Given the promising results obtained with the softwood lignin, attention was turned towards Eucalyptus nitens lignins, both in acetosolv and ionosolv form. Maximum catalyst loadings were studied using identical conditions for the oxidative degradation and valorisation of AE and IEB; the results are summarised in Table 3.

Table 3.
Results obtained in the oxidative degradation of aceto- and ionosolv eucalyptus lignins, AE and IEB, respectively, using various loadings of vanadate and molybdate catalyst systems.
The vanadate-based system led, in case of acetosolv hardwood lignin AE, to the formation of a seemingly homogenised product portfolio. Depolymerisation yields were found to be ranging from 38 to 53% (Table 3). Highest selectivity was found at a catalyst loading of 7.5% (w/w) with essentially only syringaldehyde being formed. Lower depolymerisation yields of around 38% are observed independent of catalyst loading in the case of IEB lignin (Table 3). When using 5% (w/w) vanadate-based catalyst, vanillin, and syringaldehyde were formed as single products; at higher catalyst loadings, depolymerisation yields remained constant, while product diversity increased. Together with 1-(4-hydroxy-3-methoxy-phenyl)-2-methylprop-2-en-1-one (6), 2,6-dimethoxybenzoquinone (8) was found. Interestingly, in none of the experiments the corresponding acids were found; the aqueous phase was controlled during extraction for sufficient acidity. The findings thus correspond to the activity of the vanadate system towards the pine lignin samples discussed above.
Given the overall better results obtained when applying lower concentrations of the [Mo] catalyst system in case of the softwood lignin, the eucalyptus acetosolv lignin AE and the ionosolv lignin IEB were, respectively, treated with only 7.5% and 5% (w/w) of molybdate catalyst, as these concentrations led to more selective product formation with the other catalyst. In both cases, vanillic acid was detected as the only significantly abundant depolymerisation product, achieving depolymerisation yields of 84 and 78%, respectively (Table 3).
The overall more difficult situation encountered in case of the investigated oxidative hardwood valorisation is reflected as well in the molecular weight analyses. As in the case of the softwood lignins, the presence of the metal catalysts helps to increase depolymerisation, but in contrast to the situation found in the softwood cases, re-polymerisation is less effectively suppressed. Rather high values for the polydispersities indicate that re-polymerisation poses a significant problem during the oxidative degradation, which is likely to contribute to the overall lower depolymerisation yields seen for the hardwood lignins.
3.5. Mechanistic Considerations
Given the basic reactivities of the catalysts and the polyphenolic substrates, different reaction pathways can be assumed. One route comprises an initial attack of the catalytic system on the phenolic OH-group [44,46,47]. The phenoxy radical (I) formed initially can be stabilised by the aromatic system before eventually reaching the ipso-position where it can trigger an exo-depolymerisation pathway of the lignin chains (Scheme 1). In the case of the [V]-mediated oxidation of IEB isolated monomeric quinone 8 supports this thesis.
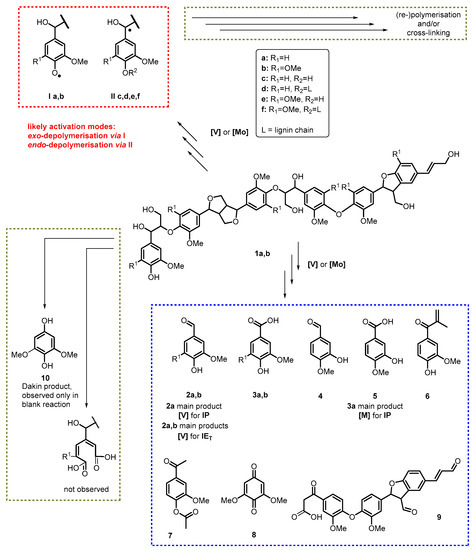
Scheme 1.
Proposed activation modes based on observed products in [V]- and [Mo]-catalysed oxidation of soft and hardwood aceto- and ionosolv lignins [48].
Formation of vanillin (2a) and syringaldehyde (2b) in the case of [V]-mediated pine and eucalyptus ionosolv lignin degradation can be seen as immediate products originating from an exo-depolymerisation process triggered by oxidative activation of the benzylic position (II) in terminal β-O-4′ motifs; previous in silico studies had revealed that the benzylic position is similarly susceptible to an activation via radicals [48]. Formation of alkenone 7 can be explained by this route as well. Isolation of isovanillic acid (5), as in case of [Mo]-mediated valorisation of IPB, and formation of vanillic acid, in case of [Mo]-treated IEB, suggests a similar mechanism under eventually less controlled conditions favouring rearrangement reactions. Functionalisation of the benzylic position is an important aspect for rendering the transformation of the lignin aromatic monomers effective by giving rise to an effective endo-polymerisation happening in parallel.
Importantly, hydroquinones as products deriving from a Dakin reaction [49] have not been observed.
31P NMR data (Table 1) indicate the presence of significant amounts of condensed units in the starting lignins, whereas material isolated after oxidative valorisation using the [V] and [Mo] catalysts systems contains less of these groups. In addition, the loss of these motifs can be interpreted in favour of an endo-depolymerisation. Repolymerisation reactions via the phenoxy radical could lead to an increase i 4-O-5′ groups, however this is not observed in this study.
Reduced amounts of aliphatic OH-groups (Table 1, Figure 1) show the activity of the catalyst systems on the aliphatic OH-groups along the lignin chain (Scheme 1); this activity is more pronounced in case of the [Mo]-based catalyst system, especially in case of softwood ionosolv lignin in which essentially all OH-groups must have been oxidised in remaining oligomeric structures (Scheme 1); structure 9 in Scheme 1 illustrates such a putatively ‘per-oxidised’ lignin fragment.
An interesting insight into the tighter interplay of the involved redox potentials can be delineated from the presence of vanillic acid (3a) in case of the [Mo]-mediated oxidation of pine and eucalyptus lignin, while the [V]-mediated valorisation of both soft- and hardwoods is possible yielding preferentially vanillin (2a) and syringaldehyde (2b), respectively. The data further suggest that the oxidation potential of the phenolic OH-group in initially formed vanillin still fits the dynamic range of the [Mo]-catalyst, while it is outside of the oxidation range of the [V]-catalyst.
Observed selectivities can be reasoned based on structural features of the lignins involved. However, the current study does not allow making clear connections between the catalyst type, the biomass source, and/or the isolation process and the observed activities and selectivities. Additional studies targeting this aspect are currently being pursued.
4. Conclusions
Acetosolv soft- and hardwood lignins as well as ionosolv soft- and hardwood lignins were transformed into monomeric aromatic compounds using either a vanadate or molybdate-based catalyst system. Monomers were generated with remarkable, catalyst-dependent selectivity and high depolymerisation yields via oxidative exo- and endo-depolymerisation processes. Using the vanadate–hydrogen peroxide system on acetosolv pine lignin, vanillin and isovanillin were produced as main products with depolymerisation yields of 31%. Using the molybdate system on the same lignin, vanillic acid was the practically exclusive product, with a depolymerisation yield of 72%. Ionosolv pine could be valorised into vanillin and vanillic acid as practically exclusive products in depolymerisation yields of 34 and 57%, respectively, using either the vanadate or the molybdate catalyst system. Similar selectivities, albeit with lower depolymerisation yields of around 50% under standardised conditions, could be obtained for eucalyptus acetosolv lignin, producing vanillin and syringaldehyde or vanillic acid as products, using either the vanadate or the molybdate system. Omitting the metal species in the reactions mixture, acetosolv eucalyptus was converted into benzoquinones as effectively only isolable aromatic monomer. Interestingly, the ionosolv hardwood lignins did not perform as well as the ionosolv softwood under the chosen conditions. A partial backbone degradation, as induced during the isolation of ionosolv lignins employing [bmim]HSO4, does not fundamentally enhance oxidative depolymerisation. In all cases, (re-)polymerisation reactions in form of elevated number average molecular weights in combination with significantly increased polydispersities of re-isolated materials have been observed. In case of softwood lignins, the presence of the metal catalysts led to a partial control/suppression of such (re-)polymerisation; in case of the hardwood samples, however, metal catalysts appeared less effective in this respect.
Author Contributions
Conceptualization, F.S., V.C., H.L. and C.C.; data curation, L.P., M.G. and H.L.; investigation, L.P., M.G. and F.S.; methodology, F.S., P.G., V.C., H.L. and C.C.; project administration, V.C., J.C.P. and C.C.; resources, P.G., V.C., V.S., J.C.P. and C.C.; supervision, V.S., J.C.P., F.S., P.G., H.L. and C.C.; validation, M.G., H.L. and C.C.; writing—original draft, M.G., F.S. and H.L.; writing—review & editing, J.C.P., V.C., H.L. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Ministry of Economy and Competitiveness” of Spain (research project “Modified aqueous media for wood biorefineries”, reference CTQ2017-82962-R).
Acknowledgments
H.L. acknowledges the MIUR Grant ‘Dipartimento di Eccellenza 2018–2022’ to the Department of Pharmacy of the University of Naples ‘Federico II’. C.C. acknowledges the Ca’Foscari FPI 2019 funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aresta, M.; Dibenedetto, A.; Dumeignil, F. Biorefineries, An Introduction; De Gruyter: Berlin, Germany; Boston, MA, USA, 2015; ISBN 978-3-11-033158-5. [Google Scholar]
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current Status, Challenges, and Future Direction. Energy Fuels 2006, 20, 1727–1737. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, M.; Kobayashi, H.; Fukuoka, A. Catalytic transformation of cellulose into platform chemicals. Appl. Catal. B Environ. 2014, 145, 1–9. [Google Scholar] [CrossRef]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803–2830. [Google Scholar] [CrossRef]
- Argyropoulos, D.S.; Crestini, C. A Perspective on Lignin Refining, Functionalization, and Utilization. ACS Sustain. Chem. Eng. 2016, 4, 5089. [Google Scholar] [CrossRef]
- Fiorani, G.; Crestini, C.; Selva, M.; Perosa, A. Advancements and Complexities in the Conversion of Lignocellulose Into Chemicals and Materials. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Mobley, J.K. Conversion of Lignin to Value-added Chemicals via Oxidative Depolymerization. In Chemical Catalysts for Biomass Upgrading; Crocker, M., Santillan-Jimenez, E., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 357–393. ISBN 978-3-527-34466-6. [Google Scholar]
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin—Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Crestini, C.; Crucianelli, M.; Orlandi, M.; Saladino, R. Oxidative strategies in lignin chemistry: A new environmental friendly approach for the functionalisation of lignin and lignocellulosic fibers. Catal. Today 2010, 156, 8–22. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Argyropoulos, D.S. Oxidative Delignification Chemistry: Fundamentals and Catalysis; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; ISBN 978-0-8412-3738-4. [Google Scholar]
- Crestini, C.; Pro, P.; Neri, V.; Saladino, R. Methyltrioxorhenium: A new catalyst for the activation of hydrogen peroxide to the oxidation of lignin and lignin model compounds. Bioorg. Med. Chem. 2005, 13, 2569–2578. [Google Scholar] [CrossRef]
- Crestini, C.; Caponi, M.C.; Argyropoulos, D.S.; Saladino, R. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds. Bioorg. Med. Chem. 2006, 14, 5292–5302. [Google Scholar] [CrossRef]
- Guadix-Montero, S.; Sankar, M. Review on Catalytic Cleavage of C–C Inter-unit Linkages in Lignin Model Compounds: Towards Lignin Depolymerisation. Top. Catal. 2018, 61, 183–198. [Google Scholar] [CrossRef]
- Chan, J.M.W.; Bauer, S.; Sorek, H.; Sreekumar, S.; Wang, K.; Toste, F.D. Studies on the Vanadium-Catalyzed Nonoxidative Depolymerization of Miscanthus giganteus-Derived Lignin. ACS Catal. 2013, 3, 1369–1377. [Google Scholar] [CrossRef]
- Son, S.; Toste, F.D. Non-Oxidative Vanadium-Catalyzed C-O Bond Cleavage: Application to Degradation of Lignin Model Compounds. Angew. Chem. Int. Ed. 2010, 49, 3791–3794. [Google Scholar] [CrossRef] [PubMed]
- Amadio, E.; Di Lorenzo, R.; Zonta, C.; Licini, G. Vanadium catalyzed aerobic carbon–carbon cleavage. Coord. Chem. Rev. 2015, 301–302, 147–162. [Google Scholar] [CrossRef]
- Bozell, J.J.; Hoberg, J.O.; Dimmel, D.R. Heteropolyacid Catalyzed Oxidation of Lignin and Lignin Models to Benzoquinones. J. Wood Chem. Technol. 2000, 20, 19–41. [Google Scholar] [CrossRef]
- Mobley, J.K.; Jennings, J.A.; Morgan, T.; Kiefer, A.; Crocker, M. Oxidation of Benzylic Alcohols and Lignin Model Compounds with Layered Double Hydroxide Catalysts. Inorganics 2018, 6, 75. [Google Scholar] [CrossRef]
- Voitl, T.; Rudolf von Rohr, P. Oxidation of Lignin Using Aqueous Polyoxometalates in the Presence of Alcohols. ChemSusChem 2008, 1, 763–769. [Google Scholar] [CrossRef]
- Weinstock, I.A.; Atalla, R.H.; Reiner, R.S.; Moen, M.A.; Hammel, K.E.; Houtman, C.J.; Hill, C.L.; Harrup, M.K. A new environmentally benign technology for transforming wood pulp into paper. Engineering polyoxometalates as catalysts for multiple processes. J. Mol. Catal. A Chem. 1997, 116, 59–84. [Google Scholar] [CrossRef]
- Mottweiler, J.; Puche, M.; Räuber, C.; Schmidt, T.; Concepción, P.; Corma, A.; Bolm, C. Copper- and Vanadium-Catalyzed Oxidative Cleavage of Lignin using Dioxygen. ChemSusChem 2015, 8, 2106–2113. [Google Scholar] [CrossRef]
- Hao, K.; Zhang, L.-L.; Song, L.; Guan, H.-Y.; Li, C.-M.; Liu, T.; Yu, Q.; Zeng, J.-M.; Wang, Z.-W. Highly active Mo-V-based bifunctional catalysts for catalytic conversion of lignin dimer model compounds at room temperature. Inorg. Chem. Commun. 2020, 116, 107910. [Google Scholar] [CrossRef]
- Drago, G.A.; Gibson, T.D. Enzyme Stability and Stabilisation: Applications and Case Studies. In Engineering and Manufacturing for Biotechnology; Hofman, M., Thonart, P., Eds.; Focus on Biotechnology; Springer: Dordrecht, The Netherlands, 2002; pp. 361–376. ISBN 978-0-306-46889-6. [Google Scholar]
- Crestini, C.; Pastorini, A.; Tagliatesta, P. Metalloporphyrins immobilized on motmorillonite as biomimetic catalysts in the oxidation of lignin model compounds. J. Mol. Catal. A Chem. 2004, 208, 195–202. [Google Scholar] [CrossRef]
- Crestini, C.; Tagliatesta, P. Metalloporphyrins in the Biomimetic Oxidation of Lignin and Lignin Model Compounds: Development of Alternative Delignification Strategies. Porphyr. Handb. Bioinorg. Bioorg. Chem. 2003, 11, 161. [Google Scholar]
- Crestini, C.; Tagliatesta, P.; Saladino, R. A biomimetic approach to lignin degradation, metalloporphyrins catalysed oxidation of lignin and lignin model compounds. Oxid. Delignification Chem. Fundam. Catal. 2001, 213–225. [Google Scholar]
- Giannì, P.; Lange, H.; Crestini, C. Lipoxygenase: Unprecedented Carbon-Centered Lignin Activation. ACS Sustain. Chem. Eng. 2018, 6, 5085–5096. [Google Scholar] [CrossRef]
- Wong, D.W.S. Structure and Action Mechanism of Ligninolytic Enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef]
- Penín, L.; Lange, H.; Santos, V.; Crestini, C.; Parajó, J.C. Characterization of Eucalyptus nitens Lignins Obtained by Biorefinery Methods Based on Ionic Liquids. Molecules 2020, 25, 425. [Google Scholar] [CrossRef]
- Penín, L.; Peleteiro, S.; Santos, V.; Alonso, J.L.; Parajó, J.C. Selective fractionation and enzymatic hydrolysis of Eucalyptus nitens wood. Cellulose 2019, 26, 1125–1139. [Google Scholar] [CrossRef]
- Penín, L.; Santos, V.; del Río, J.C.; Parajó, J.C. Assesment on the chemical fractionation of Eucalyptus nitens wood: Characterization of the products derived from the structural components. Bioresour. Technol. 2019, 281, 269–276. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31 P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Argyropoulos, D.S. 31P NMR in Wood Chemistry: A Review of Recent Progress. Res. Chem. Intermed. 1995, 21, 373–395. [Google Scholar] [CrossRef]
- Amini, M.; Haghdoost, M.M.; Bagherzadeh, M. Oxido-peroxido molybdenum(VI) complexes in catalytic and stoichiometric oxidations. Coord. Chem. Rev. 2013, 257, 1093–1121. [Google Scholar] [CrossRef]
- Conte, V.; Floris, B. Vanadium and molybdenum peroxides: Synthesis and catalytic activity in oxidation reactions. Dalton Trans. 2011, 40, 1419–1436. [Google Scholar] [CrossRef] [PubMed]
- Schwendt, P.; Tatiersky, J.; Krivosudský, L.; Šimuneková, M. Peroxido complexes of vanadium. Coord. Chem. Rev. 2016, 318, 135–157. [Google Scholar] [CrossRef]
- Conte, V.; Floris, B. Vanadium catalyzed oxidation with hydrogen peroxide. Inorg. Chim. Acta 2010, 363, 1935–1946. [Google Scholar] [CrossRef]
- Conte, V.; Coletti, A.; Floris, B.; Licini, G.; Zonta, C. Mechanistic aspects of vanadium catalysed oxidations with peroxides. Coord. Chem. Rev. 2011, 255, 2165–2177. [Google Scholar] [CrossRef]
- Galloni, P.; Mancini, M.; Floris, B.; Conte, V. A sustainable two-phase procedure for V-catalyzed toluene oxidative bromination with H2O2–KBr. Dalton Trans. 2013, 42, 11963–11970. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Floris, B.; Sabuzi, F.; Coletti, A.; Conte, V. Sustainable vanadium-catalyzed oxidation of organic substrates with H2O2. Catal. Today 2017, 285, 49–56. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Garau, G.; Palma, A.; Lauro, G.P.; Mele, E.; Senette, C.; Manunza, B.; Deiana, S. Detoxification Processes from Vanadate at the Root Apoplasm Activated by Caffeic and Polygalacturonic Acids. PLoS ONE 2015, 10, e0141041. [Google Scholar] [CrossRef] [PubMed]
- Linskens, H.F.; Jackson, J.F. (Eds.) Plant Cell Wall Analysis; Modern Methods of Plant Analysis; Springer: Berlin/Heidelberg, Germany, 1996; Volume 17, ISBN 978-3-642-64644-7. [Google Scholar]
- Crestini, C.; Jurasek, L.; Argyropoulos, D.S. On the Mechanism of the Laccase-Mediator System in the Oxidation of Lignin. Chem. Eur. J. 2003, 9, 5371–5378. [Google Scholar] [CrossRef] [PubMed]
- Dakin, H.D. The oxidation of hydroxy derivatives of benzaldehyde, acetophenone and related substances. Am. Chem. J. 1909, 42, 477–498. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).