Process Optimization for Green Synthesis of Silver Nanoparticles Using Indonesian Medicinal Plant Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Plant Materials
2.2. Preparation of Plant Extracts
2.3. Green Synthesis of AgNPs Using the Plant Extracts
2.4. Characterization of AgNPs
3. Results
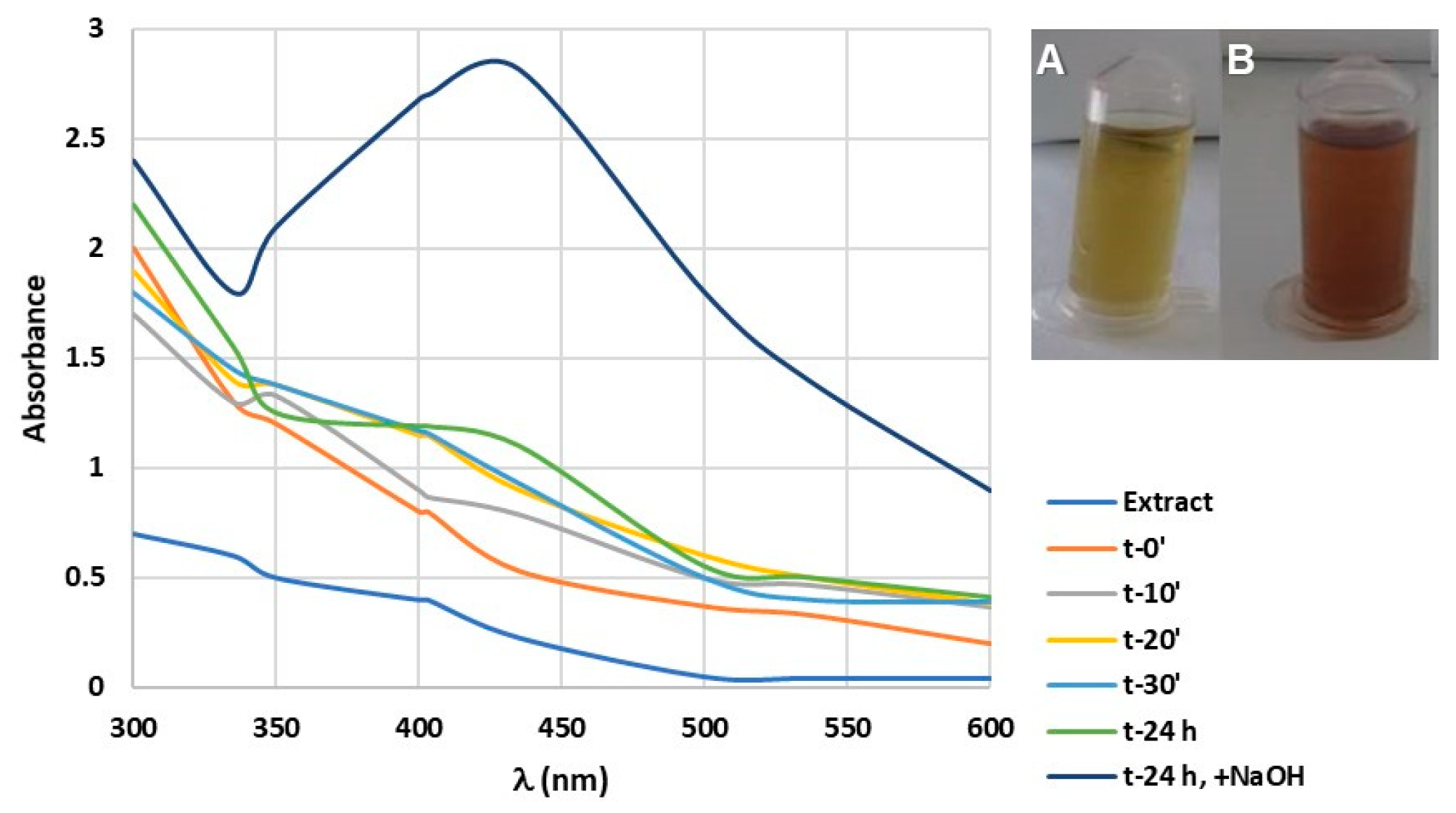
3.1. Optimization of the Extract Concentration
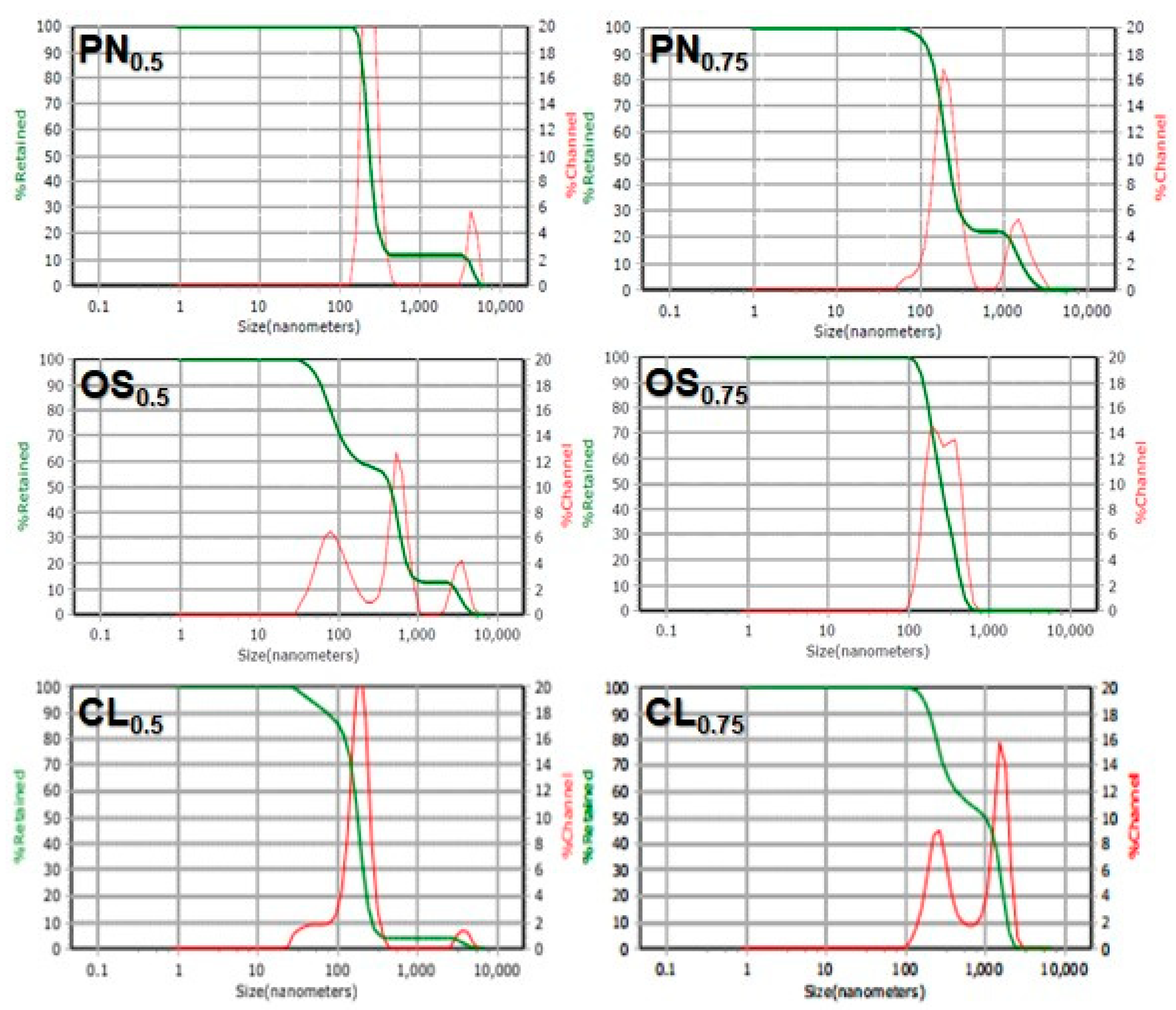
3.2. Particle Size and Particle Size Distribution of AgNPs
3.3. Morphological AgNP Evaluation by Scanning Electron Microscopy (SEM)
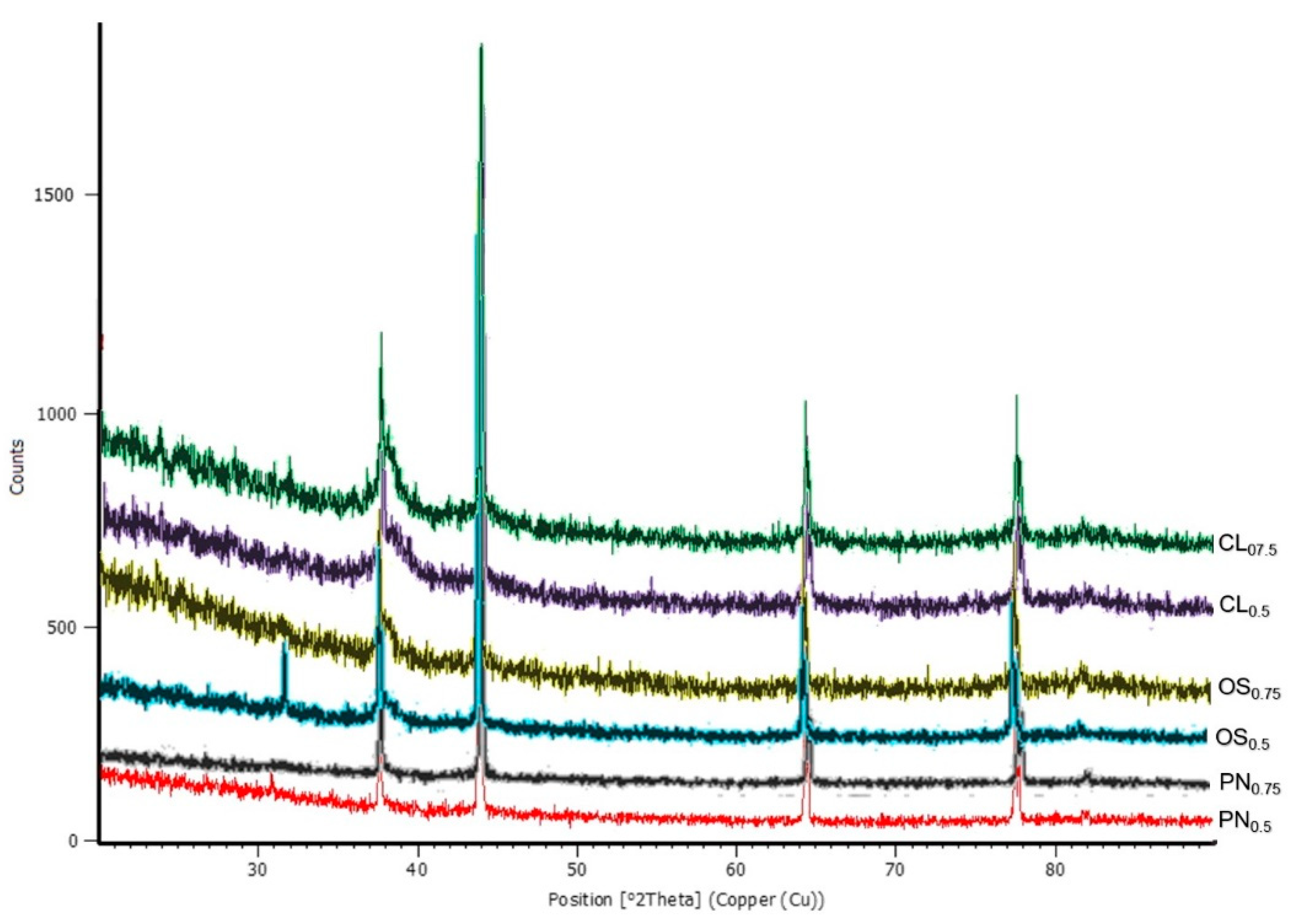
3.4. Characterization of AgNPs by X-ray Diffraction (X-RD)
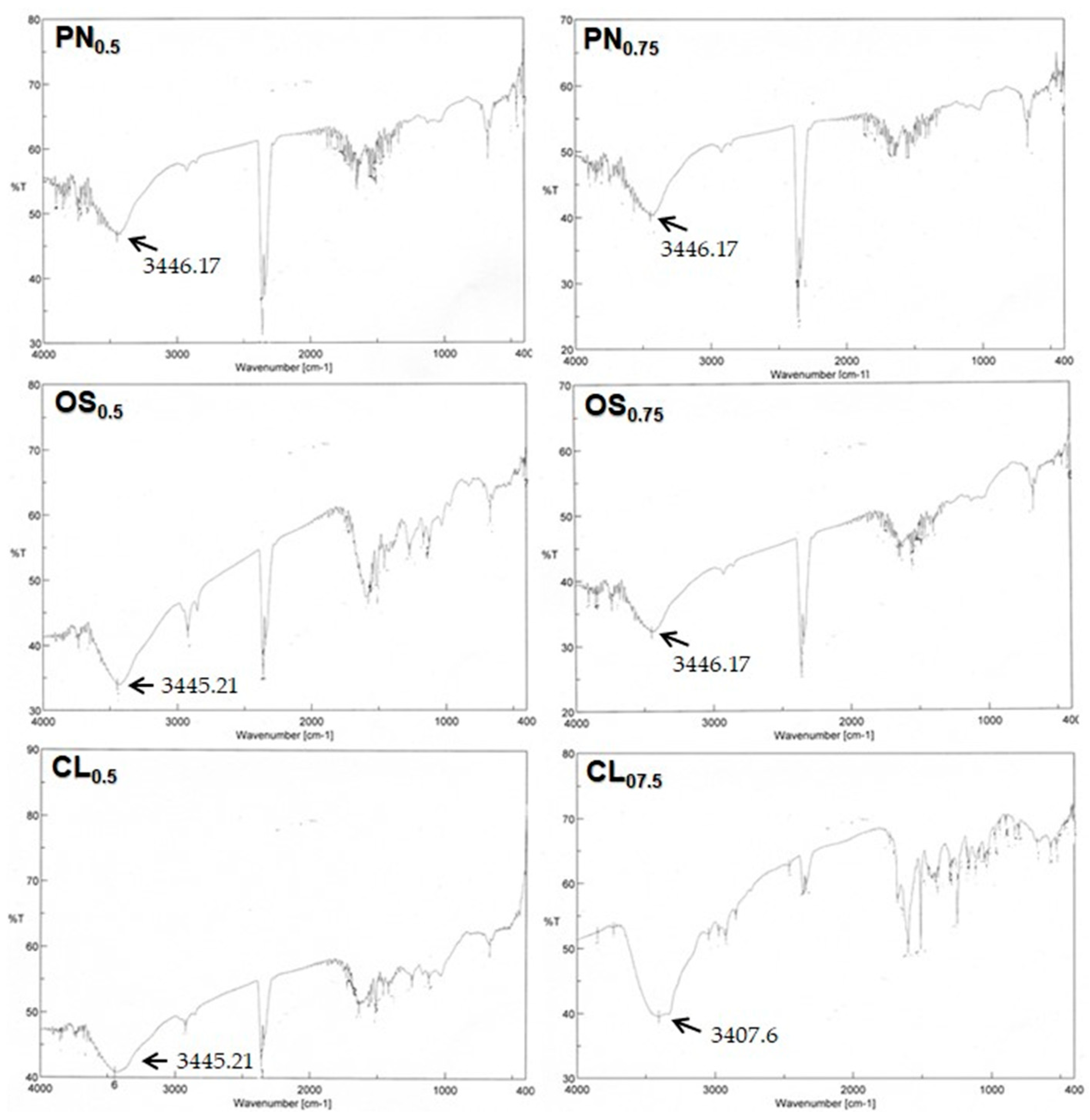
3.5. FTIR
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gardner, S.E.; Hillis, S.L.; Heilmann, K.; Segre, J.A.; Grice, E.A. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes 2013, 62, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, T.S.; Kavitha, S.; Spoorthi, J.; Bhat, D.V.; Prasad, A.S.B.; Upton, Z.; Ramachandra, L.; Acharya, R.V.; Satyamoorthy, K. Characteristics of microbial drug resistance and its correlates in chronic diabetic foot ulcer infections. J. Med. Microbiol. 2014, 63, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Kesavan, R.; Kavitha, K.; Kumpatla, S. A pilot study on the effects of a polyherbal formulation cream on diabetic foot ulcers. Indian J. Med. Res. 2011, 134, 168. [Google Scholar] [PubMed]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, K.; Apelqvist, J.; Schaper, N.C.; IWGDF. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab. Res. Rev. 2012, 28, 225–231. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Balzarro, M.; Rubilotta, E.; Trabacchin, N.; Soldano, A.; Cerrato, C.; Migliorini, F.; Mancini, V.; Pastore, A.L.; Carbone, A.; Cormio, L. Early and late efficacy on wound healing of silver nanoparticle gel in males after circumcision. J. Clin. Med. 2020, 9, 1822. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Velusamy, P.; Kumar, G.V.; Jeyanthi, V.; Das, J.; Pachaiappan, R. Bio-inspired green nanoparticles: Synthesis, mechanism, and antibacterial application. Toxicol. Res. 2016, 32, 95–102. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Reda, M.; Ashames, A.; Edis, Z.; Bloukh, S.; Bhandare, R.; Abu Sara, H. Green synthesis of potent antimicrobial silver nanoparticles using different plant extracts and their mixtures. Processes 2019, 7, 510. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Sukweenadhi, J.; Setiawan, F.; Yunita, O.; Kartini, K.; Avanti, C. Antioxidant activity screening of seven Indonesian herbal extract. Biodiversitas 2020, 21, 2062–2067. [Google Scholar] [CrossRef]
- Kartini, K.; Setiawan, F.; Sukweenadhi, J.; Yunita, O.; Avanti, C. Selection of potential Indonesian plant species for antioxidant. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Ranilla, L.G.; Apostolidis, E.; Shetty, K. Antimicrobial activity of an Amazon medicinal plant (Chancapiedra) (Phyllanthus niruri L.) against Helicobacter pylori and lactic acid bacteria. Phytother. Res. 2012, 26, 791–799. [Google Scholar] [PubMed]
- Ibrahim, D.; Hong, L.S.; Kuppan, N. Antimicrobial activity of crude methanolic extract from Phyllanthus niruri. Nat. Prod. Commun. 2013, 8, 493–496. [Google Scholar] [CrossRef] [Green Version]
- Alshawsh, M.A.; Abdulla, M.A.; Ismail, S.; Amin, Z.A.; Qader, S.W.; Hadi, H.A.; Harmal, N.S. Free radical scavenging, antimicrobial and immunomodulatory activities of Orthosiphon stamineus. Molecules 2012, 17, 5385–5395. [Google Scholar] [CrossRef]
- Malahubban, M.; Alimon, A.; Sazili, A.; Fakurazi, S.; Zakry, F. Phytochemical analysis of Andrographis paniculata and Orthosiphon stamineus leaf extracts for their antibacterial and antioxidant potential. Trop. Biomed. 2013, 30, 467–480. [Google Scholar]
- Gupta, A.; Mahajan, S.; Sharma, R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnol. Rep. 2015, 6, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Niamsa, N.; Sittiwet, C. Antimicrobial activity of Curcuma longa aqueous extract. J. Pharmacol. Toxicol. 2009, 4, 173–177. [Google Scholar]
- Lestari, T.P.; Tahlib, F.A.; Sukweenadhi, J.; Kartini, K.; Avanti, C. Physical characteristic and antibacterial activity of silver nanoparticles from green synthesis using ethanol extracts of Phaleria macrocarpa (Scheff.) Boerl Leaves. Tradit. Med. J. 2019, 24, 16–21. [Google Scholar] [CrossRef]
- Dewi, K.T.A.; Kartini, K.; Sukweenadhi, J.; Avanti, C. Karakter fisik dan aktivitas antibakteri nanopartikel perak hasil green synthesis menggunakan ekstrak air daun sendok (Plantago major L.). Pharm. Sci. Res. 2019, 6, 69–81. [Google Scholar] [CrossRef]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 47. [Google Scholar] [CrossRef] [Green Version]
- Geetha, A.R.; George, E.; Srinivasan, A.; Shaik, J. Optimization of green synthesis of silver nanoparticles from leaf extracts of Pimenta dioica (Allspice). Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I. Green synthesis of silver nanoparticles using extract of Parkia speciosa Hassk pods assisted by microwave irradiation. J. Adv. Res. 2016, 7, 961–969. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of nanomaterials: Tools and challenges. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; De Almeida, M.; Pereira, E.; West, P. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef]
- Bagalkotkar, G.; Sagineedu, S.; Saad, M.; Stanslas, J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: A review. J. Pharm. Pharmacol. 2006, 58, 1559–1570. [Google Scholar]
- Saidan, N.H.; Hamil, M.S.R.; Memon, A.H.; Abdelbari, M.M.; Hamdan, M.R.; Mohd, K.S.; Majid, A.M.S.A.; Ismail, Z. Selected metabolites profiling of Orthosiphon stamineus Benth leaves extracts combined with chemometrics analysis and correlation with biological activities. BMC Complement. Altern. Med. 2015, 15, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Extracts | Concentrations (%w/v) | Color of Solution * | Silver Luster ** | Yields | |
|---|---|---|---|---|---|
| %w/w | mg | ||||
| PN | 0.125 | Blackish brown | - | - | - |
| 0.25 | Blackish brown | - | - | - | |
| 0.50 | Blackish brown | +++ | 15.76 | 78.8 | |
| 0.75 | Blackish brown | +++ | 6.88 | 51.6 | |
| 1.00 | Dark green | - | - | - | |
| PN | 0.125 | Reddish-brown | - | - | - |
| 0.25 | Reddish-brown | - | - | - | |
| 0.50 | Reddish-brown | +++ | 1.27 | 6.35 | |
| 0.75 | Reddish-brown | +++ | 0.64 | 4.78 | |
| 1.00 | Reddish-brown | - | - | - | |
| CL | 0.125 | Brownish-yellow | - | - | - |
| 0.25 | Brownish-yellow | - | - | - | |
| 0.50 | Reddish-brown | +++ | 0.53 | 2.64 | |
| 0.75 | Reddish-brown | +++ | 2.46 | 12.3 | |
| 1.00 | Blackish brown | - | - | - | |
| Plant Extracts | Particle Size (nm) | Poly-Dispersity Index (PDI) |
|---|---|---|
| PN0.5 | 715.0 | 0.0085 |
| PN0.75 | 522.0 | 1.2370 |
| OS0.5 | 729.0 | 0.1716 |
| OS0.75 | 277.9 | 0.2378 |
| CL0.5 | 318.0 | 0.0431 |
| CL0.75 | 918.0 | 0.6040 |
| PN0.5 | PN0.75 | OS0.5 | OS0.75 | CL0.5 | CL0.75 | AgNPs [23] |
|---|---|---|---|---|---|---|
| 37.6454 | 37.6274 | 37.6248 | 37.6420 | 37.6406 | 37.6494 | 38.45 |
| 44.0146 | 44.0020 | 43.9073 | 43.8388 | 44.0151 | 43.8857 | 46.35 |
| 64.5091 | 64.4893 | 64.3259 | 64.3173 | 64.4910 | 64.5224 | 64.75 |
| 77.5067 | 77.7218 | 77.5186 | 77.5217 | 77.7313 | 77.7441 | 78.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartini, K.; Alviani, A.; Anjarwati, D.; Fanany, A.F.; Sukweenadhi, J.; Avanti, C. Process Optimization for Green Synthesis of Silver Nanoparticles Using Indonesian Medicinal Plant Extracts. Processes 2020, 8, 998. https://doi.org/10.3390/pr8080998
Kartini K, Alviani A, Anjarwati D, Fanany AF, Sukweenadhi J, Avanti C. Process Optimization for Green Synthesis of Silver Nanoparticles Using Indonesian Medicinal Plant Extracts. Processes. 2020; 8(8):998. https://doi.org/10.3390/pr8080998
Chicago/Turabian StyleKartini, Kartini, Amarisa Alviani, Dia Anjarwati, Adinda Finna Fanany, Johan Sukweenadhi, and Christina Avanti. 2020. "Process Optimization for Green Synthesis of Silver Nanoparticles Using Indonesian Medicinal Plant Extracts" Processes 8, no. 8: 998. https://doi.org/10.3390/pr8080998







