Tracing the Scientific History of Fe0-Based Environmental Remediation Prior to the Advent of Permeable Reactive Barriers
Abstract
:1. Introduction
2. Methodology
3. Fe0 in Organic Synthesis: The Béchamp Reduction
4. Fe0 for Safe Drinking Water Provision
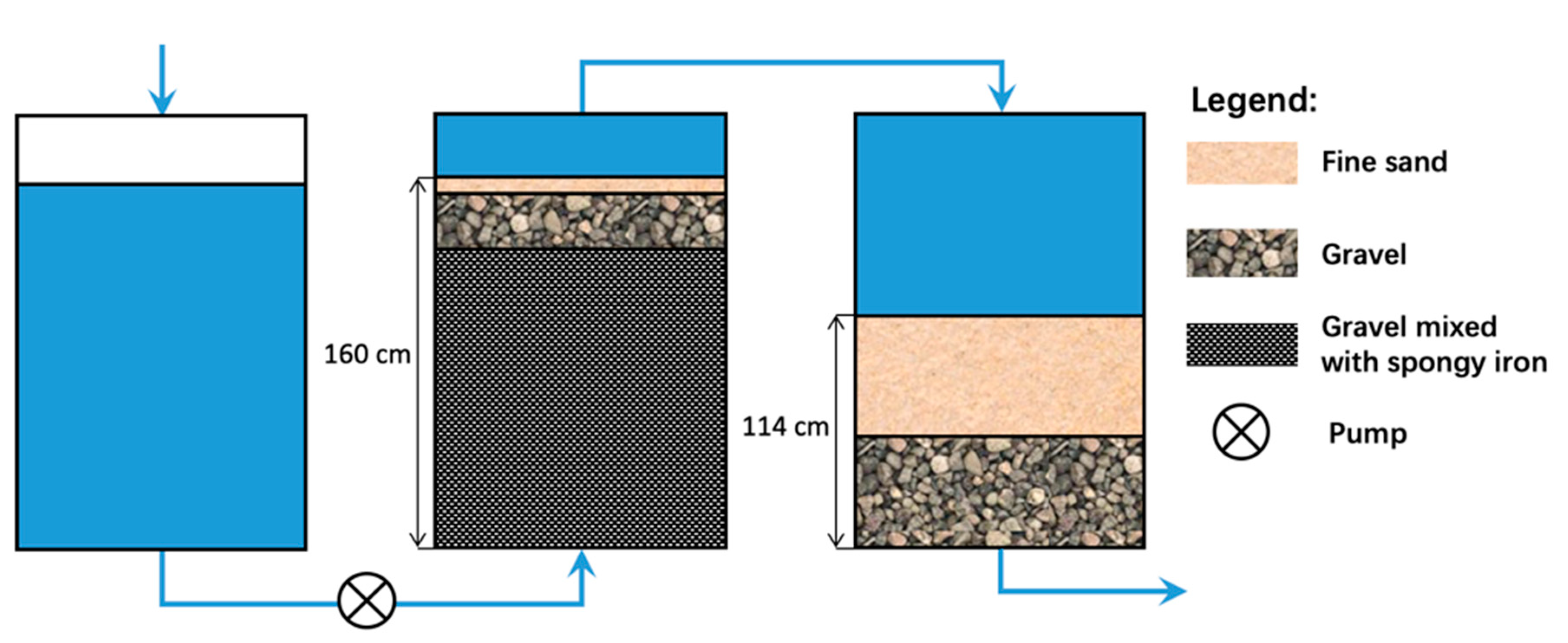
4.1. The Bischof Process
4.2. The Anderson Process
4.3. The Emmons Process
5. Fe0 for Agricultural Wastewater
6. Fe0 for Domestic and Industrial Wastewaters
7. Significance for the Fe0 PRB Technology
8. Shaping the Design of Better Fe0-Based Systems
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howe, K.J.; Hand, D.W.; Crittenden, J.C.; Trussell, R.R. Tchobanoglous, In Principles of Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 674. [Google Scholar]
- Detay, M.; Alessandrello, E.; Come, P.; Groom, I. Groundwater contamination and pollution in micronesia. J. Hydrol 1989, 112, 149–170. [Google Scholar] [CrossRef]
- Mackay, M.D.; Cherry, J.A. Groundwater contamination: Pump-and-treat remediation. Environ. Sci. Technol 1989, 23, 630–636. [Google Scholar] [CrossRef]
- McMurty, D.C.; Elton, R.O. New approach to in-situ treatment of contaminated groundwaters. Environ. Progr 1985, 4, 168–170. [Google Scholar] [CrossRef]
- Starr, R.C.; Cherry, J.A. In situ remediation of contaminated Ground water: The funnel-and-Gate System. Ground Water 1994, 32, 465–476. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ "iron wall" for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Lee, G.; Rho, S.; Jahng, D. Design considerations for groundwater remediation using reduced metals. Korean J. Chem. Eng. 2004, 21, 621–628. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef] [Green Version]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freiberg Online Geosci. 2015, 32, 1–80. [Google Scholar]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar]
- Richardson, J.P.; Nicklow, J.W. In situ permeable reactive barriers for groundwater contamination. Soil Sediment Contam. 2002, 11, 241–268. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Metals 1923, 29, 529–591. [Google Scholar]
- Caré, S.; Nguyen, Q.T.; L’Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cement Concrete Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Caré, S.; Crane, R.; Calabrò, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modeling the permeability loss of metallic iron water filtration systems. CLEAN—Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Bailey, E.H.; Mooney, S.J. Quantification of changes in zero valent iron morphology using X-ray computed tomography. J. Environ. Sci. 2013, 25, 2344–2351. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Wang, C.; Li, X.-Z. Electron efficiency of zero-valent iron for groundwater remediation and wastewater treatment. Chem. Eng. J. 2013, 215–216, 90–95. [Google Scholar] [CrossRef]
- He, F.; Gong, L.; Fan, D.; Tratnyek, P.G.; Lowry, G.V. Quantifying the efficiency and selectivity of organohalide dechlorination by zerovalent iron. Environ. Sci. Process. Impacts 2020, 22, 528. [Google Scholar] [CrossRef]
- Noubactep, C. Flaws in the design of Fe(0)-based filtration systems? Chemosphere 2014, 117, 104–107. [Google Scholar] [CrossRef]
- Noubactep, C. Research on metallic iron for environmental remediation: Stopping growing sloppy science. Chemosphere 2016, 153, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Hu, R.; Cui, X.; Gwenzi, W.; Noubactep, C. Understanding the operating mode of Fe0/Fe-sulfide/H2O systems for water treatment. Processes 2020, 8, 409. [Google Scholar] [CrossRef] [Green Version]
- Gillham, R.W. Development of the granular iron permeable reactive barrier technology (good science or good fortune). In Advances in Environmental Geotechnics, Proceedings of the International Symposium on Geoenvironmental Engineering in Hangzhou, China, 8–10 September, 2007; Chen, Y., Tang, X., Zhan, L., Eds.; Springer: Berlin, Germany; London, UK, 2008; pp. 5–15. [Google Scholar]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef]
- Schreier, C.G.; Reinhard, M. Transformation of chlorinated organic compounds by iron and manganese powders in buffered water and in landfill leachate. Chemosphere 1994, 29, 1743–1753. [Google Scholar] [CrossRef]
- Gould, J.P.; Escovar, I.B.; Khudenko, B.M. Examination of the zinc cementation on cadmium in aqueous solutions. Water Sci. Technol. 1987, 19, 333–344. [Google Scholar] [CrossRef]
- Noubactep, C. Elemental metals for environmental remediation: Learning from cementation process. J. Hazard. Mater. 2010, 181, 1170–1174. [Google Scholar] [CrossRef] [Green Version]
- Oldright, G.L.; Keyes, H.E.; Miller, V.; Sloan, W.A. Precipitation of lead and copper from solution on sponge iron. BuMines B 1928, 281, 1–131. [Google Scholar]
- Gould, J.P. The kinetics of hexavalent chromium reduction by metallic iron. Water Res. 1982, 16, 871–877. [Google Scholar] [CrossRef]
- March, J. Advanced Organic Chemistry: Reaction Mechanism and Structure; McGraw-Hill Book Co.: New York, NY, USA, 1968. [Google Scholar]
- Werner, J. Amination by reduction. Unit Processes Review. Ind. Eng. Chem. 1961, 53, 77–78. [Google Scholar] [CrossRef]
- Khudenko, B.M. Feasibility evaluation of a novel method for destruction of organics. Water Sci. Technol. 1991, 23, 1873–1881. [Google Scholar] [CrossRef]
- Béchamp, A. De l’action des protosels de fer sur la nitronaphtaline et la nitrobenzine: Nouvelle méthode de formation des bases organiques artificielles de Zinin. Ann. Chim. Phys. 1854, 42, 186–196. [Google Scholar]
- Werner, J. Amination by reduction. Ind. Eng. Chem. 1951, 43, 1917–1919. [Google Scholar] [CrossRef]
- Popat, V.; Padhiyar, N. Kinetic study of Bechamp process for p-nitrotoluene reduction to p-toluidine. Int. J. Chem. Eng. Applications 2013, 4, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. Relevant reducing agents in remediation Fe0/H2O systems. Clean—Soil Air Water 2013, 41, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Kochenov, S.V.; Korolev, K.G.; Dubinchuk, V.T.; Medvedev, Y.L. Experimental data on the conditions of precipitation of uranium from aqueous solutions. Geokhimiya 1977, 11, 1711–1716. [Google Scholar]
- Davis, F. An Elementary Handbook on Potable Water; Silver, Burdett & Co.: New York, NY, USA; Boston, MA, USA; Chicago, IL, USA, 1891; p. 118. [Google Scholar]
- Hatton, F. On the oxidation of organic matter in water by filtration through various media; and on the reduction of nitrates by sewage, spongy iron, and other agents. J. Chem. Soc. Trans. 1881, 39, 258–276. [Google Scholar] [CrossRef] [Green Version]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar]
- Bischof, G. The Purification of Water: Embracing the Action of Spongy Iron on Impure Water; Bell and Bain: Glasgow, UK, 1873; pp. 1–19. [Google Scholar]
- Bischof, G. On putrescent organic matter in potable water. Proc. R. Soc. Lond. 1877, 26, 258–261. [Google Scholar]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Anderson, W. On the purification of water by agitation with iron and by sand filtration. J. Soc. Arts 1886, 35, 29–38. [Google Scholar] [CrossRef]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Work. Assoc. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Lacy, W.J. Removal of radioactive material from water by slurrying with powdered metal. J. Am. Water Work. Assoc. 1952, 44, 824–828. [Google Scholar] [CrossRef]
- Emmons, A.H.; Lauderdale, J.R.A. Process for Water Decontamination. US Patent 2,752,309, 26 June 1956. [Google Scholar]
- Swope, H.G. Mixed bed ion exchange for the removal of radioactivity. J. Am. Water Work. Assoc. 1957, 49, 1085–1102. [Google Scholar] [CrossRef]
- Levin, H.; Diamond, W.J.; Brown, B.J. Influence of Ionic Strength on ion Exchange. Ind. Eng. Chem. 1959, 51, 313–318. [Google Scholar] [CrossRef]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. 2007, 133, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042. [Google Scholar]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Phosphate removal from agricultural tile drainage with iron enhanced sand. Water 2017, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Frankenberger, W.T.; Amrhein, C.; Fan, T.W.; Flaschi, D.; Glater, J.; Kartinen, E.; Kovac, K.; Lee, E.; Ohlendorf, H.M.; Owens, L. Advanced treatment technologies in the remediation of seleniferous drainage waters and sediments. Irrig. Drain. Syst. 2004, 18, 19–41. [Google Scholar] [CrossRef]
- Harza Engineering Co. Selenium Removal Study. Report to Panoche Drainage District; Harza Engineering Co.: Firebaugh, CA, USA, 1986. [Google Scholar]
- Anderson, M.A. Fundamental aspects of selenium removal by Harza process. In Rep San Joaquin Valley Drainage Program; US Department Interior: Sacramento, CA, USA, 1989. [Google Scholar]
- Wakatsuki, T.; Esumi, H.; Omura, S. High performance and N, P removable on-site domestic wastewater treatment system by multi-soil-layering method. Water Sci. Technol. 1993, 27, 31–40. [Google Scholar] [CrossRef]
- Latrach, L.; Ouazzani, N.; Hejjaj, A.; Mahi, M.; Masunaga, T.; Mandi, L. Two-stage vertical flow multi-soil-layering (MSL) technology for efficient removal of coliforms and human pathogens from domestic wastewater in rural areas under arid climate. Int. J. Hyg. Environ. Health 2018, 22, 64–80. [Google Scholar] [CrossRef]
- Masunaga, T.; Sato, K.; Zennami, T.; Fujii, S.; Wakatsuki, T. Direct treatment of polluted river water by the multi-soil-layering method. J. Water Environ. Technol. 2003, 1, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Luo, A.C.; Sato, K.; Wakatsuki, T.; Masunaga, T. An introduction of a multi-soil-layering system: A novel green technology for wastewater treatment in rural areas. Water Environ. J. 2009, 23, 255–262. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water treatment using metallic iron: A tutorial review. Processes 2019, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Makota, S.; Nde-Tchoupe, A.I.; Mwakabona, H.T.; Tepong-Tsindé, R.; Noubactep, C.; Nassi, A.; Njau, K.N. Metallic iron for water treatment: Leaving the valley of confusion. Appl. Water Sci. 2017, 7, 4177–4196. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, G.W.; Hoff, J.T.; Gillham, R.W. Sampling bias caused by materials used to monitor halocarbons in groundwater. Environ. Sci. Technol. 1990, 24, 135–142. [Google Scholar] [CrossRef]
- Sweeney, K.H.; Fischer, J.R. Reductive Degradation of Halogenated Pesticides. U.S. Patent 3, 640,821, 8 February 1972. [Google Scholar]
- Phillips, D.H.; van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Lee, T.R.; Sexton, M.R.; Acree, S.D.; Puls, R.W.; Blowes, D.W.; Kalinowski, C.; Tilton, J.M.; Woods, L.L. Geochemical and isotope study of trichloroethene degradation in a zero-valent iron permeable reactive barrier: A twenty-two-year performance evaluation. Environ. Sci. Technol. 2019, 53, 296–306. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Ruppert, H.; Noubactep, C. Designing the next generation of Fe0-based filters for decentralized safe drinking water treatment: A conceptual framework. Processes 2020, 8, 745. [Google Scholar] [CrossRef]
- Moraci, N.; Lelo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Canadian Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Antia, D.D.J. Water treatment and desalination using the eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz[mH2O], and n-Fex[Cation]nOyHz[Anion]m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- van Craenenbroeck, W. Easton & Anderson and the water supply of Antwerp (Belgium). Ind. Archaeol. Rev. 1998, 20, 105–116. [Google Scholar]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited. The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. An analysis of the evolution of reactive species in Fe0/H2O systems. J. Hazard. Mater. 2009, 168, 1626–1631. [Google Scholar] [CrossRef] [Green Version]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A Novel and Facile Method to Characterize the Suitability of Metallic Iron for Water Treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef] [Green Version]
- CNanseu-Njiki, P.; Gwenzi, W.; Pengou, M.; Rahman, M.A.; Noubactep, C. Fe0/H2O Filtration Systems for Decentralized Safe Drinking Water: Where to from Here? Water 2019, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Groysman, A. Corrosion for Everybody; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010. [Google Scholar]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Sleiman, N.; Deluchat, V.; Wazne, M.; Mallet, M.; Courtin-Nomade, A.; Kazpard, V.; Baudu, M. Phosphate removal from aqueous solution using ZVI/sand bed reactor: Behavior and mechanism. Water Res. 2016, 99, 56–65. [Google Scholar] [PubMed]
- Ullah, S.; Guo, X.; Luo, X.; Zhang, X.; Li, Y.; Liang, Z. The coupling of sand with ZVI/oxidants achieved proportional and highly efficient removal of arsenic. Front. Environ. Sci. Eng. 2020, 14, 94. [Google Scholar] [CrossRef]
- Hussam, A. Contending with a development disaster: Sono filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar]
- Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a newly designed steel-wool-based household filter for safe drinking water provision: Hydraulic conductivity and efficiency for pathogen removal. Processes 2019, 7, 966. [Google Scholar] [CrossRef] [Green Version]
| Process | Anno | Rationale for Using Fe0 | Species of Concern | Reference |
|---|---|---|---|---|
| Béchamp process (*) | 1854 | Reducing agent | Nitro phenols | [37] |
| Bischof process | 1871 | Generator of adsorbents | Color, pathogens, nitrate | [45] |
| Anderson process | 1885 | Generator of flocs | Color, pathogens, nitrate | [48] |
| Emmons process | 1951 | Generator of adsorbents | Radionuclides | [49] |
| Harza process | 1986 | Generator of adsorbents | Selenium | [59] |
| Wakatsuki process | 1989 | Generator of adsorbents | Organics, nutrients | [61] |
| Khudenko process | 1991 | Generator of reducing agents | Organics | [36] |
| Frigon process | 1992 | Generator of adsorbents | Phosphate | [54] |
| Fe0 PRBs | 1994 | Reducing agent | Chlorinated hydrocarbons | [28] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, V.; Yang, H.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. Tracing the Scientific History of Fe0-Based Environmental Remediation Prior to the Advent of Permeable Reactive Barriers. Processes 2020, 8, 977. https://doi.org/10.3390/pr8080977
Cao V, Yang H, Ndé-Tchoupé AI, Hu R, Gwenzi W, Noubactep C. Tracing the Scientific History of Fe0-Based Environmental Remediation Prior to the Advent of Permeable Reactive Barriers. Processes. 2020; 8(8):977. https://doi.org/10.3390/pr8080977
Chicago/Turabian StyleCao, Viet, Huichen Yang, Arnaud Igor Ndé-Tchoupé, Rui Hu, Willis Gwenzi, and Chicgoua Noubactep. 2020. "Tracing the Scientific History of Fe0-Based Environmental Remediation Prior to the Advent of Permeable Reactive Barriers" Processes 8, no. 8: 977. https://doi.org/10.3390/pr8080977





