Characterization of HCN-Derived Thermal Polymer: Implications for Chemical Evolution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Elemental Analysis
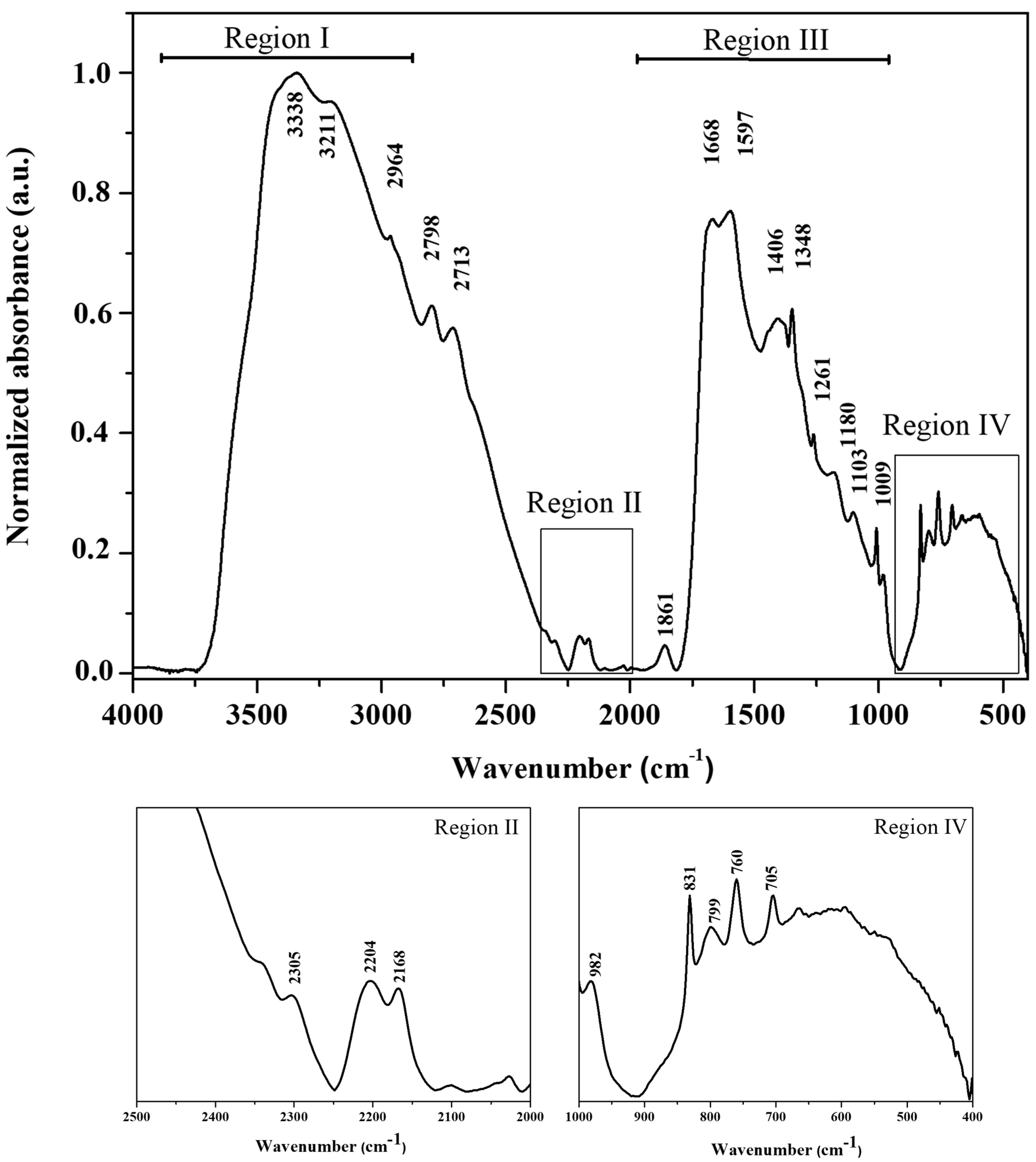
3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
3.3. TG, DTG and DSC Analysis
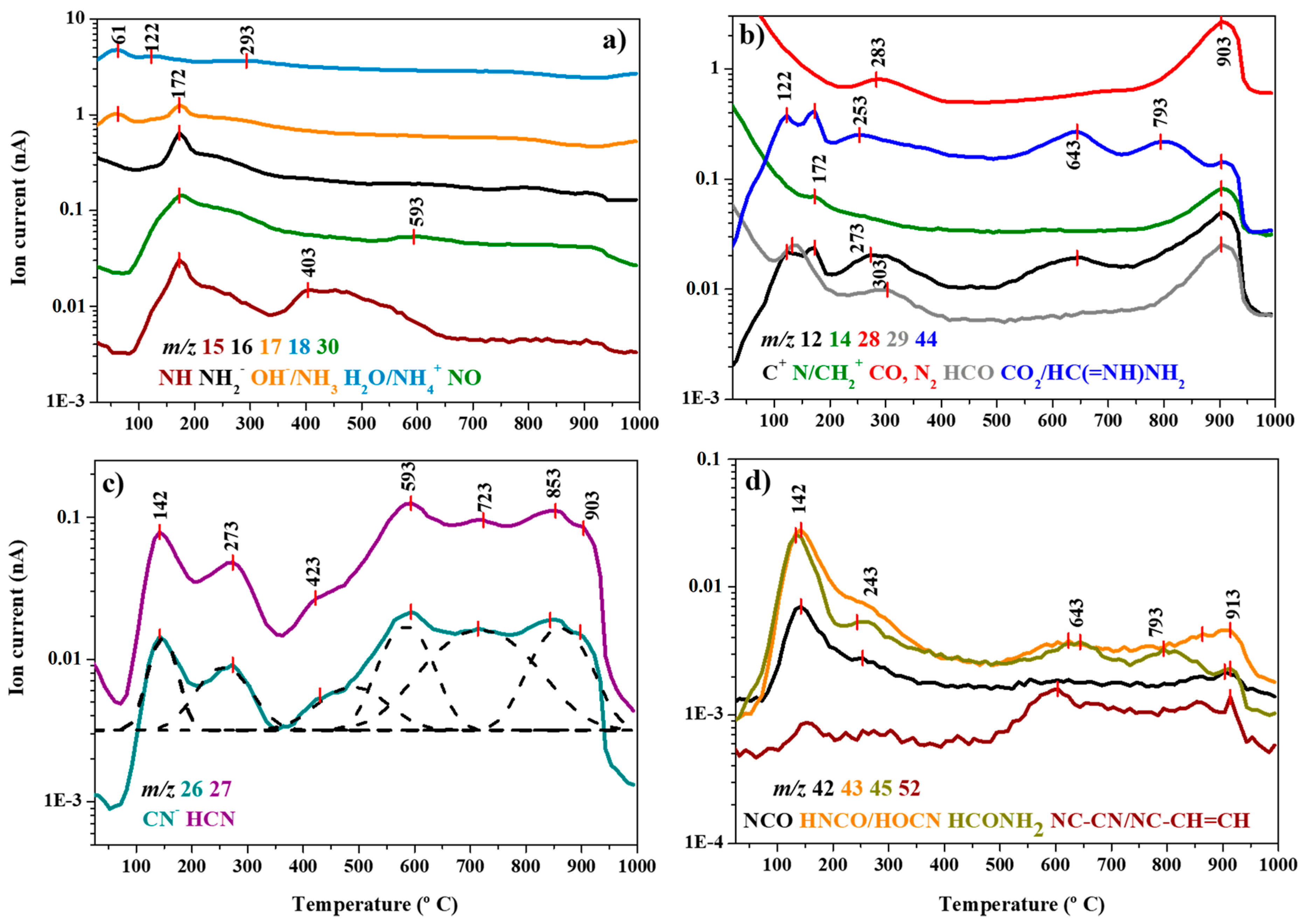
3.4. Mass Spectrometric Thermal Analysis
3.5. GC-MS Analysis of Hydrolyzed Samples
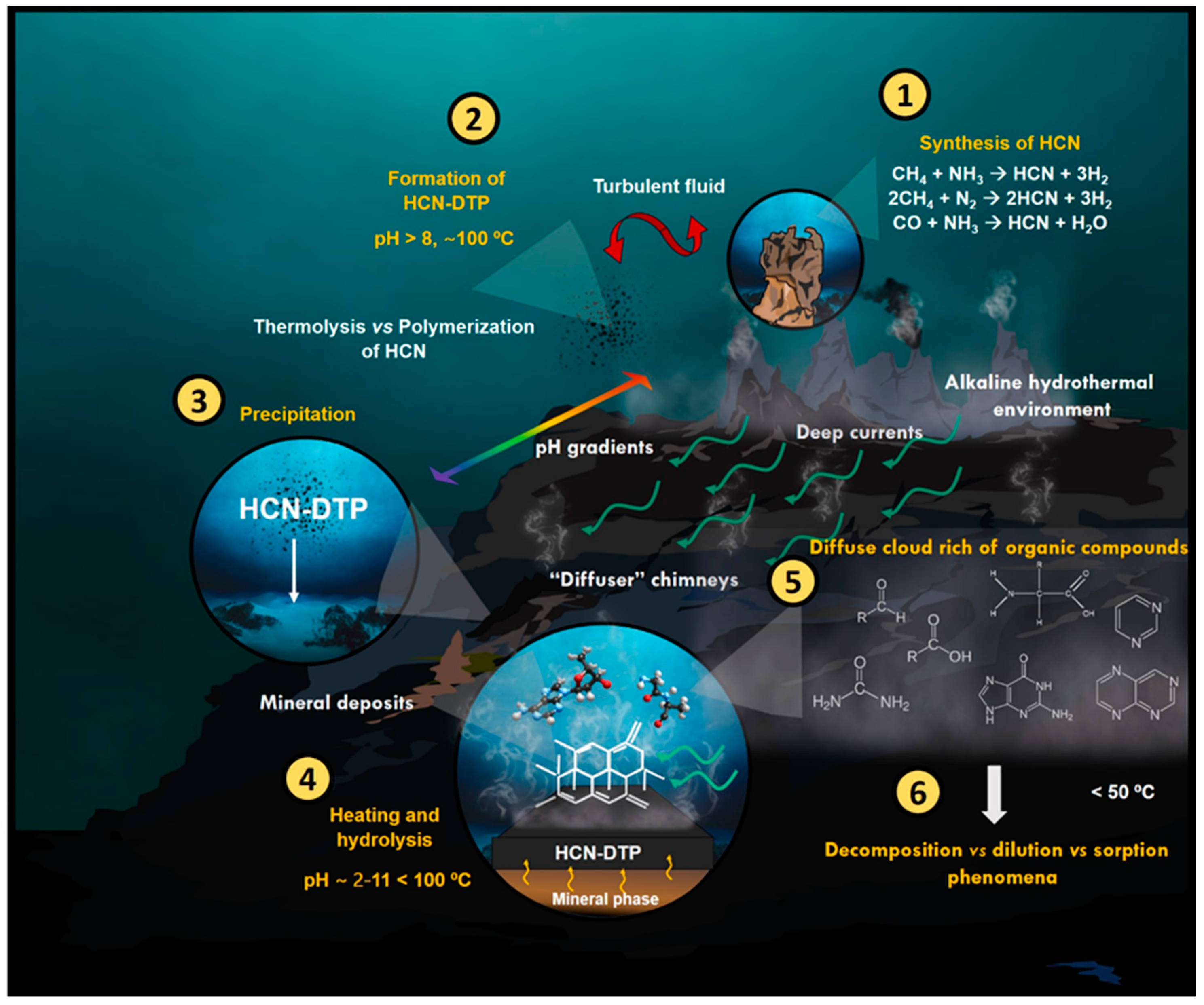
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sutherland, J.D. The Origin of Life-Out of the Blue. Angew. Chem. Int. Ed. 2016, 55, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bermejo, M.; Zorzano, M.P.; Osuna-Esteban, S. Simple Organics and Biomonomers Identified in HCN Polymers: An Overview. Life 2013, 3, 421–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, J.P.; Hagan, W.J. HCN and chemical evolution: The possible role of cyano compounds in prebiotic synthesis. Tetrahedron 1984, 40, 1093–1120. [Google Scholar] [CrossRef]
- Matthews, C.N.; Minard, R.D. Hydrogen cyanide polymers, comets and the origin of life. Faraday Discuss. 2006, 133, 393–401. [Google Scholar] [CrossRef]
- Holm, N.G.; Neubeck, A. Reduction of nitrogen compounds in oceanic basement and its implications for HCN formation and abiotic organic synthesis. Geochem. Trans. 2009, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Kasting, J.F.; Zahnle, K. Revisiting HCN formation in Earth’s early atmosphere. Earth Planet. Sci. Lett. 2011, 308, 417–423. [Google Scholar] [CrossRef]
- Parkos, D.; Pikus, A.; Alexeenko, A.; Melosh, H.J. HCN production from impact ejecta on the early Earth. In Proceedings of the 30th Internatinal Symposium on Rarefied Gas Dynamics: RGD 30, Victoria, BC, Canada, 10–15 July 2016; AIP Publishing: Melville, NY, USA; Volume 1786, p. 170001. [Google Scholar]
- Ferus, M.; Kubelík, P.; Knížek, A.; Pastorek, A.; Sutherland, J.; Civiš, S. High energy radical chemistry formation of HCN-rich atmospheres on early Earth. Sci. Rep. 2017, 7, 6275. [Google Scholar] [CrossRef] [Green Version]
- Rimmer, P.B.; Shorttle, O. Origin of life’s building blocks in carbon-and nitrogen-rich surface hydrothermal vents. Life 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Colín-García, M.; Negrón-Mendoza, A.; Ramos-Bernal, S. Organics produced by irradiation of frozen and liquid HCN solutions: Implications for Chemical Evolution Studies. Astrobiology 2009, 9, 279–288. [Google Scholar] [CrossRef]
- Mumma, M.J.; Charnley, S.B. The chemical composition of comets—Emerging taxonomies and natal heritage. Annu. Rev. Astron. Astrophys. 2011, 49, 471–524. [Google Scholar] [CrossRef]
- Pizzarello, S. Hydrogen cyanide in the Murchison meteorite. Astrophys. J. Lett. 2012, 754, L27. [Google Scholar] [CrossRef] [Green Version]
- Stribling, R.; Miller, S.L. Energy yields for hydrogen cyanide and formaldehyde syntheses: The hcn and amino acid concentrations in the primitive ocean. Orig. Life Evol. Biosph. 1987, 17, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The cold origin of life: A. Implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. he cold origin of life: A. Implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. Orig. Life Evol. Biosph. 2002, 32, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Fábián, B.; Szőri, M.; Jedlovszky, P. Floating Patches of HCN at the Surface of their aqueous solutions—Can they make “HCN World” Plausible? J. Phys. Chem. C 2014, 118, 21469–21482. [Google Scholar] [CrossRef] [Green Version]
- Liebman, S.A.; Pesce-Rodriguez, R.A.; Matthews, C.N. Organic analysis of hydrogen cyanide polymers: Prebiotic and extraterrestrial chemistry. Adv. Space Res. 1995, 15, 71–80. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Rogero, C.; Menor-Salván, C.; Osuna-Esteban, S.; Martín-Gago, J.A. New insights into the characterization of ‘insoluble black HCN polymers. Chem. Biodivers. 2012, 9, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bermejo, M.; José, L.; Carretero-González, J.; García-Fernández, L.; Aguilar, M.R. A Comparative Study on HCN Polymers Synthesized by Polymerization of NH4CN or Diaminomaleonitrile in Aqueous Media: New Perspectives for Prebiotic Chemistry and Materials Science. Chem. Eur. J. 2019, 25, 11437–11455. [Google Scholar] [CrossRef]
- Ferris, J.P.; Wos, J.D.; Nooner, D.W.; Oró, J. Chemical evolution: XXI. The Amino Acids Released on Hydrolysis of HCN Oligomers. J. Mol. Evol. 1974, 3, 225–231. [Google Scholar] [CrossRef]
- Matthews, C.N.; Moser, R.E. Peptide synthesis from hydrogen cyanide and water. Nature 1967, 215, 1230–1234. [Google Scholar] [CrossRef]
- Oró, J.; Kimball, A.P. Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide. Arch. Biochem. Biophys. 1961, 94, 217–227. [Google Scholar] [CrossRef]
- Thissen, H.; Koegler, A.; Salwiczek, M.; Easton, C.D.; Qu, Y.; Lithgow, T.; Evans, R.A. Prebiotic-chemistry inspired polymer coatings for biomedical and material science applications. NPG Asia Mater. 2015, 7, e225. [Google Scholar] [CrossRef] [Green Version]
- Thissen, H.; Evans, R.; Koegler, A. Hydrogen Cyanide-Based Polymer Surface Coatings and Hydrogels. U.S. Patent 9,587,141, 17 May 2017. [Google Scholar]
- Toh, R.J.; Evans, R.; Thissen, H.; Voelcker, N.H.; d’Ischia, M.; Ball, V. Deposition of Aminomalononitrile-Based Films: Kinetics, Chemistry, and Morphology. Langmuir 2019, 35, 9896–9903. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Manini, P.; Moracci, M.; Saladino, R.; Ball, V.; Thissen, H.; Evans, R.A.; Puzzarini, C.; Barone, V. Astrochemistry and Astrobiology: Materials Sciencein Wonderland? Int. J. Mol. Sci. 2019, 20, 4079. [Google Scholar]
- Mas, I.; de la Fuente, J.L.; Ruiz-Bermejo, M. Temperature effect on aqueous NH4CN polymerization: Relationship between kinetic behaviour and structural properties. Eur. Polym. J. 2020, 132, 109719. [Google Scholar] [CrossRef]
- José, L.; Ruiz-Bermejo, M.; Nna-Mvondo, D.; Minard, R.D. Further progress into the thermal characterization of HCN polymers. Polym. Degrad. Stab. 2014, 110, 241–251. [Google Scholar]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Marín-Yaseli, M.R. The influence of reaction conditions in aqueous HCN polymerization on the polymer thermal degradation properties. J. Anal. Appl. Pyrolysis 2017, 124, 103–112. [Google Scholar]
- Cataldo, F.; Patanè, G.; Compagnini, G. Synthesis of HCN polymer from thermal decomposition of formamide. J. Macromol. Sci. A Pure Appl. Chem. 2009, 46, 1039–1048. [Google Scholar] [CrossRef]
- Cataldo, F.; Lilla, E.; Ursini, O.; Angelini, G. TGA–FT-IR study of pyrolysis of poly (hydrogen cyanide) synthesized from thermal decomposition of formamide. Implications in cometary emissions. J. Anal. Appl. Pyrol. 2010, 87, 34–44. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in prebiotic synthesis. II. Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J. Mol. Biol. 1967, 30, 223–253. [Google Scholar]
- Ferris, J.P.; Ryan, T.J. Chemical evolution. XIV. Oxidation of diaminomaleonitrile and its possible role in hydrogen cyanide oligomerization. J. Org. Chem. 1973, 38, 3302–3307. [Google Scholar] [CrossRef]
- Ferris, J.P.; Edelson, E.H. Chemical evolution. 31. Mechanism of the condensation of cyanide to hydrogen cyanide oligomers. J. Org. Chem. 1978, 43, 3989–3995. [Google Scholar] [CrossRef]
- Ferris, J.P.; Donner, D.B.; Lotz, W. Chemical evolution. IX. Mechanism of the oligomerization of hydrogen cyanide and its possible role in the origins of life. J. Am. Chem. Soc. 1972, 94, 6968–6974. [Google Scholar] [CrossRef]
- Völker, T. Polymere blausäure. Angew. Chem. 1960, 72, 379–384. [Google Scholar]
- Ferris, J.P.; Edelson, E.H.; Auyeung, J.M.; Joshi, P.C. Structural studies on HCN oligomers. J. Mol. Evol. 1981, 17, 69–77. [Google Scholar]
- Umemoto, K.; Takahasi, M.; Yokota, K. Studies on the structure of HCN oligomers. Orig. Life Evol. Biosph. 1987, 17, 283–293. [Google Scholar]
- Mamajanov, I.; Herzfeld, J. HCN polymers characterized by solid state NMR: Chains and sheets formed in the neat liquid. J. Chem. Phys. 2009, 130, 134503. [Google Scholar]
- Mamajanov, I.; Herzfeld, J. HCN polymers characterized by SSNMR: Solid state reaction of crystalline tetramer (diaminomaleonitrile). J. Chem. Phys. 2009, 130, 134504. [Google Scholar]
- Mukhin, L.E.V. Evolution of organic compounds in volcanic regions. Nature 1974, 251, 50–51. [Google Scholar] [CrossRef]
- Mukhin, L. Volcanic processes and synthesis of simple organic compounds on primitive earth. Orig. Life 1976, 7, 355–368. [Google Scholar] [CrossRef]
- Dowler, M.J.; Ingmanson, D.E. Thiocyanate in Red Sea brine and its implications. Nature 1979, 279, 51–52. [Google Scholar] [CrossRef]
- Corliss, J.B.; Baross, J.A.; Hoffman, S.E. An hypothesis concerning the relationships between submarine hot springs and the origin of life on earth. Oceanol. Acta Spec. Issue 1981, 1, 59–69. [Google Scholar]
- Baross, J.A.; Hoffman, S.E. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 1985, 15, 327–345. [Google Scholar] [CrossRef]
- Ferris, J.P. Chemical Markers of Prebiotic Chemistry in Hydrothermal Systems. In Marine Hydrothermal Systems and the Origin of Life; Holm, N.G., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 109–134. [Google Scholar]
- Aubrey, A.D.; Cleaves, H.J.; Bada, J.L. The Role of Submarine Hydrothermal Systems in the Synthesis of Amino Acids. Orig. Life Evol. Biosph. 2009, 39, 91–108. [Google Scholar] [CrossRef]
- Lowe, C.U.; Rees, M.W.; Markham, R. Synthesis of Complex Organic Compounds from Simple Precursors: Formation of Amino-Acids, Amino-Acid Polymers, Fatty Acids and Purines from Ammonium Cyanide. Nature 1963, 199, 219–222. [Google Scholar] [CrossRef]
- Hennet, R.-C.; Holm, N.; Engel, M. Abiotic synthesis of amino acids under hydrothermal conditions and the origin of life: A perpetual phenomenon? Naturwissenschaften 1992, 79, 361–365. [Google Scholar] [CrossRef]
- Islam, M.N.; Kaneko, T.; Kobayashi, K. Determination of amino acids formed in a supercritical water flow reactor simulating submarine hydrothermal systems. Jpn. Soc. Anal. Chem. 2002, 17, i1631–i1634. [Google Scholar]
- Colín-García, M.; Heredia, A.; Cordero, G.; Camprubí, A.; Negrón-Mendoza, A.; Ortega-Gutiérrez, F.; Beraldi, H.; Ramos-Bernal, S. Hydrothermal vents and prebiotic chemistry: A review. Bol. Soc. Geol. Mex. 2016, 68, 599–620. [Google Scholar] [CrossRef]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubí, E.; Lane, N. The Origin of Life in Alkaline Hydrothermal Vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Villafañe-Barajas, S.A.; Colín-García, M.; Negrón-Mendoza, A.; Ruiz-Bermejo, M. An experimental study of the thermolysis of hydrogen cyanide: The role of hydrothermal systems in chemical evolution. Int. J. Astrobiol. 2020, 1–10. [Google Scholar] [CrossRef]
- de la Fuente, J.L.; Ruiz-Bermejo, M.; Menor-Salván, C.; Osuna-Esteban, S. Thermal characterization of HCN polymers by TG–MS, TG, DTA and DSC methods. Polym. Degrad. Stab. 2011, 96, 943–948. [Google Scholar]
- Fernández, A.; Ruiz-Bermejo, M.; de la Fuente, J.L. Modelling the kinetics and structural property evolution of a versatile reaction: Aqueous HCN polymerization. Phys. Chem. Chem. Phys. 2018, 20, 17353–17366. [Google Scholar]
- Marín-Yaseli, M.R.; Moreno, M.; de la Fuente, J.L.; Briones, C.; Ruiz-Bermejo, M. Experimental conditions affecting the kinetics of aqueous HCN polymerization as revealed by UV–vis spectroscopy. Spectrochim. Acta Part A 2018, 191, 389–397. [Google Scholar]
- Azamar, J.; Draganić, I. Equipo para la preparación de compuestos tóxicos en solución acuosa y en atmósfera controlada: Cianuros para experimentos en Química de Radiaciones; Informe Técnico Q5; Departamento de Química, CEN, UNAM: Mexico City, Mexico, 1982; p. 16. [Google Scholar]
- Ferris, J.P.; Joshi, P.C.; Edelson, E.H.; Lawless, J.G. HCN: A plausible source of purines, pyrimidines and amino acids on the primitive earth. J. Mol. Evol. 1978, 11, 293–311. [Google Scholar] [CrossRef]
- Colín-García, M.; Villafañe-Barajas, S.; Camprubí, A.; Ortega-Gutiérrez, F.; Colás, V.; Negrón-Mendoza, A. 5.4 Prebiotic Chemistry in Hydrothermal Vent Systems. In Handbook of Astrobiology; Kolb, V., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 297–329. [Google Scholar]
- Matthews, C.N. Hydrogen cyanide polymers: From laboratory to space. Planet. Space Sci. 1995, 43, 1365–1370. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; González-Toril, E.; Mompeán, C.; Ruiz-Bermejo, M. The Role of Aqueous Aerosols in the “Glyoxylate Scenario”: An Experimental Approach. Chem. Eur. J. 2016, 22, 12785–12799. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; Cid, C.; Yagüe, A.I.; Ruiz-Bermejo, M. Detection of Macromolecular Fractions in HCN Polymers Using Electrophoretic and Ultrafiltration Techniques. Chem. Biodivers. 2017, 14, e1600241. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Repr. as Paperback; Wiley: Chichester, UK, 2010; p. 366. [Google Scholar]
- Schwartz, A.W.; Voet, A.B.; Veen, M. Recent progress in the prebiotic chemistry of HCN. Orig. Life 1984, 14, 91–98. [Google Scholar]
- Minard, R.D.; Hatcher, P.G.; Gourley, R.C.; Matthews, C.N. Structural investigations of hydrogen cyanide polymers: New insights using TMAH thermochemolysis/GC-MS. Orig. Life Evol. Biosph. 1998, 28, 461–473. [Google Scholar]
- Marín-Yaseli, M.R.; Mompeán, C.; Ruiz-Bermejo, M. A prebiotic synthesis of pterins. Chem. Eur. J. 2015, 21, 13531–13534. [Google Scholar] [CrossRef]
- Borquez, E.; Cleaves, H.J.; Lazcano, A.; Miller, S.L. An investigation of prebiotic purine synthesis from the hydrolysis of HCN polymers. Orig. Life Evol. Biosph. 2005, 35, 79–90. [Google Scholar] [CrossRef]
- Moser, R.E.; Claggett, A.R.; Matthews, C.N. Peptide formation from aminomalononitrile (HCN trimer). Tetrahedron Lett. 1968, 9, 1605–1608. [Google Scholar] [CrossRef]
- Das, T.; Ghule, S.; Vanka, K. Insights into the origin of life: Did it begin from HCN and H2O? ACS Cent. Sci. 2019, 5, 1532–1540. [Google Scholar] [CrossRef] [Green Version]
- Toner, J.D.; Catling, D.C. Alkaline lake settings for concentrated prebiotic cyanide and the origin of life. Geochim. Cosmochim. Acta 2019, 260, 124–132. [Google Scholar] [CrossRef]
- Smirnov, A.; Hausner, D.; Laffers, R.; Strongin, D.R.; Schoonen, M.A. Abiotic ammonium formation in the presence of Ni-Fe metals and alloys and its implications for the Hadean nitrogen cycle. Geochem. Trans. 2008, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- LaRowe, D.E.; Regnier, P. Thermodynamic potential for the abiotic synthesis of adenine, cytosine, guanine, thymine, uracil, ribose, and deoxyribose in hydrothermal systems. Orig. Life Evol. Biosph. 2008, 38, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Lupton, J.E.; Delaney, J.R.; Johnson, H.P.; Tivey, M.K. Entrainment and vertical transport of deep-ocean water by buoyant hydrothermal plumes. Nature 1985, 316, 621–623. [Google Scholar] [CrossRef]
- Little, S.A.; Stolzenbach, K.D.; Von Herzen, R.P. Measurements of plume flow from a hydrothermal vent field. J. Geophys. Res. 1987, 92, 2587–2596. [Google Scholar] [CrossRef]
- Bemis, K.; Lowell, R.; Farough, A. Diffuse Flow on and Around Hydrothermal Vents at Mid-Ocean Ridges. Oceanography 2012, 25, 182–191. [Google Scholar] [CrossRef]
- Mittelstaedt, E.; Escartín, J.; Gracias, N.; Olive, J.-A.; Barreyre, T.; Davaille, A.; Cannat, M.; Garcia, R. Quantifying diffuse and discrete venting at the Tour Eiffel vent site, Lucky Strike hydrothermal field. Geochem. Geophys. Geosyst. 2012, 13, 1–18. [Google Scholar] [CrossRef]
- Holm, N.G.; Hennet, R.J.-C. Hydrothermal systems: Their varieties, dynamics, and suitability for prebiotic chemistry. In Marine Hydrothermal Systems and the Origin of Life; Springer: Dordrecht, The Netherlands, 1992; pp. 15–31. [Google Scholar]
- Allen, D.E.; Seyfried, W.E. Serpentinization and heat generation: Constraints from Lost City and Rainbow hydrothermal systems. Geochim. Cosmochim. Acta 2004, 68, 1347–1354. [Google Scholar] [CrossRef]
- Yamaoka, K.; Kawahata, H.; Gupta, L.P.; Ito, M.; Masuda, H. Thermal stability of amino acids in siliceous ooze under alkaline hydrothermal conditions. Org. Geochem. 2007, 38, 1897–1909. [Google Scholar] [CrossRef]
- Stüeken, E.E.; Anderson, R.E.; Bowman, J.S.; Brazelton, W.J.; Colangelo-Lillis, J.; Goldman, A.D.; Som, S.M.; Baross, J.A. Did life originate from a global chemical reactor? Geobiology 2013, 11, 101–126. [Google Scholar] [CrossRef]
- Omran, A.; Pasek, M. A Constructive Way to Think about Different Hydrothermal Environments for the Origins of Life. Life 2020, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Burcar, B.T.; Barge, L.M.; Trail, D.; Watson, E.B.; Russell, M.J.; McGown, L.B. RNA Oligomerization in Laboratory Analogues of Alkaline Hydrothermal Vent Systems. Astrobiology 2015, 15, 509–522. [Google Scholar] [CrossRef]
- Mompeán, C.; Marín-Yaseli, M.R.; Espigares, P.; González-Toril, E.; Zorzano, M.-P.; Ruiz-Bermejo, M. Prebiotic chemistry in neutral/reduced-alkaline gas-liquid interfaces. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Holm, N.G.; Dumont, M.; Ivarsson, M.; Konn, C. Alkaline fluid circulation in ultramafic rocks and formation of nucleotide constituents: A hypothesis. Geochem. Trans. 2006, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Stage I (25–150 °C) Evaporation | Stage II (150–450 °C) Low Thermal Decomposition | Stage III (450–1000 °C) High Thermal Decomposition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak | DTG | DSC | Peak | DTG | DSC | Peak | DTG | DSC | |||
| Tmax (°C) | dW/dt (wt %/°C) | Tpeak (°C) | Tmax (°C) | dW/dt (wt %/°C) | Tpeak (°C) | Tmax (°C) | dW/dt (wt %/°C) | Tpeak (°C) | |||
| 1 | 56 | 0.11 | 71 | 3 | 167 | 0.15 | 167 | 5 | 636 | 0.05 | 636 |
| 2 | 124 | 0.20 | 127 | 4 | 279 | 0.11 | 785 | ||||
| 288 | 0.11 | 301 | 6 | 906 | 0.34 | 910 | |||||
| 7 | 921 | 0.27 | 938 | ||||||||
| Probable Species | MS Peaks (m/z) | DTG Peaks (°C) | ||||||
|---|---|---|---|---|---|---|---|---|
| 55 | 124 | 167 | 279–288 | 642 | 906 | 921 | ||
| C+ | 12 | |||||||
| N, CH2+ | 14 | |||||||
| NH | 15 | |||||||
| NH2 | 16 | |||||||
| OH-, NH3 | 17 | |||||||
| H2O/NH4+ | 18 | |||||||
| -CN | 26 | |||||||
| HCN | 27 | |||||||
| CO, N2 | 28 | |||||||
| N2H, HCO | 29 | |||||||
| NO | 30 | |||||||
| NCO | 42 | |||||||
| HNCO, HOCN | 43 | |||||||
| CO2, +C(=NH)NH | 44 | |||||||
| HCONH2 | 45 | |||||||
| NC-CN, NC-CH=CH | 52 | |||||||
| STAGES | STAGE I | STAGE II | STAGE III | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Colín-García, M. Characterization of HCN-Derived Thermal Polymer: Implications for Chemical Evolution. Processes 2020, 8, 968. https://doi.org/10.3390/pr8080968
Villafañe-Barajas SA, Ruiz-Bermejo M, Rayo-Pizarroso P, Colín-García M. Characterization of HCN-Derived Thermal Polymer: Implications for Chemical Evolution. Processes. 2020; 8(8):968. https://doi.org/10.3390/pr8080968
Chicago/Turabian StyleVillafañe-Barajas, Saúl A., Marta Ruiz-Bermejo, Pedro Rayo-Pizarroso, and María Colín-García. 2020. "Characterization of HCN-Derived Thermal Polymer: Implications for Chemical Evolution" Processes 8, no. 8: 968. https://doi.org/10.3390/pr8080968
APA StyleVillafañe-Barajas, S. A., Ruiz-Bermejo, M., Rayo-Pizarroso, P., & Colín-García, M. (2020). Characterization of HCN-Derived Thermal Polymer: Implications for Chemical Evolution. Processes, 8(8), 968. https://doi.org/10.3390/pr8080968







