Upgrading of Biogas to Methane Based on Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
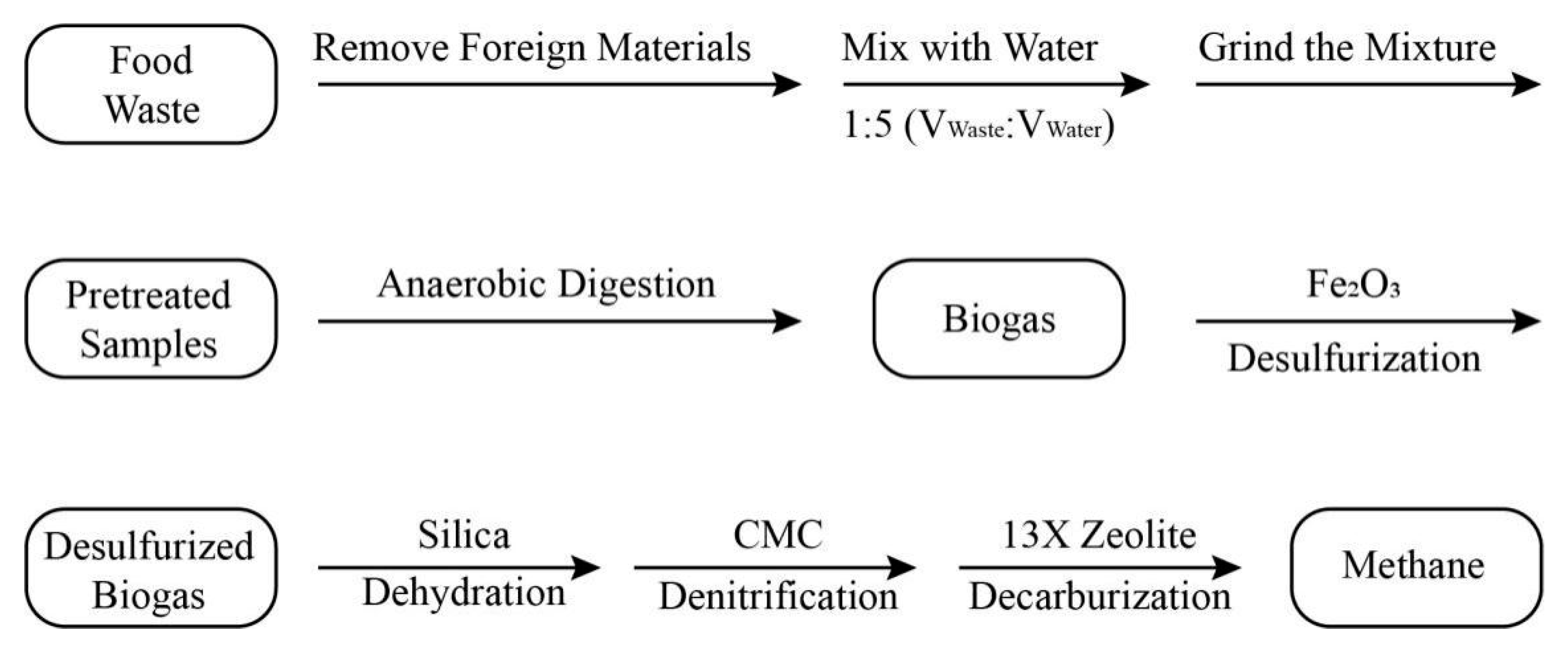
2.2.1. Production of Biogas
Pretreatment of Raw Materials
Production of Biogas
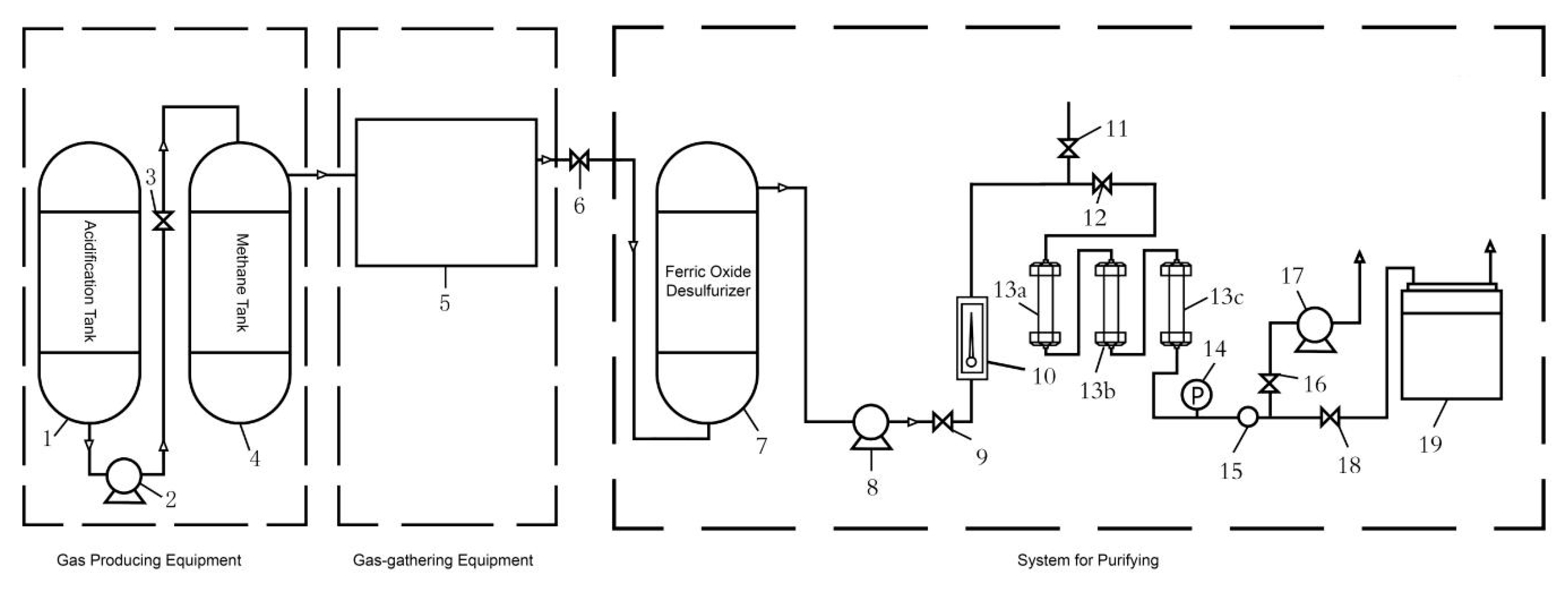
2.2.2. Purification of Biogas
Desulfurization of Biogas
Measurement of CO2 Adsorption Capacity
Regenerating of Adsorbents
3. Results and Discussion
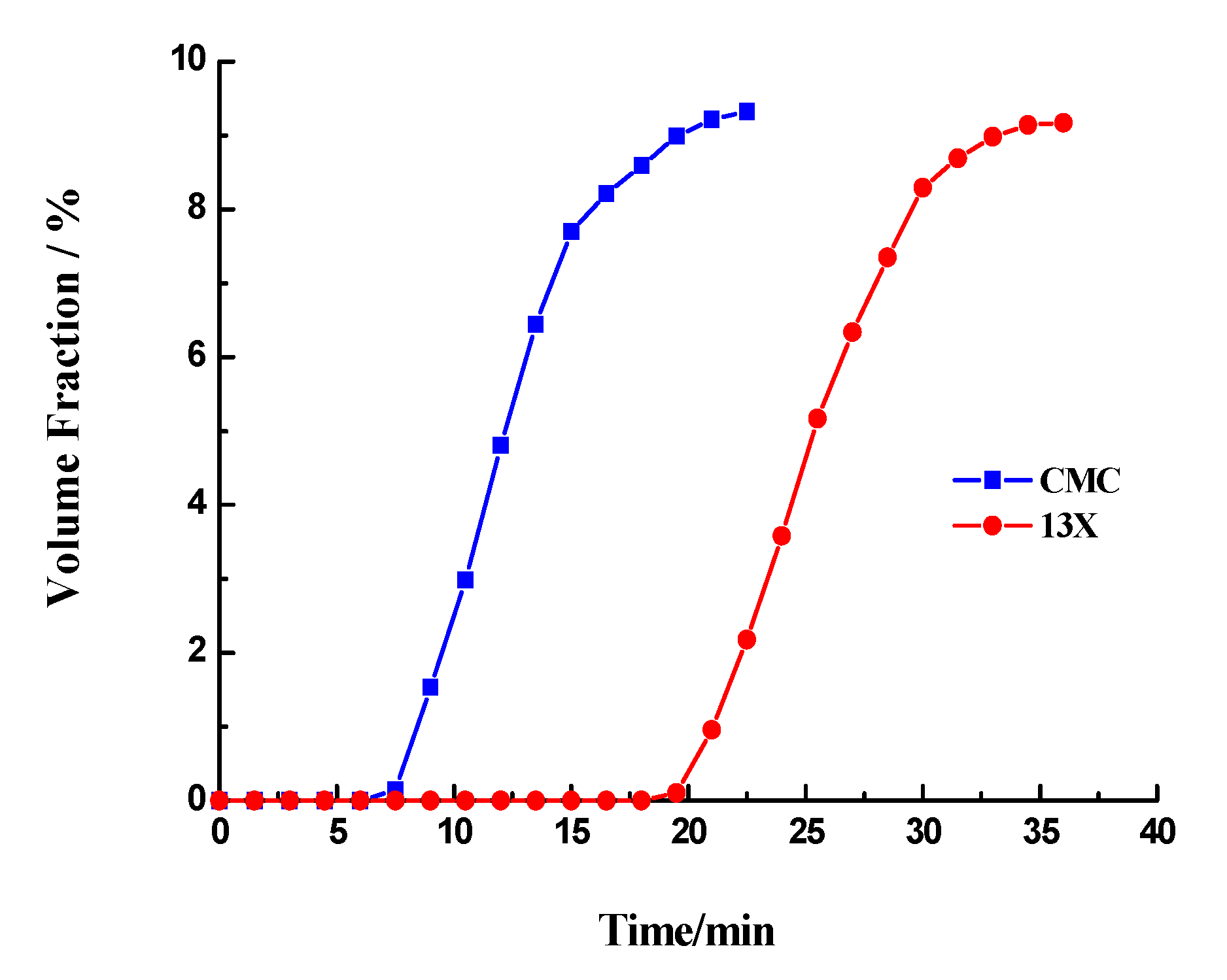
3.1. CO2 Adsorption on 13X and CMS
3.2. Purification of the Humid Desulfurized Biogas
3.3. Purification of the Dry Desulfurized Biogas
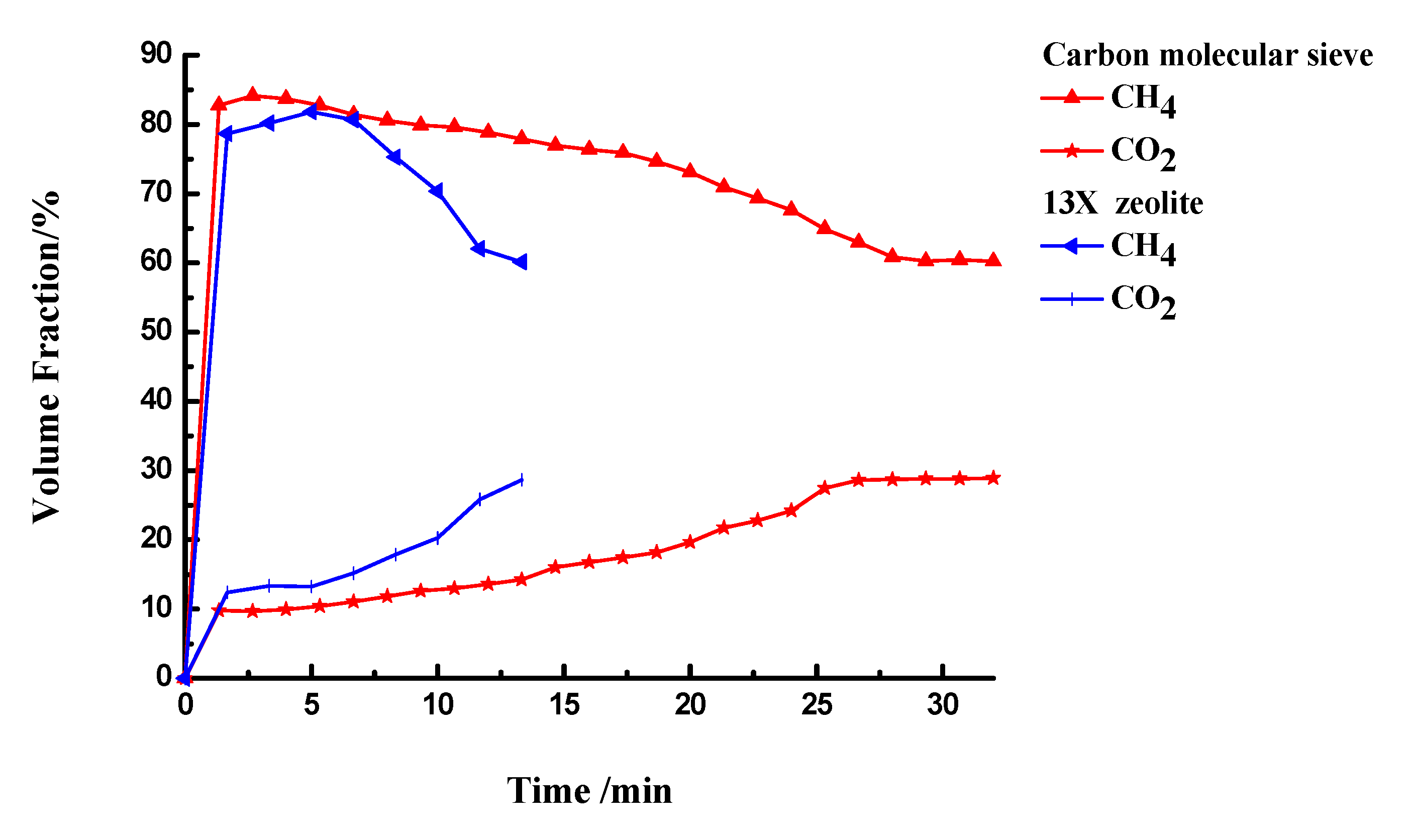
3.3.1. CO2 Separation
3.3.2. Desorption and Adsorption Cycling
3.4. Upgrading Biogas to CH4
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Gustafsson, M.; Cruz, I.; Svensson, N.; Karlsson, M. Scenarios for upgrading and distribution of compressed and liquefied biogas-Energy, environmental, and economic analysis. J. Clean Prod. 2020, 256, 120473. [Google Scholar] [CrossRef]
- Dhanya, B.S.; Mishra, A.; Chandel, A.K.; Verma, M.L. Development of sustainable approaches for converting the organic waste to bioenergy. Sci. Total Environ. 2020, 723, 138109. [Google Scholar] [CrossRef] [PubMed]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granstrom, K. A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Karagoz, Y. Analysis of the impact of gasoline, biogas and biogas plus hydrogen fuels on emissions and vehicle performance in the WLTC and NEDC. Int. J. Hydrog. Energy 2019, 44, 31621–31632. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. An economic and environmental assessment on landfill gas to vehicle fuel conversion for waste hauling operations. Resour. Conserv. Recycl. 2019, 142, 155–166. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, X.; Jiang, Z. Composition and sources of aerosol organic matter in a highly anthropogenic influenced semi-enclosed bay: Insights from excitation-emission matrix spectroscopy and isotopic evidence. Atmos. Res. 2020, 241, 104958. [Google Scholar] [CrossRef]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle emissions trapping materials: Successes, challenges, and the path forward. Appl. Catal. B-Environ. 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Augelletti, R.; Conti, M.; Annesini, M.C. Pressure swing adsorption for biogas upgrading. A new process confifiguration for the separation of biomethane and carbon dioxide. J. Clean Prod. 2017, 140, 1390–1398. [Google Scholar] [CrossRef]
- Rodero, M.D.R.; Carvajal, A.; Arbib, Z.; Lara, E.; de Prada, C.; Lebrero, R.; Munoz, R.A. Performance evaluation of a control strategy for photosynthetic biogas upgrading in a semi-industrial scale photobioreactor. Bioresour. Technol. 2020, 307, 123207. [Google Scholar] [CrossRef] [PubMed]
- Ferella, F.; Cucchiella, F.; D’Adamo, I.; Gallucci, K. A techno-economic assessment of biogas upgrading in a developed market. J. Clean Prod. 2019, 210, 945–957. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valori. 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Ferella, F.; Puca, A.; Taglieri, G.; Rossi, L.; Gallucci, K. Separation of carbon dioxide for biogas upgrading to biomethane. J. Clean Prod. 2017, 164, 1205–1218. [Google Scholar] [CrossRef]
- Tagliabue, M.; Farrusseng, D.; Valencia, S.; Aguado, S.; Ravon, U.; Rizzo, C.; Corma, A.; Mirodatos, C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. [Google Scholar] [CrossRef]
- Rocha, L.A.M.; Andreassen, K.A.; Grande, C.A. Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure. Chem. Eng. Sci. 2017, 164, 148–157. [Google Scholar] [CrossRef]
- Lozano-Castello, D.; Alcaniz-Monge, J.; Cazorla-Amoro, D.; Linares-Solano, A.; Zhu, W.; Kapteijn, F.; Moulijn, J.A. Adsorption properties of carbon molecular sieves prepared from an activated carbon by pitch pyrolysis. Carbon 2005, 43, 1643–1651. [Google Scholar] [CrossRef]
- Yu, H.; Cho, S.; Bai, B.C.; Yi, K.B.; Lee, Y. Effects of fluorination on carbon molecular sieves for CH4/CO2 gas separation behavior. Int. J. Greenh. Gas Control 2012, 10, 278–284. [Google Scholar] [CrossRef]
- Wahby, A.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. CO2 adsorption on carbon molecular sieves. Microporous Mesoporous Mater. 2012, 164, 280–287. [Google Scholar] [CrossRef]
- Wahby, A.; Ramos-Fernandez, J.M.; Martinez-Escandell, M. High-surface-area carbon molecular sieves for selective CO2 adsorption. Chemsuschem 2010, 3, 974–981. [Google Scholar] [CrossRef]
- Liang, Z.; Marshall, M.; Chaffee, A.L. CO2 adsorption-based separation by metal organic framework (Cu-BTC) versus zeolite (13X). Energy Fuels 2009, 23, 2785–2789. [Google Scholar] [CrossRef]
- Peter, S.A.; Baron, G.V.; Gascon, J.; Kapteijn, F.; Denayer, J.F.M. Dynamic desorption of CO2 and CH4 from amino-MIL-53(Al) adsorbent. Adsorption 2013, 19, 1235–1244. [Google Scholar] [CrossRef]
- Alonso-Vicario, A.; Ochoa-Gomez, J.R.; Gil-Rio, S.; Gomez-Jimenez-Aberasturi, O.; Ramirez-Lopez, C.A.; Torrecilla-Soria, J.; Dominguez, A. Purification and upgrading of biogas by pressure swing adsorption on syntheticand natural zeolites. Microporous Mesoporous Mater. 2010, 134, 100–107. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Song, C. “Molecular Basket” sorbents for separation of CO2 and H2S from various gas streams. J. Am. Chem. Soc. 2009, 131, 5777–5783. [Google Scholar] [CrossRef]
- Montanari, T.; Busca, G. On the mechanism of adsorption and separation of CO2 on LTA zeolites: An IR investigation. Vib. Spectrosc. 2008, 46, 45–51. [Google Scholar] [CrossRef]

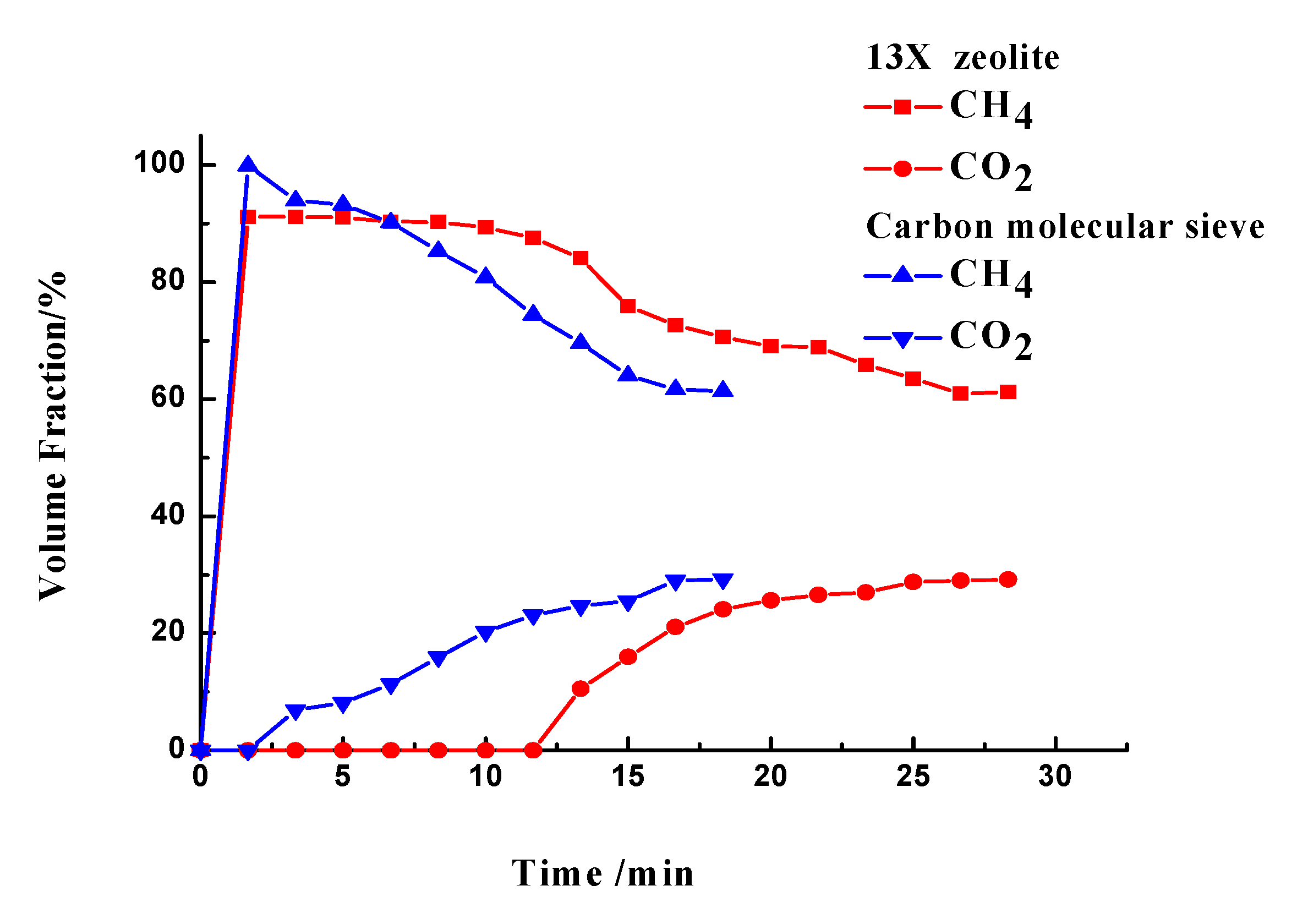
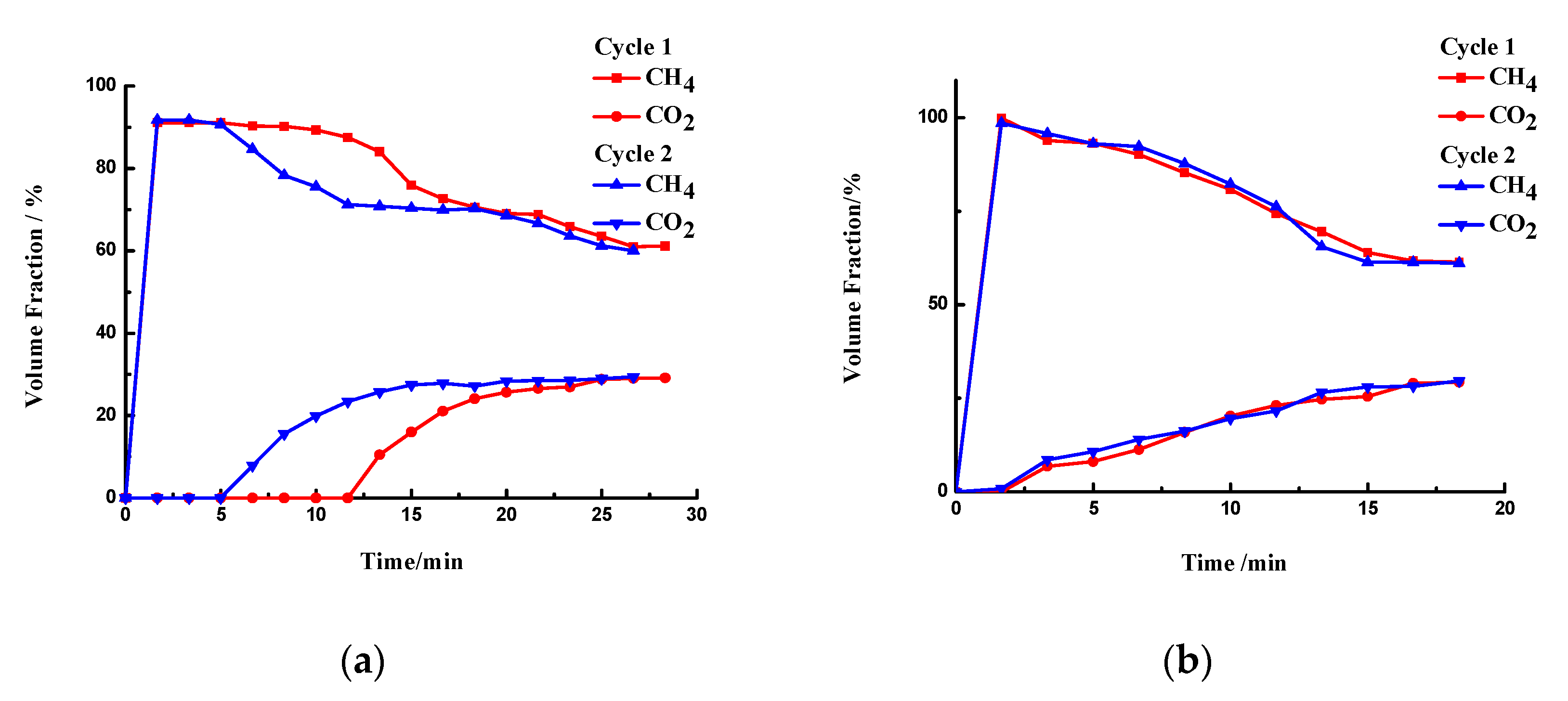

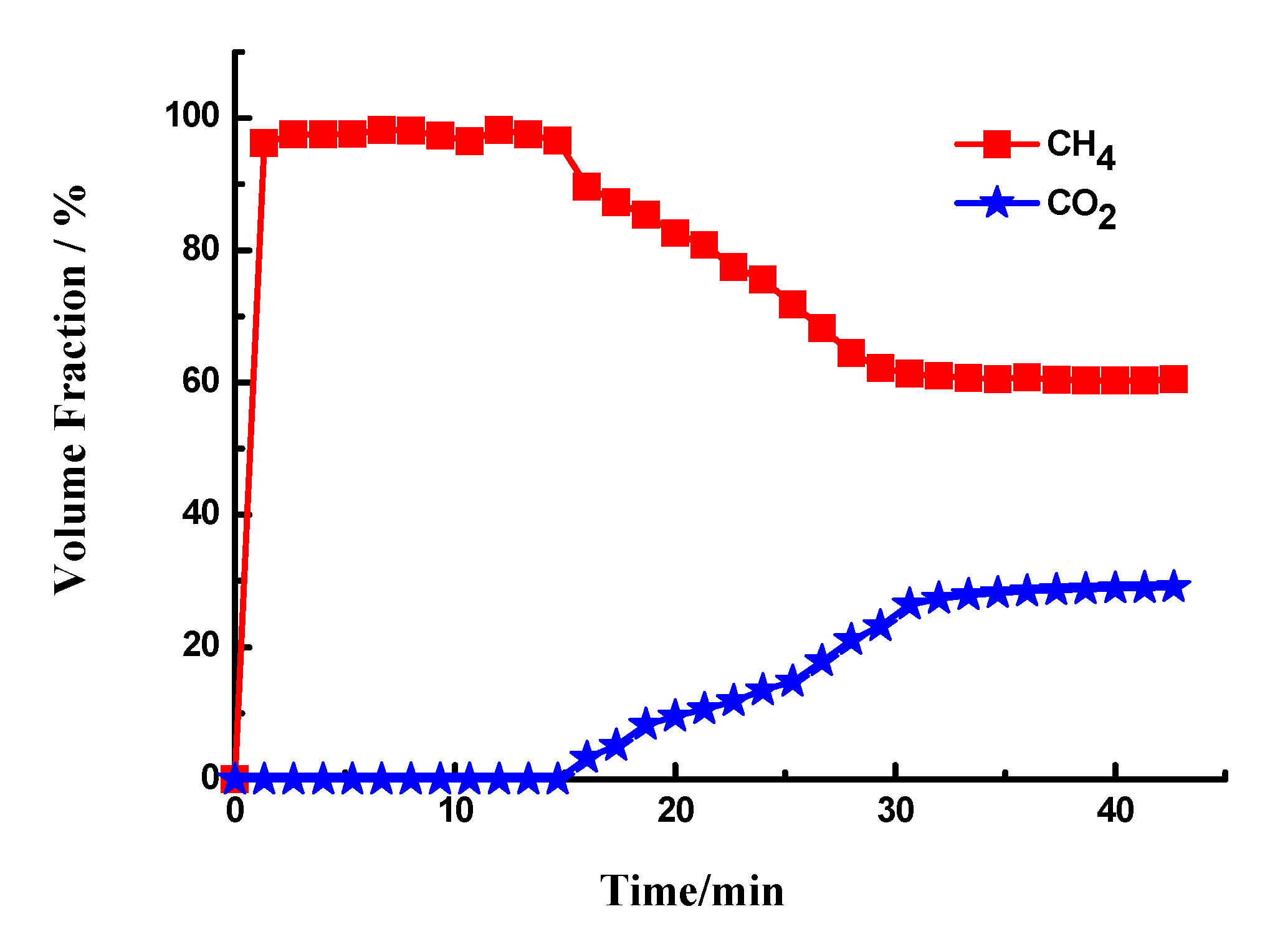
| Samples | Gas Composition (V/V) | Pressure (MPa) | Temperature (°C) | CO2 Adsorption Capacity (mgCO2/g) | Reference |
|---|---|---|---|---|---|
| CMS | CH4/CO2 90/10 | 1 | 41.5 | 35.2 a | [16] |
| CMS | CO2 100 | 0.1 | 25 | 115 b | [17] |
| CMS | CO2 100 | 0.1 | 25 | 70.8 b | [18] |
| CMS | CO2 100 | 0.1 | 25 | 132 b | [19] |
| CMS | CO2 100 | 0.1 | 25 | 207 b | [20] |
| 13X | CO2 100 | 0.1 | 25 | 210 b | [21] |
| 13X | CH4/CO2 60/40 | 0.1 | 30 | 182 a | [22] |
| 13X | CH4/CO2/H2S 59.95/39.95/0.1 | 0.7 | 25 | 298.5 a | [23] |
| 13X | CH4/CO2 35/65 | 0.2 | 25 | 24 a | [14] |
| 13X | CO2 100 | 0.16 | 25 | 223 b | [24] |
| CMS | 13X | A-Slica | |
|---|---|---|---|
| shape | Cylindrical | Spherical | Spherical |
| diameter/mm | 1.7~1.8 | 3~5 | 2~4 |
| Gas | CH4 | CO2 | N2 | O2 | H2S |
|---|---|---|---|---|---|
| Volume fraction/% | 60.4 | 29.1 | 7.35 | 1.6 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, Q.; Qi, P. Upgrading of Biogas to Methane Based on Adsorption. Processes 2020, 8, 941. https://doi.org/10.3390/pr8080941
Liu J, Chen Q, Qi P. Upgrading of Biogas to Methane Based on Adsorption. Processes. 2020; 8(8):941. https://doi.org/10.3390/pr8080941
Chicago/Turabian StyleLiu, Jun, Qiang Chen, and Peng Qi. 2020. "Upgrading of Biogas to Methane Based on Adsorption" Processes 8, no. 8: 941. https://doi.org/10.3390/pr8080941
APA StyleLiu, J., Chen, Q., & Qi, P. (2020). Upgrading of Biogas to Methane Based on Adsorption. Processes, 8(8), 941. https://doi.org/10.3390/pr8080941





