Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review
Abstract
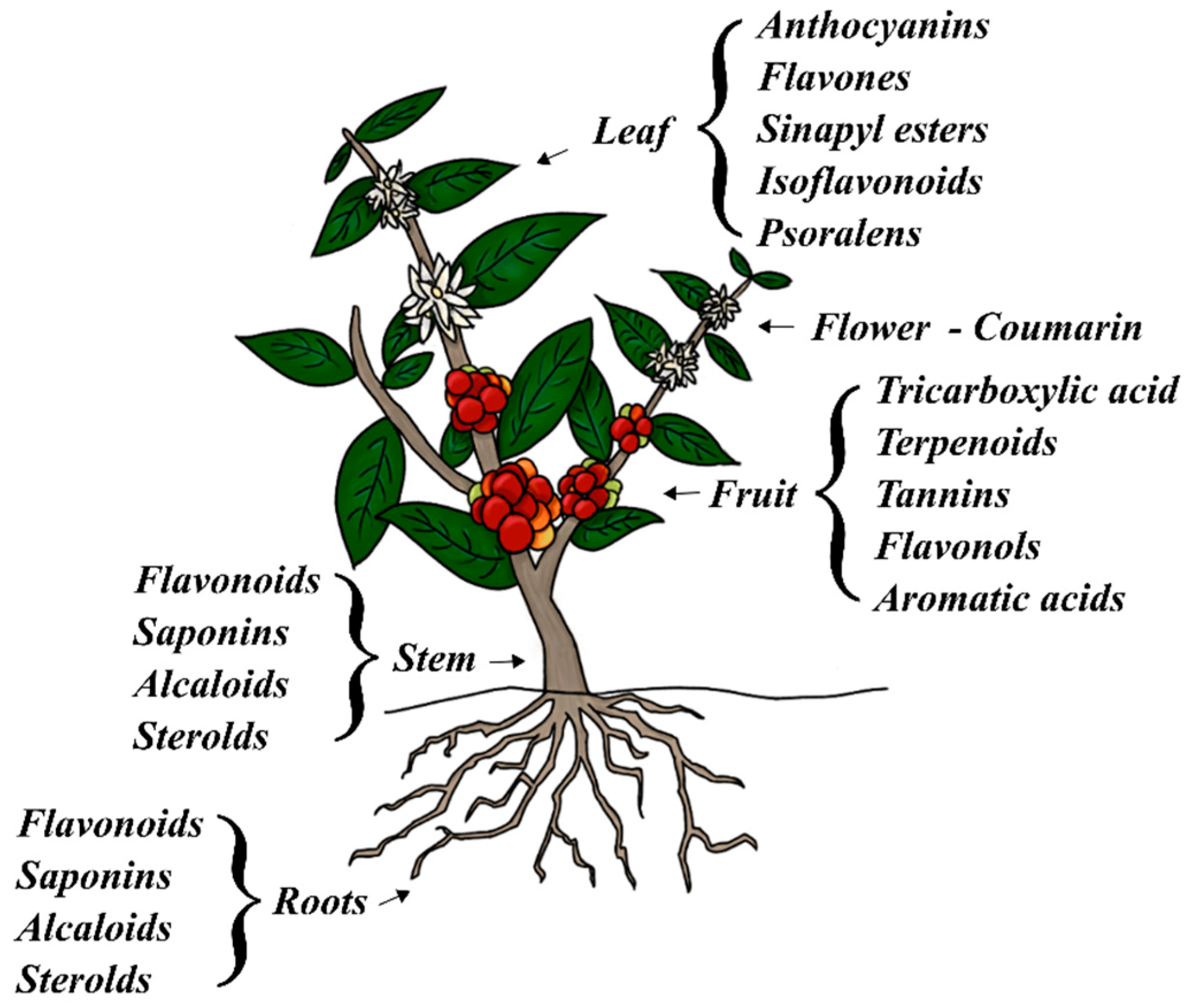
1. Introduction
1.1. Corrosion Inhibition Fundamentals
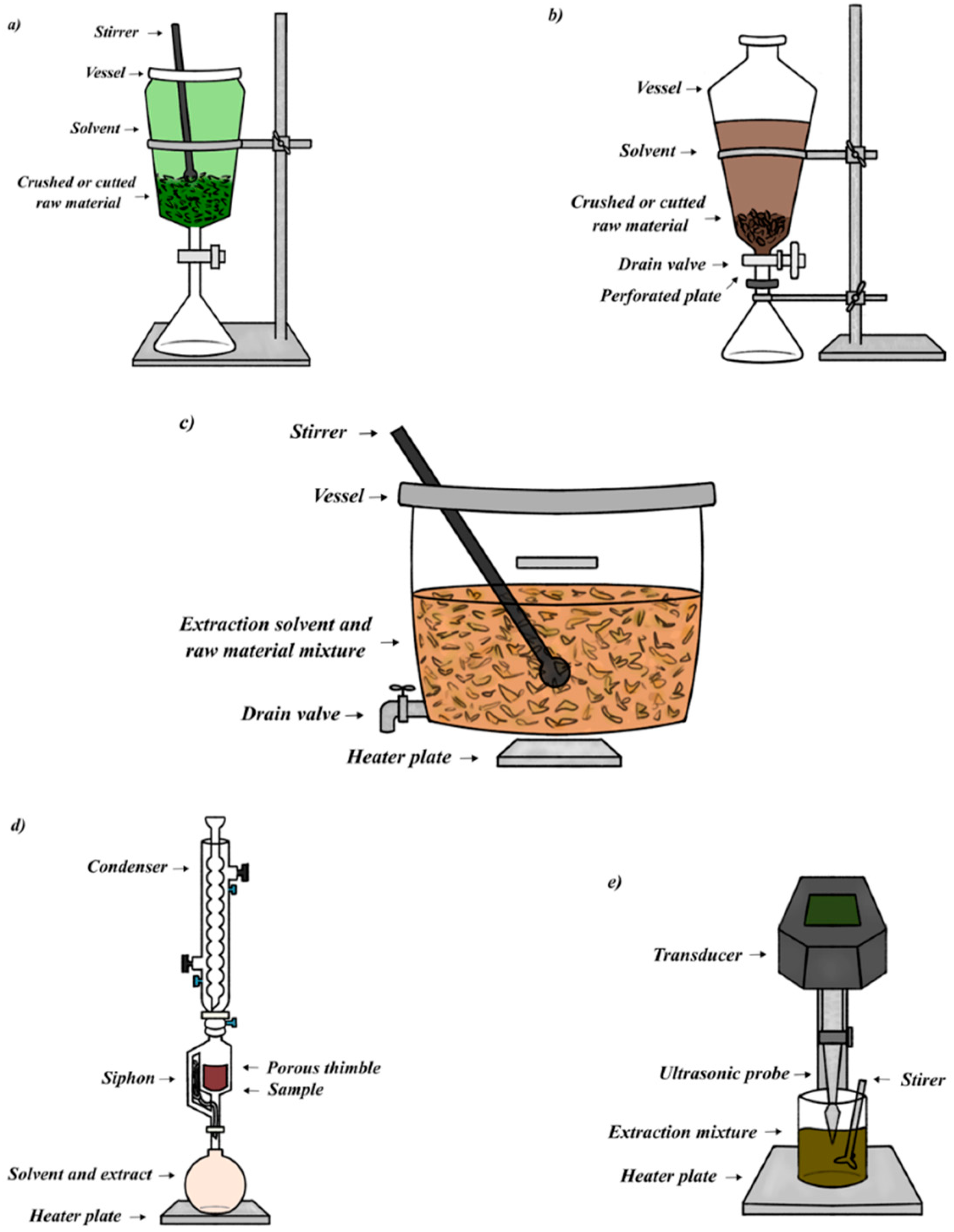
1.2. Extraction Methods
1.3. Characterization Techniques
1.4. Adsorption Mechanism and Quantum Chemistry Methods
2. Plant-Based Corrosion Inhibitors for Mild Steel
2.1. Solvent Effect
2.2. Temperature and Immersion Time Effect
2.3. Adsorption Mechanism and Theoretical Characterization
3. Plant-Based Corrosion Inhibitors in Other Metals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Atomic force microscope | AFM |
| Density functional theory | DFT |
| Dispersion-corrected density functional theory | DFT-D |
| Electrochemical frequency | EFM |
| Electrochemical impedance spectroscopy | EIS |
| Electrostatic potential | ESP |
| Energy-dispersive X-ray spectroscopy | EDS |
| Fourier transform infrared spectroscopy | FT-IR |
| Gas chromatography mass spectrometry | GC–MS |
| Graphene oxide | GO |
| Hydrochloric acid | HCl |
| Highest occupied molecular orbital | HOMO |
| Linear polarization resistance | LPR |
| Lowest unoccupied molecular orbital | LUMO |
| Molecular dynamics | MD |
| Monte Carlo | MC |
| Potentiodynamic polarization | PP |
| Proton-nuclear magnetic resonance | 1H NMR |
| Scanning electron microscope | SEM |
| Self-consistent reaction field | SCRF |
| Tamarindus indiaca extract | Ti.E/TAM |
| Ultraviolet–visible spectroscopy | UV–VIS |
| Weight loss method | WL |
| X-ray photoelectron spectroscopy | XPS |
| Zinc nitrate | ZS |
References
- Parthipan, P.; Elumalai, P.; Narenkumar, J.; Machuca, L.L.; Murugan, K.; Karthikeyan, O.P.; Rajasekar, A. Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int. Biodeterior. Biodegrad. 2018, 132, 66–73. [Google Scholar] [CrossRef]
- Loto, R.T.; Olowoyo, O. Synergistic effect of sage and jojoba oil extracts on the corrosion inhibition of mild steel in dilute acid solution. Procedia Manuf. 2019, 35, 310–314. [Google Scholar] [CrossRef]
- Anupama, K.K.; Ramya, K.; Joseph, A. Electrochemical measurements and theoretical calculations on the inhibitive interaction of Plectranthus amboinicus leaf extract with mild steel in hydrochloric acid. Measurement 2017, 95, 297–305. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Behnamian, Y.; Song, S.; Wang, R.; Gao, Z.; Hu, W.; Xia, D.-H. Metal pitting corrosion characterized by scanning acoustic microscopy and binary image processing. Corros. Sci. 2020, 170, 108685. [Google Scholar] [CrossRef]
- Mai, W.; Soghrati, S.; Buchheit, R.G. A phase field model for simulating the pitting corrosion. Corros. Sci. 2016, 110, 157–166. [Google Scholar] [CrossRef]
- Kıcır, N.; Tansuğ, G.; Erbil, M.; Tüken, T. Investigation of ammonium (2,4-dimethylphenyl)-dithiocarbamate as a new, effective corrosion inhibitor for mild steel. Corros. Sci. 2016, 105, 88–99. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, V.; Quraishi, M.A. Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: Electrochemical, SEM, AFM, and XPS studies. J. Mol. Liq. 2016, 216, 164–173. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Ladan, M.; Basirun, W.J.; Kazi, S.N.; Rahman, F.A. Corrosion protection of AISI 1018 steel using Co-doped TiO2/polypyrrole nanocomposites in 3.5% NaCl solution. Mater. Chem. Phys. 2017, 192, 361–373. [Google Scholar] [CrossRef]
- Nathiya, R.S.; Perumal, S.; Murugesan, V.; Raj, V. Evaluation of extracts of Borassus flabellifer dust as green inhibitors for aluminium corrosion in acidic media. Mater. Sci. Semicond. Process. 2019, 104, 104674. [Google Scholar] [CrossRef]
- Laabaissi, T.; Benhiba, F.; Missioui, M.; Rouifi, Z.; Rbaa, M.; Oudda, H.; Ramli, Y.; Guenbour, A.; Warad, I.; Zarrouk, A. Coupling of chemical, electrochemical and theoretical approach to study the corrosion inhibition of mild steel by new quinoxaline compounds in 1 M HCl. Heliyon 2020, 6, e03939. [Google Scholar] [CrossRef] [PubMed]
- El Aoufir, Y.; Aslam, R.; Lazrak, F.; Marzouki, R.; Kaya, S.; Skal, S.; Ghanimi, A.; Ali, I.H.; Guenbour, A.; Lgaz, H.; et al. The effect of the alkyl chain length on corrosion inhibition performances of 1,2,4-triazole-based compounds for mild steel in 1.0 M HCl: Insights from experimental and theoretical studies. J. Mol. Liq. 2020, 303, 112631. [Google Scholar] [CrossRef]
- Hashim, N.Z.N.; Anouar, E.H.; Kassim, K.; Zaki, H.M.; Alharthi, A.I.; Embong, Z. XPS and DFT investigations of corrosion inhibition of substituted benzylidene Schiff bases on mild steel in hydrochloric acid. Appl. Surf. Sci. 2019, 476, 861–877. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, J.; Liu, Q.; Li, J.; Zhang, R.; Chen, G. Effect of the alkyl chain of quaternary ammonium cationic surfactants on corrosion inhibition in hydrochloric acid solution. Comptes Rendus Chim. 2019, 22, 355–362. [Google Scholar] [CrossRef]
- Shahabi, S.; Hamidi, S.; Ghasemi, J.B.; Norouzi, P.; Shakeri, A. Synthesis, experimental, quantum chemical and molecular dynamics study of carbon steel corrosion inhibition effect of two Schiff bases in HCl solution. J. Mol. Liq. 2019, 285, 626–639. [Google Scholar] [CrossRef]
- Talebian, M.; Raeissi, K.; Atapour, M.; Fernández-Pérez, B.M.; Betancor-Abreu, A.; Llorente, I.; Fajardo, S.; Salarvand, Z.; Meghdadi, S.; Amirnasr, M.; et al. Pitting corrosion inhibition of 304 stainless steel in NaCl solution by three newly synthesized carboxylic Schiff bases. Corros. Sci. 2019, 160, 108130. [Google Scholar] [CrossRef]
- Devikala, S.; Kamaraj, P.; Arthanareeswari, M.; Patel, M.B. Green corrosion inhibition of mild steel by aqueous Allium sativum extract in 3.5% NaCl. Mater. Today Proc. 2019, 14, 580–589. [Google Scholar] [CrossRef]
- Huang, H.; Guo, X. The relationship between the inhibition performances of three benzo derivatives and their structures on the corrosion of copper in 3.5 wt% NaCl solution. Colloids Surf. Physicochem. Eng. Asp. 2020, 598, 124809. [Google Scholar] [CrossRef]
- Othman, N.K.; Yahya, S.; Ismail, M.C. Corrosion inhibition of steel in 3.5% NaCl by rice straw extract. J. Ind. Eng. Chem. 2019, 70, 299–310. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Ebenso, E.E.; Liu, W.; Pan, J.; Huang, B. Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution. J. Ind. Eng. Chem. 2015, 24, 219–228. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Ghandchi, M.S. Santolina chamaecyparissus extract as a natural source inhibitor for 304 stainless steel corrosion in 3.5% NaCl. J. Ind. Eng. Chem. 2015, 31, 231–237. [Google Scholar] [CrossRef]
- Guruprasad, A.M.; Sachin, H.P.; Swetha, G.A.; Prasanna, B.M. Corrosion inhibition of zinc in 0.1 M hydrochloric acid medium with clotrimazole: Experimental, theoretical and quantum studies. Surf. Interfaces 2020, 19, 100478. [Google Scholar] [CrossRef]
- Padash, R.; Sajadi, G.S.; Jafari, A.H.; Jamalizadeh, E.; Rad, A.S. Corrosion control of aluminum in the solutions of NaCl, HCl and NaOH using 2,6-dimethylpyridine inhibitor: Experimental and DFT insights. Mater. Chem. Phys. 2020, 244, 122681. [Google Scholar] [CrossRef]
- Al Hasan, N.H.J.; Alaradi, H.J.; Al Mansor, Z.A.K.; Al Shadood, A.H.J. The dual effect of stem extract of Brahmi (Bacopamonnieri) and Henna as a green corrosion inhibitor for low carbon steel in 0.5 M NaOH solution. Case Stud. Constr. Mater. 2019, 11, e00300. [Google Scholar] [CrossRef]
- Natarajan, S.; Kumaresh Babu, S.P. Corrosion and its inhibition in SA213-T22 TIG weldments used in power plants under neutral and alkaline environments. Mater. Sci. Eng. A 2006, 432, 47–51. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Xie, X.; Du, G. Synergistic inhibition effect of 5-aminotetrazole and 4,6-dihydroxypyrimidine on the corrosion of cold rolled steel in H3PO4 solution. Mater. Chem. Phys. 2016, 181, 33–46. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H.; Xie, X. Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H3PO4 solution. Corros. Sci. 2014, 78, 29–42. [Google Scholar] [CrossRef]
- Ismail, A.S.; Farag, A.A. Experimental, theoretical and simulation studies of extracted crab waste protein as a green polymer inhibitor for carbon steel corrosion in 2 M H3PO4. Surf. Interfaces 2020, 19, 100483. [Google Scholar] [CrossRef]
- Mahalakshmi, D.; Hemapriya, V.; Subramaniam, E.P.; Chitra, S. Synergistic effect of antibiotics on the inhibition property of aminothiazolyl coumarin for corrosion of mild steel in 0.5 M H2SO4. J. Mol. Liq. 2019, 284, 316–327. [Google Scholar] [CrossRef]
- Bagga, M.K.; Gadi, R.; Yadav, O.S.; Kumar, R.; Chopra, R.; Singh, G. Investigation of phytochemical components and corrosion inhibition property of Ficus racemosa stem extract on mild steel in H2SO4 medium. J. Environ. Chem. Eng. 2016, 4, 4699–4707. [Google Scholar] [CrossRef]
- Jiang, S.; Chai, F.; Su, H.; Yang, C. Influence of chromium on the flow-accelerated corrosion behavior of low alloy steels in 3.5% NaCl solution. Corros. Sci. 2017, 123, 217–227. [Google Scholar] [CrossRef]
- Dohare, P.; Chauhan, D.S.; Sorour, A.A.; Quraishi, M.A. DFT and experimental studies on the inhibition potentials of expired Tramadol drug on mild steel corrosion in hydrochloric acid. Mater. Discov. 2017, 9, 30–41. [Google Scholar] [CrossRef]
- El-Haddad, M.N.; Fouda, A.S.; Hassan, A.F. Data from Chemical, electrochemical and quantum chemical studies for interaction between Cephapirin drug as an eco-friendly corrosion inhibitor and carbon steel surface in acidic medium. Chem. Data Collect. 2019, 22, 100251. [Google Scholar] [CrossRef]
- Gholamhosseinzadeh, M.R.; Aghaie, H.; Zandi, M.S.; Giahi, M. Rosuvastatin drug as a green and effective inhibitor for corrosion of mild steel in HCl and H2SO4 solutions. J. Mater. Res. Technol. 2019, 8, 5314–5324. [Google Scholar] [CrossRef]
- Farahati, R.; Mousavi-Khoshdel, S.M.; Ghaffarinejad, A.; Behzadi, H. Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Prog. Org. Coat. 2020, 142, 105567. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J.; Negrón-Silva, G.E.; González-Olvera, R.; Ángeles-Beltrán, D.; Palomar-Pardavé, M.; Miralrio, A.; Castro, M. Fluconazole and fragments as corrosion inhibitors of API 5L X52 steel immersed in 1 M HCl. Corros. Sci. 2020, 174, 108853. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, J.; Cui, G.; Han, T.; Wu, Y. Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids Surf. B Biointerfaces 2020, 194, 111150. [Google Scholar] [CrossRef]
- Machado Fernandes, C.; Pina, V.G.S.S.; Alvarez, L.X.; de Albuquerque, A.C.F.; dos Santos Júnior, F.M.; Barrios, A.M.; Velasco, J.A.C.; Ponzio, E.A. Use of a theoretical prediction method and quantum chemical calculations for the design, synthesis and experimental evaluation of three green corrosion inhibitors for mild steel. Colloids Surf. Physicochem. Eng. Asp. 2020, 599, 124857. [Google Scholar] [CrossRef]
- Munis, A.; Zhao, T.; Zheng, M.; Rehman, A.U.; Wang, F. A newly synthesized green corrosion inhibitor imidazoline derivative for carbon steel in 7.5% NH4Cl solution. Sustain. Chem. Pharm. 2020, 16, 100258. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Olawale, O.; Bello, J.O.; Ogunsemi, B.T.; Uchella, U.C.; Oluyori, A.P.; Oladejo, N.K. Optimization of chicken nail extracts as corrosion inhibitor on mild steel in 2 M H2SO4. Heliyon 2019, 5, e02821. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Kumar, C.B.; Mohana, K.N. Phytochemical screening and corrosion inhibitive behavior of Pterolobium hexapetalum and Celosia argentea plant extracts on mild steel in industrial water medium. Egypt. J. Pet. 2014, 23, 201–211. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Elhajjaji, F.; Taleb, M.; Salim, R.; Boukir, A. Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L): Evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater. Today Proc. 2020, S2214785320328170. [Google Scholar] [CrossRef]
- Sedik, A.; Lerari, D.; Salci, A.; Athmani, S.; Bachari, K.; Gecibesler, İ.H.; Solmaz, R. Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: Electrochemical and surface morphological studies. J. Taiwan Inst. Chem. Eng. 2020, 107, 189–200. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential of Borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel: Electrochemical and theoretical studies. J. Mol. Liq. 2019, 277, 895–911. [Google Scholar] [CrossRef]
- Chaubey, N.; Singh, V.K.; Quraishi, M.A. Papaya peel extract as potential corrosion inhibitor for Aluminium alloy in 1 M HCl: Electrochemical and quantum chemical study. Ain Shams Eng. J. 2018, 9, 1131–1140. [Google Scholar] [CrossRef]
- Jokar, M.; Farahani, T.S.; Ramezanzadeh, B. Electrochemical and surface characterizations of morus alba pendula leaves extract (MAPLE) as a green corrosion inhibitor for steel in 1 M HCl. J. Taiwan Inst. Chem. Eng. 2016, 63, 436–452. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Experimental complemented with microscopic (electronic/atomic)-level modeling explorations of Laurus nobilis extract as green inhibitor for carbon steel in acidic solution. J. Ind. Eng. Chem. 2020, 84, 52–71. [Google Scholar] [CrossRef]
- Handa, S. An overview of extraction techniques for medicinal and aromatic plants. Extr. Technol. Med. Aromat. Plants 2008, 1, 50–52. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4. [Google Scholar] [CrossRef]
- Pham, H.; Nguyen, V.; Vuong, Q.; Bowyer, M.; Scarlett, C. Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves. Technologies 2015, 3, 285–301. [Google Scholar] [CrossRef]
- Neffati, N.; Aloui, Z.; Karoui, H.; Guizani, I.; Boussaid, M.; Zaouali, Y. Phytochemical composition and antioxidant activity of medicinal plants collected from the Tunisian flora. Nat. Prod. Res. 2017, 31, 1583–1588. [Google Scholar] [CrossRef]
- Seal, T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 157–166. [Google Scholar] [CrossRef]
- Sharghi, H.; Khalifeh, R.; Doroodmand, M.M. Copper Nanoparticles on Charcoal for Multicomponent Catalytic Synthesis of 1,2,3-Triazole Derivatives from Benzyl Halides or Alkyl Halides, Terminal Alkynes and Sodium Azide in Water as a “Green” Solvent. Adv. Synth. Catal. 2009, 351, 207–218. [Google Scholar] [CrossRef]
- Varma, R.S. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Nóbrega, E.M.; Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. The Impact of Hot Air Drying on the Physical-Chemical Characteristics, Bioactive Compounds and Antioxidant Activity of Acerola (Malphigia emarginata) Residue: Hot Air Dried Acerola Residue. J. Food Process. Preserv. 2015, 39, 131–141. [Google Scholar] [CrossRef]
- Marchante, L.; Gómez Alonso, S.; Alañón, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, M.C. Natural extracts from fresh and oven-dried winemaking by-products as valuable source of antioxidant compounds. Food Sci. Nutr. 2018, 6, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B 2016, 1011, 223–232. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Mauromicale, G. Leaf extracts of cultivated cardoon as potential bioherbicide. Sci. Hortic. 2020, 261, 109024. [Google Scholar] [CrossRef]
- Mo, S.; Luo, H.-Q.; Li, N.-B. Plant extracts as “green” corrosion inhibitors for steel in sulphuric acid. Chem. Pap. 2016, 70, 1131–1143. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Vaamonde, A.J.V.; de Damborenea, J.J.; González, J.J.D. Ciencia e Ingeniería de la Superficie de los Materiales Metálicos; Editorial CSIC-CSIC Press: Madrid, Spain, 2000; Volume 31, ISBN 84-00-07920-5. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 1-118-52739-9. [Google Scholar]
- Mansfeld, F. Fundamental aspects of the polarization resistance technique—The early days. J. Solid State Electrochem. 2009, 13, 515–520. [Google Scholar] [CrossRef]
- Saxena, A.; Thakur, K.K.; Bhardwaj, N. Electrochemical studies and surface examination of low carbon steel by applying the extract of Musa acuminata. Surf. Interfaces 2020, 18, 100436. [Google Scholar] [CrossRef]
- Vengatesh, G.; Sundaravadivelu, M. Non-toxic bisacodyl as an effective corrosion inhibitor for mild steel in 1 M HCl: Thermodynamic, electrochemical, SEM, EDX, AFM, FT-IR, DFT and molecular dynamics simulation studies. J. Mol. Liq. 2019, 287, 110906. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Armoracia rusticana as sustainable and eco-friendly corrosion inhibitor for mild steel in 0.5 M sulphuric acid: Experimental and theoretical investigations. J. Environ. Chem. Eng. 2018, 6, 5230–5238. [Google Scholar] [CrossRef]
- Finšgar, M. Electrochemical, 3D topography, XPS, and ToF-SIMS analyses of 4-methyl-2-phenylimidazole as a corrosion inhibitor for brass. Corros. Sci. 2020, 169, 108632. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Synergistic inhibition effect of red tetrazolium and uracil on the corrosion of cold rolled steel in H3PO4 solution: Weight loss, electrochemical, and AFM approaches. Mater. Chem. Phys. 2009, 115, 815–824. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. J. Environ. Chem. Eng. 2018, 6, 2290–2301. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Kaya, S. Anti-corrosion investigation of pyrimidine derivatives as green and sustainable corrosion inhibitor for N80 steel in highly corrosive environment: Experimental and AFM/XPS study. Sustain. Chem. Pharm. 2020, 16, 100257. [Google Scholar] [CrossRef]
- Bouanis, M.; Tourabi, M.; Nyassi, A.; Zarrouk, A.; Jama, C.; Bentiss, F. Corrosion inhibition performance of 2,5-bis(4-dimethylaminophenyl)-1,3,4-oxadiazole for carbon steel in HCl solution: Gravimetric, electrochemical and XPS studies. Appl. Surf. Sci. 2016, 389, 952–966. [Google Scholar] [CrossRef]
- Corrales Luna, M.; Le Manh, T.; Cabrera Sierra, R.; Medina Flores, J.V.; Lartundo Rojas, L.; Arce Estrada, E.M. Study of corrosion behavior of API 5L X52 steel in sulfuric acid in the presence of ionic liquid 1-ethyl 3-methylimidazolium thiocyanate as corrosion inhibitor. J. Mol. Liq. 2019, 289, 111106. [Google Scholar] [CrossRef]
- Zarrok, H.; Zarrouk, A.; Hammouti, B.; Salghi, R.; Jama, C.; Bentiss, F. Corrosion control of carbon steel in phosphoric acid by purpald–Weight loss, electrochemical and XPS studies. Corros. Sci. 2012, 64, 243–252. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Organic Corrosion Inhibitors. In Corrosion Inhibitors, Principles and Recent Applications; Aliofkhazraei, M., Ed.; InTech: London, UK, 2018; ISBN 978-953-51-3917-1. [Google Scholar]
- Richardson, J.A. Management of Corrosion in the Petrochemical and Chemical Industries. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3207–3229. ISBN 978-0-444-52787-5. [Google Scholar]
- Papavinasam, S. Corrosion Inhibitors. In Uhlig’s Corrosion Handbook; Revie, R.W., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 1021–1032. ISBN 978-0-470-87286-4. [Google Scholar]
- Pradipta, I.; Kong, D.; Tan, J.B.L. Natural organic antioxidants from green tea inhibit corrosion of steel reinforcing bars embedded in mortar. Constr. Build. Mater. 2019, 227, 117058. [Google Scholar] [CrossRef]
- Loto, R.T.; Loto, C.A. Data on the comparative evaluation of the corrosion inhibition of vanillin and vanillin admixed with rosmarinus officinalis on mild steel in dilute acid media. Chem. Data Collect. 2019, 24, 100290. [Google Scholar] [CrossRef]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Neske, A.; Brandán, S.A.; Gervasi, C.A. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 2018, 58, 92–99. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.-J.; Chen, L.; Zhang, S.; Ma, Y.; Ye, C.; Zhou, Y.; Pang, B.; Wu, Y.-C. Fructan from Polygonatum cyrtonema Hua as an eco-friendly corrosion inhibitor for mild steel in HCl media. Carbohydr. Polym. 2020, 238, 116216. [Google Scholar] [CrossRef]
- Hassan, K.H.; Khadom, A.A.; Kurshed, N.H. Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S. Afr. J. Chem. Eng. 2016, 22, 1–5. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Zhu, B.; Huang, C.; Li, W. Magnolia grandiflora leaves extract as a novel environmentally friendly inhibitor for Q235 steel corrosion in 1 M HCl: Combining experimental and theoretical researches. J. Mol. Liq. 2020, 113312. [Google Scholar] [CrossRef]
- Gao, L.; Peng, S.; Huang, X.; Gong, Z. A combined experimental and theoretical study of papain as a biological eco-friendly inhibitor for copper corrosion in H2SO4 medium. Appl. Surf. Sci. 2020, 511, 145446. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Onukwuli, O.D.; Omotioma, M.; Okafor, N.A. Experimental, theoretical modeling and optimization of inhibition efficiency of pigeon pea leaf extract as anti-corrosion agent of mild steel in acid environment. Mater. Chem. Phys. 2019, 233, 120–132. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Study of the synergistic effect of Mangifera indica leaves extract and zinc ions on the mild steel corrosion inhibition in simulated seawater: Computational and electrochemical studies. J. Mol. Liq. 2019, 292, 111387. [Google Scholar] [CrossRef]
- Pal, S.; Lgaz, H.; Tiwari, P.; Chung, I.-M.; Ji, G.; Prakash, R. Experimental and theoretical investigation of aqueous and methanolic extracts of Prunus dulcis peels as green corrosion inhibitors of mild steel in aggressive chloride media. J. Mol. Liq. 2019, 276, 347–361. [Google Scholar] [CrossRef]
- Saha, S.K.; Murmu, M.; Murmu, N.C.; Banerjee, P. Evaluating electronic structure of quinazolinone and pyrimidinone molecules for its corrosion inhibition effectiveness on target specific mild steel in the acidic medium: A combined DFT and MD simulation study. J. Mol. Liq. 2016, 224, 629–638. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Unravelling the mechanisms of corrosion inhibition of iron by henna extract: A density functional theory study. Corros. Sci. 2018, 142, 102–109. [Google Scholar] [CrossRef]
- Srivastava, V.; Haque, J.; Verma, C.; Singh, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Amino acid based imidazolium zwitterions as novel and green corrosion inhibitors for mild steel: Experimental, DFT and MD studies. J. Mol. Liq. 2017, 244, 340–352. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.J.; Valdelamar, M.P.; Vazquez, A.E.; Del Valle Perez, P.; Mata, R.; Miralrio, A.; Castro, M. Mycophenolic acid as a corrosion inhibitor of carbon steel in 3% wt. NaCl solution. An experimental and theoretical study. J. Mol. Struct. 2019, 1183, 168–181. [Google Scholar] [CrossRef]
- Arshadi, M.R.; Lashgari, M.; Parsafar, G.A. Cluster approach to corrosion inhibition problems: Interaction studies. Mater. Chem. Phys. 2004, 86, 311–314. [Google Scholar] [CrossRef]
- Garcia-Ochoa, E.; Guzmán-Jiménez, S.J.; Hernández, J.G.; Pandiyan, T.; Vásquez-Pérez, J.M.; Cruz-Borbolla, J. Benzimidazole ligands in the corrosion inhibition for carbon steel in acid medium: DFT study of its interaction on Fe30 surface. J. Mol. Struct. 2016, 1119, 314–324. [Google Scholar] [CrossRef]
- Cruz-Borbolla, J.; Garcia-Ochoa, E.; Narayanan, J.; Maldonado-Rivas, P.; Pandiyan, T.; Vásquez-Pérez, J.M. Electrochemical and theoretical studies of the interactions of a pyridyl-based corrosion inhibitor with iron clusters (Fe15, Fe30, Fe45, and Fe60). J. Mol. Model. 2017, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Mohammadalizadeh, M.; Khosravan, A. Theoretical investigation of corrosion inhibition effect of imidazole and its derivatives on mild steel using cluster model. Corros. Sci. 2011, 53, 3086–3091. [Google Scholar] [CrossRef]
- Mousavi, M.; Safarizadeh, H.; Khosravan, A. A new cluster model based descriptor for structure-inhibition relationships: A study of the effects of benzimidazole, aniline and their derivatives on iron corrosion. Corros. Sci. 2012, 65, 249–258. [Google Scholar] [CrossRef]
- Jamalizadeh, E.; Hosseini, S.M.A.; Jafari, A.H. Quantum chemical studies on corrosion inhibition of some lactones on mild steel in acid media. Corros. Sci. 2009, 51, 1428–1435. [Google Scholar] [CrossRef]
- Mousavi, M.; Baghgoli, T. Application of interaction energy in quantitative structure-inhibition relationship study of some benzenethiol derivatives on copper corrosion. Corros. Sci. 2016, 105, 170–176. [Google Scholar] [CrossRef]
- Aldana-González, J.; Espinoza-Vázquez, A.; Romero-Romo, M.; Uruchurtu-Chavarin, J.; Palomar-Pardavé, M. Electrochemical evaluation of cephalothin as corrosion inhibitor for API 5L X52 steel immersed in an acid medium. Arab. J. Chem. 2019, 12, 3244–3253. [Google Scholar] [CrossRef]
- Saxena, A.; Sharma, V.; Thakur, K.K.; Bhardwaj, N. Electrochemical Studies and the Surface Examination of Low Carbon Steel by Applying the Extract of Citrus sinensis. J. Bio- Tribo-Corros. 2020, 6, 41. [Google Scholar] [CrossRef]
- Abod, B.M.; Al-Alawy, R.M.; Khadom, A.A.; Kamar, F.H. Experimental and Theoretical Studies for Tobacco Leaf Extract as an Eco-friendly Inhibitor for Steel in Saline Water. J. Bio- Tribo-Corros. 2019, 5, 75. [Google Scholar] [CrossRef]
- Abdulazeez, I.; Zeino, A.; Kee, C.W.; Al-Saadi, A.A.; Khaled, M.; Wong, M.W.; Al-Sunaidi, A.A. Mechanistic studies of the influence of halogen substituents on the corrosion inhibitive efficiency of selected imidazole molecules: A synergistic computational and experimental approach. Appl. Surf. Sci. 2019, 471, 494–505. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thienylmethyl)-4-amino-1,2,4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Walczak, M.S.; Morales-Gil, P.; Lindsay, R. Determining Gibbs energies of adsorption from corrosion inhibition efficiencies: Is it a reliable approach? Corros. Sci. 2019, 155, 182–185. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A. Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: Kinetics and mathematical studies. S. Afr. J. Chem. Eng. 2018, 25, 13–21. [Google Scholar] [CrossRef]
- Hussin, M.H.; Jain Kassim, M.; Razali, N.N.; Dahon, N.H.; Nasshorudin, D. The effect of Tinospora crispa extracts as a natural mild steel corrosion inhibitor in 1 M HCl solution. Arab. J. Chem. 2016, 9, S616–S624. [Google Scholar] [CrossRef]
- Li, D.; Zhang, P.; Guo, X.; Zhao, X.; Xu, Y. The inhibition of mild steel corrosion in 0.5 M H2SO4 solution by radish leaf extract. RSC Adv. 2019, 9, 40997–41009. [Google Scholar] [CrossRef]
- Ishak, A.; Adams, F.V.; Madu, J.O.; Joseph, I.V.; Olubambi, P.A. Corrosion Inhibition of Mild Steel in 1 M Hydrochloric Acid using Haematostaphis barteri Leaves Extract. Procedia Manuf. 2019, 35, 1279–1285. [Google Scholar] [CrossRef]
- Benarioua, M.; Mihi, A.; Bouzeghaia, N.; Naoun, M. Mild steel corrosion inhibition by Parsley (Petroselium Sativum) extract in acidic media. Egypt. J. Pet. 2019, 28, 155–159. [Google Scholar] [CrossRef]
- Afia, L.; Salghi, R.; Bammou, L.; Bazzi, E.L.; Hammouti, B.; Bazzi, L.; Bouyanzer, A. Anti-corrosive properties of Argan oil on C38 steel in molar HCl solution. J. Saudi Chem. Soc. 2014, 18, 19–25. [Google Scholar] [CrossRef]
- Saeed, M.T.; Saleem, M.; Usmani, S.; Malik, I.A.; Al-Shammari, F.A.; Deen, K.M. Corrosion inhibition of mild steel in 1 M HCl by sweet melon peel extract. J. King Saud Univ. Sci. 2019, 31, 1344–1351. [Google Scholar] [CrossRef]
- Raghavendra, N.; Ishwara Bhat, J. Inhibition of Al corrosion in 0.5 M HCl solution by Areca flower extract. J. King Saud Univ. Eng. Sci. 2019, 31, 202–208. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M.; Solomon, M.M.; Udoh, A.P. Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 2016, 9, S209–S224. [Google Scholar] [CrossRef]
- Bammou, L.; Belkhaouda, M.; Salghi, R.; Benali, O.; Zarrouk, A.; Zarrok, H.; Hammouti, B. Corrosion inhibition of steel in sulfuric acidic solution by the Chenopodium ambrosioides Extracts. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 16, 83–90. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Olusegun, S.J.; Adelowo, O.T. Corrosion inhibition and adsorption mechanism studies of Hunteria umbellata seed husk extracts on mild steel immersed in acidic solutions. Alex. Eng. J. 2016, 55, 673–681. [Google Scholar] [CrossRef]
- Nwabanne, J.T.; Okafor, V.N. Adsorption and thermodynamics study of the inhibition of corrosion of mild steel in H2SO4 medium using Vernonia amygdalina. J. Miner. Mater. Charact. Eng. 2012, 11, 885. [Google Scholar]
- Dagdag, O.; Safi, Z.; Hsissou, R.; Erramli, H.; El Bouchti, M.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.E.; El Harfi, A. Epoxy pre-polymers as new and effective materials for corrosion inhibition of carbon steel in acidic medium: Computational and experimental studies. Sci. Rep. 2019, 9, 11715. [Google Scholar] [CrossRef]
- Alinnor, I. Corrosion Inhibition of Aluminium in Acidic Medium by Different Extracts of Ocimum gratissimum. Am. Chem. Sci. J. 2012, 2, 122–135. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Olusegun, S.J. Corrosion inhibition performance of lignin extract of sun flower (Tithonia diversifolia) on medium carbon low alloy steel immersed in H2SO4 solution. Leonardo J. Sci. 2012, 20, 59–70. [Google Scholar]
- Ebenso, E.E.; Alemu, H.; Umoren, S.A.; Obot, I.B. Inhibition of mild steel corrosion in sulphuric acid using alizarin yellow GG dye and synergistic iodide additive. Int. J. Electrochem. Sci. 2008, 3, 1325–1339. [Google Scholar]
- Chauhan, L.R.; Gunasekaran, G. Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros. Sci. 2007, 49, 1143–1161. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Green Eucalyptus leaf extract: A potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry 2019, 130, 107339. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, E.; Ramezanzadeh, M.; Haddadi, S.A.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M. Persian Liquorice extract as a highly efficient sustainable corrosion inhibitor for mild steel in sodium chloride solution. J. Clean. Prod. 2019, 210, 660–672. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D. Corrosion Resistance and Surface Protective Performance of Waste Material of Eucalyptus globulus for Low Carbon Steel. J. Bio- Tribo-Corros. 2020, 6, 48. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, B.; Bao, H.; Xie, Y.; Mou, Y.; Li, P.; Chen, D.; Shi, Y.; Li, X.; Yang, W. Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 2019, 128, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Prasad, D.; Haldhar, R.; Singh, G.; Kumar, A. Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Mol. Liq. 2018, 258, 89–97. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Ramezanzadeh, M.; Ramezanzadeh, B.; Bahlakeh, G. A green assisted route for the fabrication of a high-efficiency self-healing anti-corrosion coating through graphene oxide nanoplatform reduction by Tamarindus indiaca extract. J. Hazard. Mater. 2020, 390, 122147. [Google Scholar] [CrossRef]
- Deyab, M.A.; Guibal, E. Enhancement of corrosion resistance of the cooling systems in desalination plants by green inhibitor. Sci. Rep. 2020, 10, 4812. [Google Scholar] [CrossRef]
- Ben Harb, M.; Abubshait, S.; Etteyeb, N.; Kamoun, M.; Dhouib, A. Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arab. J. Chem. 2020, 13, 4846–4856. [Google Scholar] [CrossRef]
- Faiz, M.; Zahari, A.; Awang, K.; Hussin, H. Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv. 2020, 10, 6547–6562. [Google Scholar] [CrossRef]
- Mobin, M.; Basik, M.; Aslam, J. Pineapple stem extract (Bromelain) as an environmental friendly novel corrosion inhibitor for low carbon steel in 1 M HCl. Measurement 2019, 134, 595–605. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández-Domene, R.M.; Tabyaoui, M.; Bellaouchou, A.; Guenbour, A.; García-Antón, J. Green approach to corrosion inhibition of stainless steel in phosphoric acid of Artemesia herba albamedium using plant extract. J. Mater. Res. Technol. 2019, 8, 5763–5773. [Google Scholar] [CrossRef]
- Emori, W.; Zhang, R.-H.; Okafor, P.C.; Zheng, X.-W.; He, T.; Wei, K.; Lin, X.-Z.; Cheng, C.-R. Adsorption and corrosion inhibition performance of multi-phytoconstituents from Dioscorea septemloba on carbon steel in acidic media: Characterization, experimental and theoretical studies. Colloids Surf. Physicochem. Eng. Asp. 2020, 590, 124534. [Google Scholar] [CrossRef]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]
- Anyiam, C.K.; Ogbobe, O.; Oguzie, E.E.; Madufor, I.C.; Nwanonenyi, S.C.; Onuegbu, G.C.; Obasi, H.C.; Chidiebere, M.A. Corrosion inhibition of galvanized steel in hydrochloric acid medium by a physically modified starch. SN Appl. Sci. 2020, 2, 520. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Alibakhshi, E.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M. A detailed atomic level computational and electrochemical exploration of the Juglans regia green fruit shell extract as a sustainable and highly efficient green corrosion inhibitor for mild steel in 3.5 wt% NaCl solution. J. Mol. Liq. 2019, 284, 682–699. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Arinkoola, A.O.; Eletta, O.A.; Agbede, O.O.; Osho, Y.A.; Morakinyo, A.F.; Hamed, J.O. Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 2020, 6, e03205. [Google Scholar] [CrossRef]
- Wang, H.; Gao, M.; Guo, Y.; Yang, Y.; Hu, R. A natural extract of tobacco rob as scale and corrosion inhibitor in artificial seawater. Desalination 2016, 398, 198–207. [Google Scholar] [CrossRef]
- Buyuksagis, A.; Dİlek, M. The Use of Papaver somniferum L. Plant Extract as Corrosion Inhibitor. Prot. Met. Phys. Chem. Surf. 2019, 55, 1182–1194. [Google Scholar] [CrossRef]
- Ahanotu, C.C.; Onyeachu, I.B.; Solomon, M.M.; Chikwe, I.S.; Chikwe, O.B.; Eziukwu, C.A. Pterocarpus santalinoides leaves extract as a sustainable and potent inhibitor for low carbon steel in a simulated pickling medium. Sustain. Chem. Pharm. 2020, 15, 100196. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Molecular/electronic/atomic-level simulation and experimental exploration of the corrosion inhibiting molecules attraction at the steel/chloride-containing solution interface. J. Mol. Liq. 2019, 296, 111809. [Google Scholar] [CrossRef]
- Divya, P.; Subhashini, S.; Prithiba, A.; Rajalakshmi, R. Tithonia diversifolia flower Extract as green Corrosion Inhibitor for Mild Steel in Acid Medium. Mater. Today Proc. 2019, 18, 1581–1591. [Google Scholar] [CrossRef]
- Gadow, H.S.; Motawea, M.M. Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv. 2017, 7, 24576–24588. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: Detailed macro/micro-scale experimental and computational explorations. Constr. Build. Mater. 2020, 245, 118464. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Dehghani, A.; Ramezanzadeh, B.; Ramezanzadeh, M. Highly effective mild steel corrosion inhibition in 1 M HCl solution by novel green aqueous Mustard seed extract: Experimental, electronic-scale DFT and atomic-scale MC/MD explorations. J. Mol. Liq. 2019, 293, 111559. [Google Scholar] [CrossRef]
- Anupama, K.K.; Ramya, K.; Shainy, K.M.; Joseph, A. Adsorption and electrochemical studies of Pimenta dioica leaf extracts as corrosion inhibitor for mild steel in hydrochloric acid. Mater. Chem. Phys. 2015, 167, 28–41. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Enenebeaku, C.K.; Akalezi, C.O.; Okoro, S.C.; Ayuk, A.A.; Ejike, E.N. Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. J. Colloid Interface Sci. 2010, 349, 283–292. [Google Scholar] [CrossRef]
- Mourya, P.; Banerjee, S.; Singh, M.M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014, 85, 352–363. [Google Scholar] [CrossRef]
- Bouknana, D.; Hammouti, B.; Messali, M.; Aouniti, A.; Sbaa, M. Olive pomace extract (OPE) as corrosion inhibitor for steel in HCl medium. Asian Pac. J. Trop. Dis. 2014, 4, S963–S974. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Jeyaprabha, B.; Prakash, P. Adsorption and corrosion inhibiting behavior of Lannea coromandelica leaf extract on mild steel corrosion. Arab. J. Chem. 2017, 10, S2343–S2354. [Google Scholar] [CrossRef]
- Hamdy, A.; El-Gendy, N.S. Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt. J. Pet. 2013, 22, 17–25. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 2019, 282, 366–384. [Google Scholar] [CrossRef]
- Gerengi, H.; Uygur, I.; Solomon, M.; Yildiz, M.; Goksu, H. Evaluation of the inhibitive effect of Diospyros kaki (Persimmon) leaves extract on St37 steel corrosion in acid medium. Sustain. Chem. Pharm. 2016, 4, 57–66. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Prakash, P.; Jeyaprabha, B.; Shankar, K. Stigmasterol extracted from Ficus hispida leaves as a green inhibitor for the mild steel corrosion in 1 M HCl solution. Arab. J. Chem. 2019, 12, 3345–3356. [Google Scholar] [CrossRef]
- Kumar, K.P.V.; Pillai, M.S.N.; Thusnavis, G.R. Seed Extract of Psidium guajava as Ecofriendly Corrosion Inhibitor for Carbon Steel in Hydrochloric Acid Medium. J. Mater. Sci. Technol. 2011, 27, 1143–1149. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, G.; Hu, S.; Yan, Y.; Ren, Z.; Yu, L. Theoretical evaluation of corrosion inhibition performance of imidazoline compounds with different hydrophilic groups. Corros. Sci. 2011, 53, 147–152. [Google Scholar] [CrossRef]
- Khaled, K.F. Studies of iron corrosion inhibition using chemical, electrochemical and computer simulation techniques. Electrochim. Acta 2010, 55, 6523–6532. [Google Scholar] [CrossRef]
- Musa, A.Y.; Jalgham, R.T.T.; Mohamad, A.B. Molecular dynamic and quantum chemical calculations for phthalazine derivatives as corrosion inhibitors of mild steel in 1 M HCl. Corros. Sci. 2012, 56, 176–183. [Google Scholar] [CrossRef]
- Al-Nami, S. Corrosion Inhibition Effect and Adsorption Activities of methanolic myrrh extract for Cu in 2 M HNO3. Int. J. Electrochem. Sci. 2020, 1187–1205. [Google Scholar] [CrossRef]
- Esquivel López, A.; Cuevas-Arteaga, C.; Valladares-Cisneros, M.G. Universidad Autónoma del Estado de Morelos Study of the corrosion inhibition of copper in synthetic seawater by Equisetum arvense as green corrosion inhibitor. Rev. Mex. Ing. Quím. 2019, 19, 603–616. [Google Scholar] [CrossRef]
- Chung, I.-M.; Malathy, R.; Kim, S.-H.; Kalaiselvi, K.; Prabakaran, M.; Gopiraman, M. Ecofriendly green inhibitor from Hemerocallis fulva against aluminum corrosion in sulphuric acid medium. J. Adhes. Sci. Technol. 2020, 1–24. [Google Scholar] [CrossRef]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice; John Wiley and Sons: Hoboken, NJ, USA, 2011; ISBN 1-118-01417-0. [Google Scholar]
- Pedraza Basulto, G.K.; Carrillo, I.; Ortega, D.; Martinez, L.; Canto, J. Evaluation at Pipeline Corrosion at Oil Field. ECS Trans. 2015, 64, 103–110. [Google Scholar] [CrossRef]
| Technique | Equation |
|---|---|
| EIS [104] | IE (%) = (1 − ((Rp)blank/(Rp)inhibitor) * 100 |
| PP [105] | |
| WL [106] | IE (%) = ((W0 − W1)/(W0)) * 100 |
| Model | Adsorption Isotherm | Reference |
|---|---|---|
| langmuir | [121,122] | |
| temkin | [121,123,124] | |
| freundlish | [124,125] | |
| flory–huggins | [125] | |
| frumkin | [121] | |
| el-awady | [121,126] |
| Plant | Extraction Solvent | Metal | Corrosive Medium | Temperature (K) | C | Characterization | ηmax (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| ananas comosus | Water | Low-carbon steel | 1 M HCl | 308–338 | 1000 ppm | WL, EIS, PP, UV–VIS, SEM | 97.6 | [136] |
| artemisia herba-alba | Water | Stainless steel | 1 M H3PO4 | 298–353 | 1 g/L | EIS, SEM-EDS, GC–MS | 88 | [137] |
| bacopa monnieri/lawsonia inermis | Water | Low-carbon steel | 0.5 M NaOH | - | 0.4–0.8 g/L | WL, PP | 80 | [25] |
| camellia sinensis | Water | Carbon steel | 3.5% NaCl | - | 0.5–2% | LPR, LC–MS | 80 | [82] |
| cryptocarya nigra | Hexane, dichloromethane, methanol | Mild steel | 1 M HCl | - | 10–1000 mg/L | PP, EIS, SEM-EDS, FT-IR, UV–VIS | 91 | [135] |
| dioscorea septemloba | Ethanol | Carbon steel | 1 M HCl | 303.15 | 0.1–2.0 g/L | NMR, EIS, FT-IR, SEM | 72 | [138] |
| eucalyptus | Water | Mild steel | 1 M HCl | - | 800 ppm | PP, WL, EIS, FT-IR, UV–VIS, SEM, AFM | 88 | [127] |
| eucalyptus globulus | Water | Low-carbon steel | 0.5 M H2SO4 | - | 100–600 mg/L | WL, EIS, AFM, FT-IR, SEM, EFM | 93.09 | [129] |
| euphorbia heterophylla llinneo | Water | Mild steel | 1.5 M HCl | - | 1 g/L | PP | 69 | [139] |
| ficus tikoua | Water | Carbon steel | 1 M HCl | - | 200 mg/L | FT-IR, EIS, PP, SEM | 95.8 | [130] |
| glycyrrhiza glabra | Water | Mild steel | 1 M HCl | - | 200–600 ppm | EIS, PP, FT-IR, AFM | 88 | [128] |
| ipomoea batatas | N-hexane | Galvanized steel | 1 M HCl | 308.15–338.15 | 0.7 g/L | FT-IR, EIS | 64.26 | [140] |
| juglans regia | Water | Mild steel | 3.5 wt % NaCl | - | 1000 ppm | PP, EIS, FR-IR, SEM | 94.2 | [141] |
| luffa cylindrica | Ethanol | Mild steel | 1 M HCl | 303–333 | 1 g/L | WL, PP, EIS, FT-IR | 87.98 | [142] |
| nicotiana tabacum | Water | Q235 steel | 0.1 M NaOH | - | 34 mg/L | PP, FT-IR, XPS, SEM, XRD | 83.9 | [143] |
| olea europaea | Methanol, ethyl acetate, dichloromethane, hexane | Mild steel | 0.1 M NaOH + 0.5 M NaCl | - | 200–800 mg/L | EIS, GC–MS | 91.9 | [134] |
| papaver somniferum | Ethanol | AISI 304 stainless steel | 0.2 M HCl | 298.15–318.15 | 100–500 ppm | AFM, SEM-EDS | 88 | [144] |
| pterocarpus santalinoides | Water, ethanol, and methanol | Low carbon steel | 1 mol/dm3 HCl | 298.15–333.15 | 0.7 g/L | EIS, PP, LPR, SEM-EDAX, AFM | 90 | [145] |
| rosa canina | Water | Mild steel | 1 M HCl | - | 200–800 ppm | EIS, UV–VIS, PP, SEM | 86 | [146] |
| saraca ashoka | Water | Mild steel | 0.5 M H2SO4 | - | 25–100 mg/L | WL, EIS, PP, SEM, UV–VIS, FT-IR, AFM | 93.09 | [131] |
| tamarindus indiaca | Water | Mild steel | 3.5% NaCl | - | 300–1000 ppm | FE-SEM, AFM, EIS, FT-IR, GI-XRD | 96 | [147] |
| tamarindus indiaca | Water | Mild steel | 3.5% NaCl | - | 1000 ppm | FT-IR, UV–VIS, XRD, TGA, Raman, EIS, FE-SEM | - | [132] |
| taraxacum officinale | Water/ethanol | Carbon steel | Seawater | 295.15–328.15 | 100–400 mg/L | WL, PP, EIS, SEM, FT-IR, UV–VIS | 94.3 | [133] |
| tinospora crispa | Water, acetone/water | Mild steel | 1 M HCl | - | 800–1000 ppm | WL, EIS, SEM | 80 | [111] |
| tithonia diversifolia | Water | Mild steel | 1 M HCl | - | 0.1–0.7% | WL, EIS, PP, FT-IR | 94.55 | [148] |
| zingiber officinale | Methanol | Mild steel | 1 M HCl | 298.15–328.15 | 100 ppm | PP, EIS, FR-IR, UV–VIS, AFM | 92.5 | [149] |
| ziziphora | Water | Mild steel | 1 M HCl | - | 200–800 ppm | FT-IR, UV-VIS, EIS | 93 | [150] |
| Plant | Extract Constituents | Theory | Reference |
|---|---|---|---|
| Dioscorea septemloba | Dioscin, β-sitosterol, dioscorone A, and palmitic acid | DFT, MD | [138] |
| Eucalyptus | Macrocarpal E, macrocarpal A, eucalyptome, and ellagic acid | DFT, MC, MD | [127] |
| Eucalyptus globulus | Eucalyptol, globulusin-A, and globulusin-B | DFT | [129] |
| Ficus tikoua | Allantoin, 5-methoxypsoralen, methyl caffeate, and methyl 4-hydroxycinnamate | DFT | [130] |
| Juglans regia | Coumaric acid, ferulic acid, syringic acid, vanillic acid, juglone, and myricetin | DFT, MC, MD | [141] |
| Glycyrrhiza glabra | Licochalcone A, licochalcone E, liquiritigenin, 18β-glycyrrhetinic acid, glycyrrhizin, and glabridin | DFT, MC, MD | [128] |
| Rosa canina | Ascorbic acid, marein, pectin, and tannin | DFT, MC, MD | [146] |
| Saraca ashoka | Epicatechin | DFT | [131] |
| Tamarindus indiaca | Apigenin, naringenin, eriodyctoyl, and taxifolin | DFT, MC, MD | [147] |
| Tamarindus indiaca | Naringenin, apigenin, eriodictyol, and taxifolin | DFT-D | [132] |
| Ziziphora | Acacetin, chrysin, and thymonin | DFT, MC, MD | [150] |
| Plant | Extraction Solvent | Metal | Corrosion Medium | ηmax (%) | Temp (K) | C | Characterization | Reference |
|---|---|---|---|---|---|---|---|---|
| Borassus flabellifer | Water, methanol | Al | 1 M H2SO4 | 66.8 | 303–333 | 0.1–0.4 g/L | EIS, SEM | [11] |
| Commiphora myrrha | Methanol | Cu | 2 M HNO3 | 91 | 298.15–318.15 | 50–300 ppm | EIS, AFM, FT-IR, SEM, PP, WL | [167] |
| Equisetum arvense | Methanol | Cu | Seawater | 87.5 | 300 | 250–1000 ppm | EIS, FT-IR, PP, GC–MS | [168] |
| Hemerocallis fulva | Methanol | Al | 1 M H2SO4 | 89 | 303–333 | 200–600 ppm | WL, PP, SEM-EDS, AFM | [169] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralrio, A.; Espinoza Vázquez, A. Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes 2020, 8, 942. https://doi.org/10.3390/pr8080942
Miralrio A, Espinoza Vázquez A. Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes. 2020; 8(8):942. https://doi.org/10.3390/pr8080942
Chicago/Turabian StyleMiralrio, Alan, and Araceli Espinoza Vázquez. 2020. "Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review" Processes 8, no. 8: 942. https://doi.org/10.3390/pr8080942
APA StyleMiralrio, A., & Espinoza Vázquez, A. (2020). Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes, 8(8), 942. https://doi.org/10.3390/pr8080942






