The Attractiveness of the Ternary Rh-Pd-Pt Alloys for CO Oxidation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Precursors
2.2. Synthesis of Catalysts
2.3. Characterization Techniques
2.4. Testing the Catalytic Activity in the Oxidation of CO
3. Results and Discussion
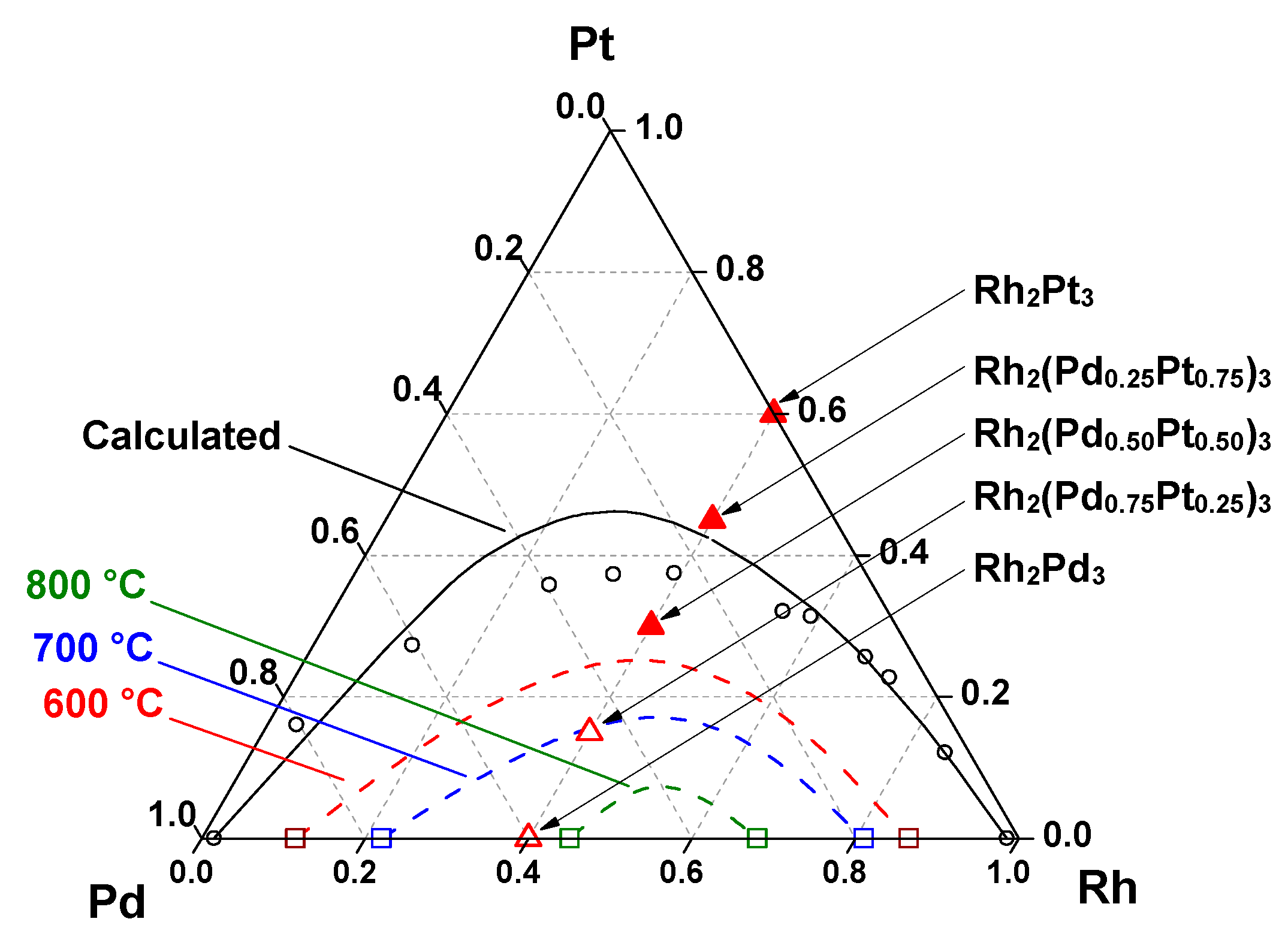
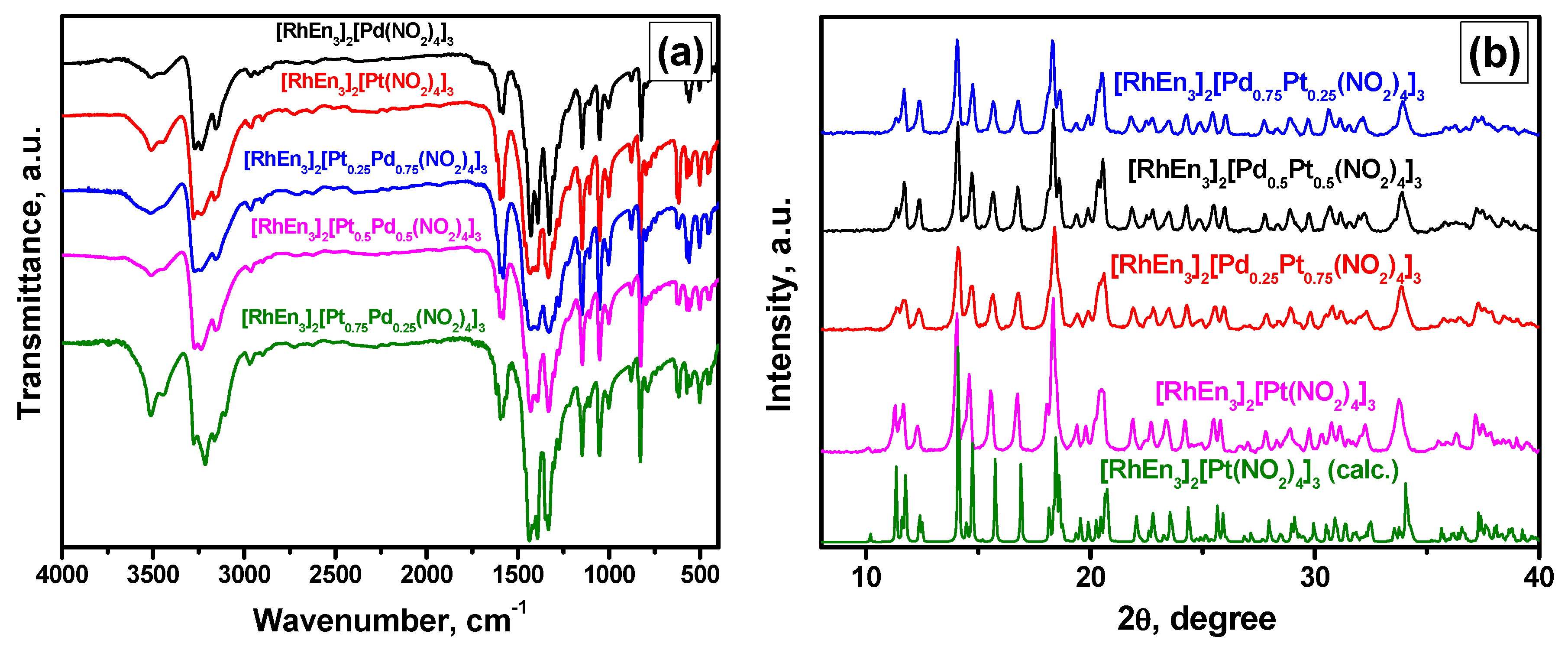
3.1. Characterization of the Precursors
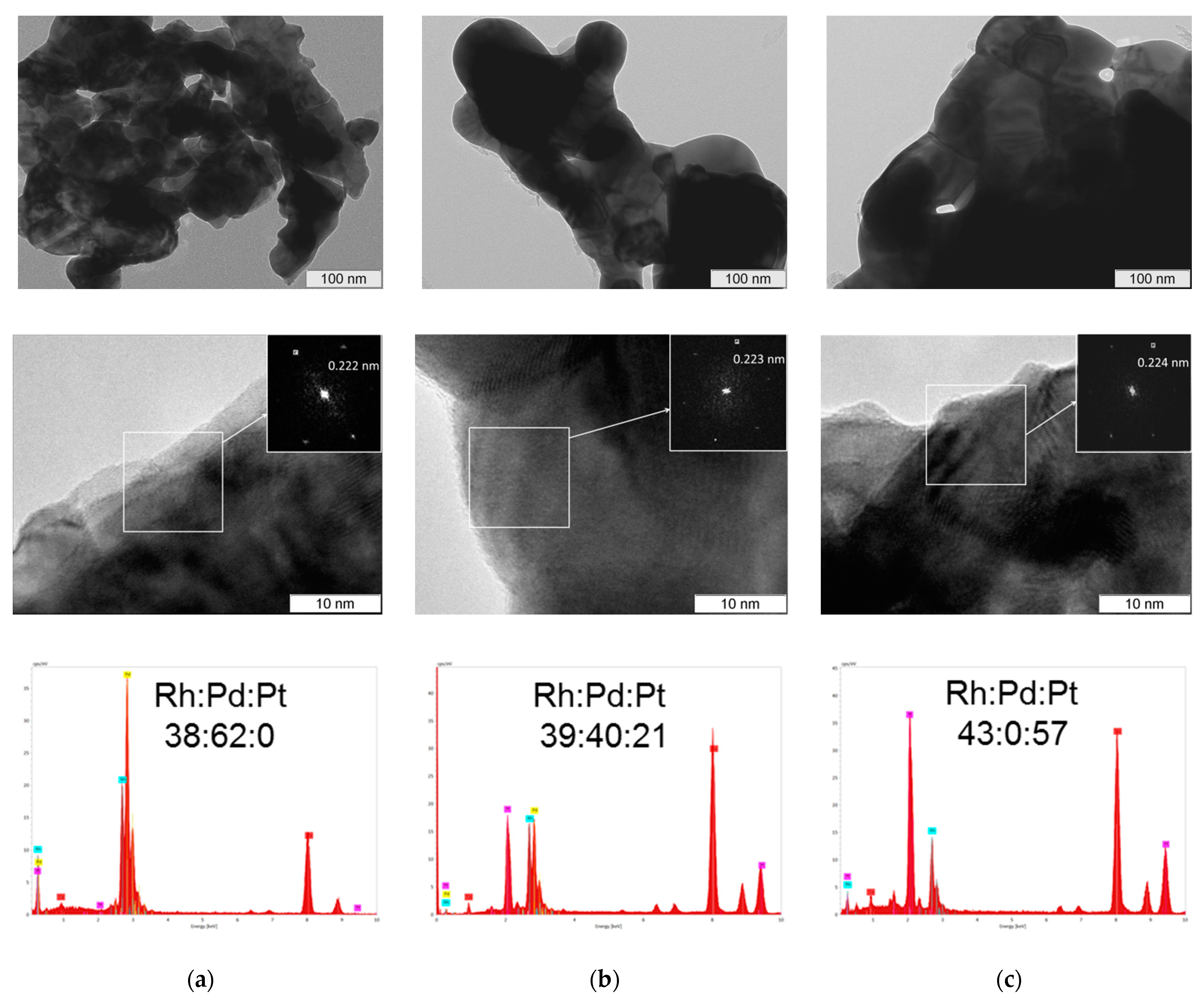
3.2. Characterization of the Alloys
| Sample | Lattice Parameter, a, Å | d111 (calc.) **, Å | d111 (TEM), Å | Rh:Pd:Pt (EDX) |
|---|---|---|---|---|
| Rh | 3.803 * | 2.196 | - | - |
| Pd | 3.890 * | 2.246 | - | - |
| Pt | 3.923 * | 2.265 | - | - |
| Rh2Pd3 | 3.856 | 2.226 | 2.22 | 38:62:0 |
| Rh2(Pd0.75Pt0.25)3 | 3.861 | 2.229 | 2.23 | 37:53:10 |
| Rh2(Pd0.50Pt0.50)3 | 3.865 | 2.231 | 2.23 | 39:40:21 |
| Rh2(Pd0.25Pt0.75)3 | 3.87 | 2.234 | 2.24 | 38:22:40 |
| Rh2Pt3 | 3.877 | 2.238 | 2.24 | 43:00:57 |
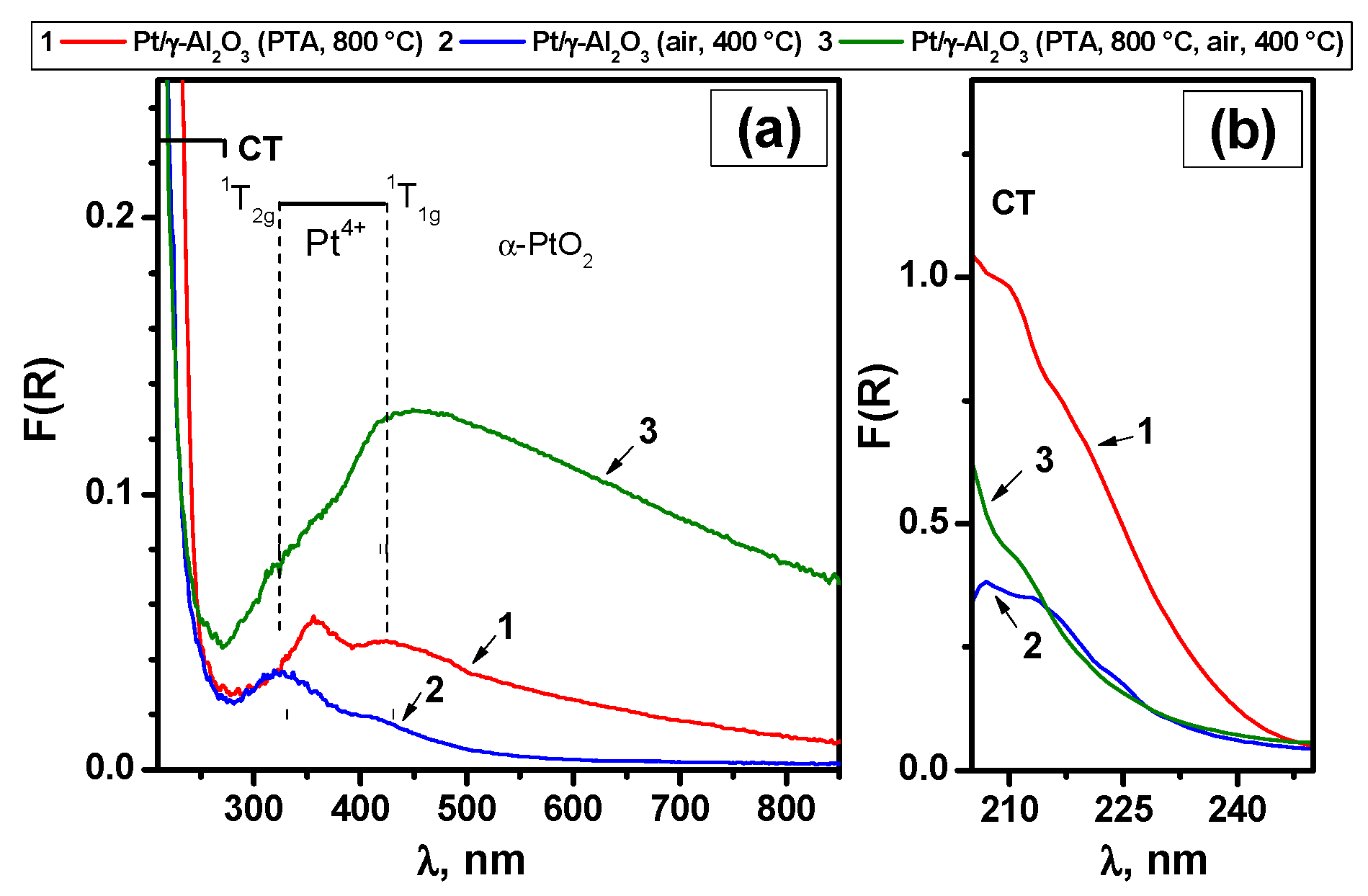
3.3. Characterization and Testing of the Alumina-Supported Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, D.; Xu, H.; Fortunelli, A. Tuning the catalytic activity of Au–Pd nanoalloys in CO oxidation via composition. J. Catal. 2014, 314, 47–55. [Google Scholar] [CrossRef]
- Kang, N.; Ng, M.S.; Shan, S.; Wu, J.; Zhao, W.; Yin, J.; Fang, W.; Luo, J.; Petkov, V.; Zhong, C.-J. Synergistic catalytic properties of bifunctional nanoalloy catalysts in rechargeable lithium-oxygen battery. J. Power Sources 2016, 326, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ruiz, V.-F.; González-Olvera, R.; Díaz-Pardo, R.; Betancourt, I.; Zumeta-Dubé, I.; Díaz, D.; Farfán, N.; Arellano-Jiménez, M.J. Mechanochemically obtained Pd–Ag nanoalloys. Structural considerations and catalytic activity. Materialia 2018, 4, 166–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, F.; Fu, M.-L. Composite of Au-Pd nanoalloys/reduced graphene oxide toward catalytic selective organic transformation to fine chemicals. Chem. Phys. Lett. 2018, 691, 61–67. [Google Scholar] [CrossRef]
- Taran, S. Composition effect on melting behaviors of Cu-Au-Pt trimetallic nanoalloys. Comput. Theor. Chem. 2019, 1166, 112576. [Google Scholar] [CrossRef]
- Campos-Roldán, C.A.; Calvillo, L.; Granozzi, G.; Alonso-Vante, N. Alkaline hydrogen electrode and oxygen reduction reaction on PtxNi nanoalloys. J. Electroanal. Chem. 2020, 857, 113449. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Guo, X. Ternary boron-, phosphorus- and oxygen-doped amorphous nickel nanoalloys for enhanced activity towards the oxygen evolution reaction. Electrochem. Commun. 2020, 111, 106649. [Google Scholar] [CrossRef]
- Cizmeci, M.; Musavi, A.; Tekin, A.; Kayahan, M. Comparison of two palladium catalysts on different supports during hydrogenation. J. Am. Oil Chem. Soc. 2006, 83, 1063–1068. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Jayasri, V.; Mahaboob Basha, P. Vapor phase hydrogenation of o-chloronitrobenzene (o-CNB) over alumina supported palladium catalyst—A kinetic study. Reac. Kinet. Catal. Lett. 2007, 91, 291–298. [Google Scholar] [CrossRef]
- Fukuyama, T.; Kippo, T.; Ryu, I.; Sagae, T. Addition of allyl bromide to phenylacetylene catalyzed by palladium on alumina and its application to a continuous flow synthesis. Res. Chem. Intermediat. 2009, 35, 1053–1057. [Google Scholar] [CrossRef]
- Arora, S.; Kapoor, P.; Singla, M.L. Catalytic studies of palladium nanoparticles immobilized on alumina synthesized by a simple physical precipitation method. Reac. Kinet. Mech. Catal. 2010, 99, 157–165. [Google Scholar] [CrossRef]
- Hong, U.G.; Hwang, S.; Seo, J.G.; Yi, J.; Song, I.K. Hydrogenation of Succinic Acid to γ-Butyrolactone over Palladium Catalyst Supported on Mesoporous Alumina Xerogel. Catal. Lett. 2010, 138, 28–33. [Google Scholar] [CrossRef]
- Berenblyum, A.S.; Podoplelova, T.A.; Shamsiev, R.S.; Katsman, E.A.; Danyushevsky, V.Y. On the mechanism of catalytic conversion of fatty acids into hydrocarbons in the presence of palladium catalysts on alumina. Petroleum Chem. 2011, 51, 336–341. [Google Scholar] [CrossRef]
- Thomazeau, C.; Cseri, T.; Bisson, L.; Aguilhon, J.; Pham Minh, D.; Boissière, C.; Durupthy, O.; Sanchez, C. Nano Design of Alumina Supported Monometallic Catalysts: A Promising Way to Improve the Selective Hydrogenation of Poly-Unsaturated Hydrocarbons. Top. Catal. 2012, 55, 690–699. [Google Scholar] [CrossRef]
- Chen, L.; Feng, T.; Wang, P.; Xiang, Y.; Ou, B. Catalytic properties of Pd supported on hexaaluminate coated alumina in low temperature combustion of coal mine ventilation air methane. Kinet. Catal. 2013, 54, 767–772. [Google Scholar] [CrossRef]
- Voskanyan, P.S. Effect of the nature of a support on the catalytic activity of a palladium catalyst in the synthesis of vinyl acetate by gas-phase ethylene acetoxylation. Catal. Ind. 2013, 5, 90–97. [Google Scholar] [CrossRef]
- Weng, X.; Yuan, X.; Li, H.; Li, X.; Chen, M.; Wan, H. The study of the active surface for CO oxidation over supported Pd catalysts. Sci. China Chem. 2014, 58, 174–179. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Fadaeerayeni, S.; Fard, M.R. The effect of calcination temperature on physicochemical properties of alumina as a support for acetylene selective hydrogenation catalyst. Res. Chem. Intermediat. 2015, 42, 4797–4811. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Vedyagin, A.A.; Tsapina, A.M.; Kaichev, V.V.; Trigub, A.L.; Trenikhin, M.V.; Shlyapin, D.A.; Tsyrulnikov, P.G.; Lavrenov, A.V. A study on structural features of bimetallic Pd-M/C (M: Zn, Ga, Ag) catalysts for liquid-phase selective hydrogenation of acetylene. Appl. Catal. A-Gen. 2018, 563, 18–27. [Google Scholar] [CrossRef]
- Iost, K.N.; Borisov, V.A.; Temerev, V.L.; Surovikin, Y.V.; Pavluchenko, P.E.; Trenikhin, M.V.; Arbuzov, A.B.; Shlyapin, D.A.; Tsyrulnikov, P.G.; Vedyagin, A.A. Carbon support hydrogenation in Pd/C catalysts during reductive thermal treatment. Int. J. Hydrog. Energ. 2018, 43, 17656–17663. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Khramov, E.V.; Smirnova, N.S.; Prosvirin, I.P.; Bukhtiyarov, A.V.; Trenikhin, M.V.; Gulyaeva, T.I.; Vedyagin, A.A.; Shlyapin, D.A.; Lavrenov, A.V. Study on the active phase formation of Pd-Zn/Sibunit catalysts during the thermal treatment in hydrogen. Appl. Surf. Sci. 2019, 483, 730–741. [Google Scholar] [CrossRef]
- Monteiro, R.S.; Dieguez, L.C.; Schmal, M. The role of Pd precursors in the oxidation of carbon monoxide over Pd/Al2O3 and Pd/CeO2/Al2O3 catalysts. Catal. Today 2001, 65, 77–89. [Google Scholar] [CrossRef]
- Demoulin, O.; Navez, M.; Ruiz, P. The Activation of a Pd/γ-alumina Catalyst During Methane Combustion: Investigation of the Phenomenon and of Potential Causes. Catal. Lett. 2005, 103, 149–153. [Google Scholar] [CrossRef]
- Gopinath, R.; Babu, N.S.; Kumar, J.V.; Lingaiah, N.; Prasad, P.S. Influence of Pd Precursor and Method of Preparation on Hydrodechlorination Activity of Alumina Supported Palladium Catalysts. Catal. Lett. 2007, 120, 312–319. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Zhao, B.; Zhou, R. A new insight into the role of transition metals doping with CeO2–ZrO2 and its application in Pd-only three-way catalysts for automotive emission control. Fuel 2012, 92, 360–368. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Liu, C. Perspective on CO oxidation over Pd-based catalysts. Catal. Sci. Technol. 2015, 5, 69–81. [Google Scholar] [CrossRef]
- Vedyagin, A.; Volodin, A.; Kenzhin, R.; Chesnokov, V.; Mishakov, I. CO Oxidation over Pd/ZrO2 Catalysts: Role of Support’s Donor Sites. Molecules 2016, 21, 1289. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Stoyanovskii, V.O.; Rogov, V.A.; Kriventsov, V.V.; Mishakov, I.V. The role of chemisorbed water in formation and stabilization of active sites on Pd/Alumina oxidation catalysts. Catal. Today 2018, 307, 102–110. [Google Scholar] [CrossRef]
- Ferri, D.; Elsener, M.; Kröcher, O. Methane oxidation over a honeycomb Pd-only three-way catalyst under static and periodic operation. Appl. Catal. B-Environ. 2018, 220, 67–77. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-Based Bimetallic Catalysis: From Model Surfaces to Supported Catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef]
- Kogan, S.B.; Herskowitz, M. Geometric and electronic factors in paraffin dehydrogenation on bimetallic platinum catalysts. Reac. Kinet. Catal. Lett. 2005, 85, 341–345. [Google Scholar] [CrossRef]
- Antolini, E. Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells. J. Mater. Sci. 2003, 38, 2995–3005. [Google Scholar] [CrossRef]
- Seselj, N.; Engelbrekt, C.; Zhang, J. Graphene-supported platinum catalysts for fuel cells. Sci. Bull. 2015, 60, 864–876. [Google Scholar] [CrossRef] [Green Version]
- Fuente, A.M.; Pulgar, G.; González, F.; Pesquera, C.; Blanco, C. Activated carbon supported Pt catalysts: Effect of support texture and metal precursor on activity of acetone hydrogenation. Appl. Catal. A-Gen. 2001, 208, 35–46. [Google Scholar] [CrossRef]
- Román-Martínez, M.C.; Cazorla-Amorós, D.; Linares-Solano, A.; de Lecea, C.S.-M. Carbon dioxide hydrogenation catalyzed by alkaline earth- and platinum-based catalysts supported on carbon. Appl. Catal. A-Gen. 1994, 116, 187–204. [Google Scholar] [CrossRef]
- Du, J.-P.; Song, C.; Song, J.-L.; Zhao, J.-H.; Zhu, Z.-P. Cyclohexane dehydrogenation over the platinum catalysts supported on carbon nanomaterials. J. Fuel Chem. Technol. 2009, 37, 468–472. [Google Scholar] [CrossRef]
- Arevalo-Bastante, A.; Álvarez-Montero, M.A.; Bedia, J.; Gómez-Sainero, L.M.; Rodriguez, J.J. Gas-phase hydrodechlorination of mixtures of chloromethanes with activated carbon-supported platinum catalysts. Appl. Catal. B-Environ. 2015, 179, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A-Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Shelef, M.; Graham, G.W. Why Rhodium in Automotive Three-Way Catalysts? Catal. Rev. 2006, 36, 433–457. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Ziółkowski, J.J.; Jaworska-Galas, Z.; Migta, W.; Wrzyszcz, J. Homogeneous and alumina supported rhodium complex catalysed hydrogenation. J. Mol. Catal. 1994, 88, 13–21. [Google Scholar] [CrossRef]
- Alini, S.; Bottino, A.; Capannelli, G.; Carbone, R.; Comite, A.; Vitulli, G. The catalytic hydrogenation of adiponitrile to hexamethylenediamine over a rhodium/alumina catalyst in a three phase slurry reactor. J. Mol. Catal. A-Chem. 2003, 206, 363–370. [Google Scholar] [CrossRef]
- Hebben, N.; Diehm, C.; Deutschmann, O. Catalytic partial oxidation of ethanol on alumina-supported rhodium catalysts: An experimental study. Appl. Catal. A-Gen. 2010, 388, 225–231. [Google Scholar] [CrossRef]
- Hung, C.M. Characterization and performance of Pt-Pd-Rh cordierite monolith catalyst for selectivity catalytic oxidation of ammonia. J. Hazard. Mater. 2010, 180, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Zapf, R.; Thiele, R.; Wichert, M.; O’Connell, M.; Ziogas, A.; Kolb, G. Application of rhodium nanoparticles for steam reforming of propane in microchannels. Catal. Commun. 2013, 41, 140–145. [Google Scholar] [CrossRef]
- Choi, K.; Joo, J.M.; Lee, C. Rhodium-catalyzed tandem addition–cyclization of alkynylimines. Tetrahedron 2015, 71, 5910–5917. [Google Scholar] [CrossRef]
- Zhang, Q.; Mixdorf, J.C.; Reynders, G.J.; Nguyen, H.M. Rhodium-catalyzed benzylic fluorination of trichloroacetimidates. Tetrahedron 2015, 71, 5932–5938. [Google Scholar] [CrossRef]
- Özhava, D.; Özkar, S. Nanoalumina-supported rhodium(0) nanoparticles as catalyst in hydrogen generation from the methanolysis of ammonia borane. Mol. Catal. 2017, 439, 50–59. [Google Scholar] [CrossRef]
- Kiss, B.; Manning, T.D.; Hesp, D.; Didier, C.; Taylor, A.; Pickup, D.M.; Chadwick, A.V.; Allison, H.E.; Dhanak, V.R.; Claridge, J.B.; et al. Nano-structured rhodium doped SrTiO3–Visible light activated photocatalyst for water decontamination. Appl. Catal. B-Environ. 2017, 206, 547–555. [Google Scholar] [CrossRef]
- Coq, B.; Figueras, F. Bimetallic palladium catalysts: Influence of the co-metal on the catalyst performance. J. Mol. Catal. A-Chem. 2001, 173, 117–134. [Google Scholar] [CrossRef]
- Hungría, A.B.; Iglesias-Juez, A.; Martínez-Arias, A.; Fernández-García, M.; Anderson, J.A.; Conesa, J.C.; Soria, J. Effects of Copper on the Catalytic Properties of Bimetallic Pd–Cu/(Ce,Zr)Ox/Al2O3 and Pd–Cu/(Ce,Zr)Ox Catalysts for CO and NO Elimination. J. Catal. 2002, 206, 281–294. [Google Scholar] [CrossRef]
- Fernández-García, M.; Martínez-Arias, A.; Iglesias-Juez, A.; Hungría, A.B.; Anderson, J.A.; Conesa, J.C.; Soria, J. Behavior of bimetallic Pd-Cr/Al2O3 and Pd-Cr/(Ce,Zr)Ox/Al2O3 catalysts for CO and NO elimination. J. Catal. 2003, 214, 220–233. [Google Scholar] [CrossRef]
- Ershov, B.G.; Anan’ev, A.V.; Abkhalimov, E.V.; Kochubei, D.I.; Kriventsov, V.V.; Plyasova, L.M.; Molina, I.Y.; Kozitsyna, N.Y.; Nefedov, S.E.; Vargaftik, M.N.; et al. Bimetallic Pd-M (M = Co, Ni, Zn, Ag) nanoparticles containing transition metals: Synthesis, characterization, and catalytic performance. Nanotechnol. Russ. 2011, 6, 323–329. [Google Scholar] [CrossRef]
- Hinokuma, S.; Katsuhara, Y.; Ando, E.; Ikeue, K.; Machida, M. PdFe/CeO2 bimetal catalysts prepared by dual arc-plasma deposition. Catal. Today 2013, 201, 92–97. [Google Scholar] [CrossRef]
- Hilli, Y.; Kinnunen, N.M.; Suvanto, M.; Savimäki, A.; Kallinen, K.; Pakkanen, T.A. Preparation and characterization of Pd–Ni bimetallic catalysts for CO and C3H6 oxidation under stoichiometric conditions. Appl. Catal. A-Gen. 2015, 497, 85–95. [Google Scholar] [CrossRef]
- Shan, S.; Petkov, V.; Prasai, B.; Wu, J.; Joseph, P.; Skeete, Z.; Kim, E.; Mott, D.; Malis, O.; Luo, J.; et al. Catalytic activity of bimetallic catalysts highly sensitive to the atomic composition and phase structure at the nanoscale. Nanoscale 2015, 7, 18936–18948. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.F.; Wang, Y.X. Effects of Alloyed Metal on the Catalysis Activity of Pt for Ethanol Partial Oxidation: Adsorption and Dehydrogenation on Pt3M (M = Pt, Ru, Sn, Re, Rh, and Pd). J. Phys. Chem. C 2011, 115, 20565–20571. [Google Scholar] [CrossRef] [Green Version]
- Shubin, Y.V.; Vedyagin, A.A.; Plyusnin, P.E.; Kirilovich, A.K.; Kenzhin, R.M.; Stoyanovskii, V.O.; Korenev, S.V. The peculiarities of Au–Pt alloy nanoparticles formation during the decomposition of double complex salts. J. Alloys Compnd. 2018, 740, 935–940. [Google Scholar] [CrossRef]
- Nunan, J.G.; Williamson, W.B.; Robota, H.J.; Henk, M.G. Impact of Pt-Rh and Pd-Rh Interactions on Performance of Bimetal Catalysts. SAE Tech. Paper 1995, 950258. [Google Scholar] [CrossRef]
- Araya, P.; Díaz, V. Synergism in the reaction of CO with O2 on bimetallic Rh-Pd catalysts supported on silica. J. Chem. Soc. Faraday Trans. 1997, 93, 3887–3891. [Google Scholar] [CrossRef]
- Wu, X.; Xu, L.; Weng, D. The thermal stability and catalytic performance of Ce-Zr promoted Rh-Pd/γ-Al2O3 automotive catalysts. Appl. Surf. Sci. 2004, 221, 375–383. [Google Scholar] [CrossRef]
- De Sarkar, A.; Khanra, B. CO oxidation and NO reduction over supported Pt-Rh and Pd-Rh nanocatalysts: A comparative study. J. Mol. Catal. A-Chem. 2005, 229, 25–29. [Google Scholar] [CrossRef]
- Hangas, J.; Chen, A.E. Comparative Analytical Study of Two Pt–Rh Three-way Catalysts. Catal. Lett. 2006, 108, 103–111. [Google Scholar] [CrossRef]
- Renzas, J.R.; Huang, W.; Zhang, Y.; Grass, M.E.; Somorjai, G.A. Rh1−xPdx Nanoparticle Composition Dependence in CO Oxidation by NO. Catal. Lett. 2010, 141, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Büchel, R.; Pratsinis, S.E.; Baiker, A. Mono-and bimetallic Rh and Pt NSR-catalysts prepared by controlled deposition of noble metals on support or storage component. Appl. Catal. B-Environ. 2012, 113–114, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Kenzhin, R.M.; Slavinskaya, E.M.; Mishakov, I.V.; Plyusnin, P.E.; Shubin, Y.V. Stabilization of active sites in alloyed Pd–Rh catalysts on γ-Al2O3 support. Catal. Today 2014, 238, 80–86. [Google Scholar] [CrossRef]
- Zhan, Z.; Song, L.; Liu, X.; Jiao, J.; Li, J.; He, H. Effects of synthesis methods on the performance of Pt + Rh/Ce0.6Zr0.4O2 three-way catalysts. J. Environ. Sci. 2014, 26, 683–693. [Google Scholar] [CrossRef]
- Shang, H.; Wang, Y.; Cui, Y.; Fang, R.; Hu, W.; Gong, M.; Chen, Y. Catalytic performance of Pt–Rh/CeZrYLa+LaAl with stoichiometric natural gas vehicles emissions. Chinese J. Catal. 2015, 36, 290–298. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Plyusnin, P.E.; Rybinskaya, A.A.; Shubin, Y.V.; Mishakov, I.V.; Korenev, S.V. Synthesis and study of Pd-Rh alloy nanoparticles and alumina-supported low-content Pd-Rh catalysts for CO oxidation. Mater. Res. Bull. 2018, 102, 196–202. [Google Scholar] [CrossRef]
- Shubin, Y.V.; Plyusnin, P.E.; Korenev, S.V. Determination of the equilibrium miscibility gap in the Pd–Rh alloy system using metal nanopowders obtained by decomposition of coordination compounds. J. Alloys Compnd. 2015, 622, 1055–1060. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Stoyanovskii, V.O.; Plyusnin, P.E.; Shubin, Y.V.; Slavinskaya, E.M.; Mishakov, I.V. Effect of metal ratio in alumina-supported Pd-Rh nanoalloys on its performance in three way catalysis. J. Alloys Compnd. 2018, 749, 155–162. [Google Scholar] [CrossRef]
- Kostin, G.A.; Plyusnin, P.E.; Filatov, E.Y.; Kuratieva, N.V.; Vedyagin, A.A.; Kal’nyi, D.B. Double complex salts [PdL4][RuNO(NO2)4OH] (L = NH3, Py) synthesis, structure and preparation of bimetallic metastable solid solution Pd0.5Ru0.5. Polyhedron 2019, 159, 217–225. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ohira, K.; Kokubu, M.; Flanagan, T.B. Thermodynamic properties for solution of hydrogen in Pd-Pt-Rh ternary alloys. J. Alloys Compnd. 1997, 253, 212–215. [Google Scholar] [CrossRef]
- Lukaszewski, M.; Grden, M.; Czerwinski, A. Cyclic voltammetric behavior of Pd-Pt-Rh ternary alloys. J. Solid State Electr. 2005, 9, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, S.Z.; Deng, H.Q. The Rh influence on the surface distribution of the ternary alloy Pt-Pd-Rh. Appl. Surf. Sci. 2007, 253, 6074–6079. [Google Scholar] [CrossRef]
- Luyten, J.; Creemers, C. Surface segregation in ternary Pt-Pd-Rh alloys studied with Monte Carlo simulations and the modified embedded atom method. Surf. Sci. 2008, 602, 2491–2495. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Anuradha, R.; Park, S.D.; Park, T.S.; Cha, W.S.; Jang, H.T. Effect of Bimetallic Pt-Rh and Trimetallic Pt-Pd-Rh Catalysts for Low Temperature Catalytic Combustion of Methane. B. Korean Chem. Soc. 2010, 31, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.M. Cordierite-supported Pt-Pd-Rh ternary composite for selective catalytic oxidation of ammonia. Powder Technol. 2010, 200, 78–83. [Google Scholar] [CrossRef]
- Bagot, P.A.J.; Kruska, K.; Haley, D.; Carrier, X.; Marceau, E.; Moody, M.P.; Smith, G.D.W. Oxidation and Surface Segregation Behavior of a Pt-Pd-Rh Alloy Catalyst. J. Phys. Chem. C 2014, 118, 26130–26138. [Google Scholar] [CrossRef]
- Sarker, M.S.I.; Nakamura, T.; Sato, S. Composition-controlled ternary Rh-Pd-Pt solid-solution alloy nanoparticles by laser irradiation of mixed solution of metallic ions. J. Mater. Res. 2014, 29, 856–864. [Google Scholar] [CrossRef]
- Liu, T.D.; Xu, L.Y.; Shao, G.F.; Tu, N.N.; Tao, J.P.; Wen, Y.H. Structural optimization of Pt-Pd-Rh trimetallic nanoparticles using improved genetic algorithm. J. Alloys Compnd. 2016, 663, 466–473. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.Q.; Jia, H.S. Pt-Rh-Pd Alloy Group Gauze Catalysts Used for Ammonia Oxidation. Rare Met. Mater. Eng. 2017, 46, 339–343. [Google Scholar]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Mishakov, I.V.; Medvedev, D.A.; Noskov, A.S. Characterization of active sites of Pd/Al2O3 model catalysts with low Pd content by luminescence, EPR and ethane hydrogenolysis. Appl. Catal. B-Environ. 2011, 103, 397–403. [Google Scholar] [CrossRef]
- Stoyanovskii, V.O.; Vedyagin, A.A.; Aleshina, G.I.; Volodin, A.M.; Noskov, A.S. Characterization of Rh/Al2O3 catalysts after calcination at high temperatures under oxidizing conditions by luminescence spectroscopy and catalytic hydrogenolysis. Appl. Catal. B-Environ. 2009, 90, 141–146. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Y.; Seo, C.Y.; Schwank, J.W.; McCabe, R.W. Aging, re-dispersion, and catalytic oxidation characteristics of model Pd/Al2O3 automotive three-way catalysts. Appl. Catal. B-Environ. 2015, 163, 499–509. [Google Scholar] [CrossRef]
- Morgan, K.; Goguet, A.; Hardacre, C. Metal Redispersion Strategies for Recycling of Supported Metal Catalysts: A Perspective. Acs Catal. 2015, 5, 3430–3445. [Google Scholar] [CrossRef] [Green Version]
- Lupescu, J.A.; Schwank, J.W.; Fisher, G.B.; Chen, X.; Peczonczyk, S.L.; Drews, A.R. Pd model catalysts: Effect of aging duration on lean redispersion. Appl. Catal. B-Environ. 2016, 185, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Lupescu, J.A.; Schwank, J.W.; Fisher, G.B.; Hangas, J.; Peczonczyk, S.L.; Paxton, W.A. Pd model catalysts: Effect of air pulse length during redox aging on Pd redispersion. Appl. Catal. B-Environ. 2018, 223, 76–90. [Google Scholar] [CrossRef]
- Seo, C.Y.; Chen, X.Y.; Sun, K.; Allard, L.F.; Fisher, G.B.; Schwank, J.W. Palladium redispersion at high temperature within the Pd@SiO2 core@shell structure. Catal. Commun. 2018, 108, 73–76. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Scofield, C.F.; Neto, A.A.; Cardoso, M.J.B.; Zotin, F.M.Z. Thermal deactivation of Pt/Rh commercial automotive catalysts. Chem. Eng. J. 2010, 160, 85–92. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Stoyanovskii, V.O.; Kenzhin, R.M.; Slavinskaya, E.M.; Plyusnin, P.E.; Shubin, Y.V. Purification of gasoline exhaust gases using bimetallic Pd–Rh/δ-Al2O3 catalysts. Reac. Kinet. Mech. Catal. 2019, 127, 137–148. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Krumm, S. An Interactive Windows Program for Profile Fitting and Size/Strain Analysis. Mater. Sci. Forum 1996, 228, 183–190. [Google Scholar] [CrossRef]
- Stoyanovskii, V.O.; Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Slavinskaya, E.M.; Plyusnin, P.E.; Shubin, Y.V. Optical Spectroscopy Methods in the Estimation of the Thermal Stability of Bimetallic Pd–Rh/Al2O3 Three-Way Catalysts. Top. Catal. 2018, 62, 296–304. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Gavrilov, M.S.; Volodin, A.M.; Stoyanovskii, V.O.; Slavinskaya, E.M.; Mishakov, I.V.; Shubin, Y.V. Catalytic Purification of Exhaust Gases Over Pd–Rh Alloy Catalysts. Top. Catal. 2013, 56, 1008–1014. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Stoyanovskii, V.O.; Shubin, Y.V.; Plyusnin, P.E.; Mishakov, I.V. Effect of metal-metal and metal-support interaction on activity and stability of Pd-Rh/alumina in CO oxidation. Catal. Today 2017, 293-294, 73–81. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Shubin, Y.V.; Kenzhin, R.M.; Plyusnin, P.E.; Stoyanovskii, V.O.; Volodin, A.M. Prospect of Using Nanoalloys of Partly Miscible Rhodium and Palladium in Three-Way Catalysis. Top. Catal. 2018, 62, 305–314. [Google Scholar] [CrossRef]
- Pd-Pt Binary Phase Diagram 0-100, at. Pd-Pt Binary Phase Diagram 0-100 at.% Pt: Datasheet from "PAULING FILE Multinaries Edition—2012" in SpringerMaterials; Villars2016:sm_isp_c_0979992; Springer: Berlin/Heidelberg, Germany; Material Phases Data System (MPDS): Vitznau, Switzerland; National Institute for Materials Science (NIMS): Tsukuba, Japan.
- Pt-Rh Binary Phase Diagram 0-100, at. Pt-Rh Binary Phase Diagram 0-100 at.% Rh: Datasheet from "PAULING FILE Multinaries Edition—2012" in SpringerMaterials; Villars2016:sm_isp_c_0979899; Springer: Berlin/Heidelberg, Germany; Material Phases Data System (MPDS): Vitznau, Switzerland; National Institute for Materials Science (NIMS): Tsukuba, Japan.
- Bharadwaj, S.R.; Tripathi, S.N. The Pd-Pt (palladium-platinum) system. J. Alloy Phase Diagr. 1990, 6, 118–121. [Google Scholar]
- Bharadwaj, S.R.; Kerkar, A.S.; Tripathi, S.N.; Dharwadkar, S.R. The palladium-platinum phase diagram. J. Less Common Met. 1991, 169, 167–172. [Google Scholar] [CrossRef]
- Shubin, Y.V.; Korenev, S.V.; Sharafutdinov, M.R. High-temperature X-ray diffraction study of thermolysis of the double complex salt [Rh(NH3)5Cl][PtCl4]. Russ. Chem. Bull. 2006, 55, 1109–1113. [Google Scholar] [CrossRef]
- Zhao, J.C.; Jackson, M.R.; Peluso, L.A.; Brewer, L.N. A diffusion-multiple approach for mapping phase diagrams, hardness, and elastic modulus. JOM 2002, 54, 42–45. [Google Scholar] [CrossRef]
- Zhao, J.C. Reliability of the diffusion-multiple approach for phase diagram mapping. J. Mater. Sci. 2004, 39, 3913–3925. [Google Scholar] [CrossRef]
- Luyten, J.; De Keyzer, J.; Wollants, P.; Creemers, C. Construction of modified embedded atom method potentials for the study of the bulk phase behaviour in binary Pt-Rh, Pt-Pd, Pd-Rh and ternary Pt-Pd-Rh alloys. Calphad 2009, 33, 370–376. [Google Scholar] [CrossRef]
- Powder Diffraction File PDF-2; International Centre for Diffraction Data (ICDD): Newtown Square, PA, USA, 2009.
- Tripathi, S.N.; Bharadwqj, S.R. The Pd-Rh (Palladium-Rhodium) system. J. Phase Equilibria 1994, 15, 208–212. [Google Scholar] [CrossRef]
- Koffyberg, F.P. Optical bandgaps and electron affinities of semiconducting Rh2O3(I) and Rh2O3(III). J. Phys. Chem. Solids 1992, 53, 1285–1288. [Google Scholar] [CrossRef]
- Aita, C.R. Optical behavior of sputter-deposited platinum-oxide films. J. Appl. Phys. 1985, 58, 3169–3173. [Google Scholar] [CrossRef]
- Zhensheng, J.; Chanjuan, X.; Qingmei, Z.; Feng, Y.; Jiazheng, Z.; Jinzhen, X. Catalytic behavior of nanoparticle α-PtO2 for ethanol oxidation. J. Mol. Catal. A-Chem. 2003, 191, 61–66. [Google Scholar] [CrossRef]







| Precursor | Content (Measured/Calculated), % | |||||
|---|---|---|---|---|---|---|
| C | N | H | Pt | Rh | Pd | |
| [RhEn3]2[Pd(NO2)4]3 | 9.5/10.02 | 20.4/23.38 | 3.1/3.37 | - | 14.1/14.32 | 21.2/22.21 |
| [RhEn3]2[Pt(NO2)4]3 | 8.4/8.46 | 19.6/19.73 | 2.8/2.84 | 35.0/34.35 | 12.0/12.08 | - |
| [RhEn3]2[Pd0.5Pt0.5(NO2)4]3 | 9.0/9.18 | 21.3/21.40 | 3.0/3.08 | 19.3/18.63 | 13.3/13.10 | 9.5/10.16 |
| Compound | νs(NH) | ν(CH) | δ(NH2) | νa(NO2) | ν(CN) | δ(ONO) |
|---|---|---|---|---|---|---|
| [RhEn3]2[Pd(NO2)4]3 | 3271 3237 3153 | 2965 | 1581 | 1428 1390 1327 1299 | 1050 | 823 |
| [RhEn3]2[Pt(NO2)4]3 | 3278 3234 3161 | 2962 | 1597 1582 1146 | 1432 1394 1331 | 1049 | 827 |
| [RhEn3]2[Pd0.75Pt0.25(NO2)4]3 | 3271 3237 3153 | 2964 | 1597 1581 1145 | 1428 1391 1328 | 1050 | 824 |
| [RhEn3]2[Pd0.5Pt0.5(NO2)4]3 | 3273 3237 3147 | 2964 | 1596 1581 1145 | 1429 1393 1330 | 1050 | 826 |
| [RhEn3]2[Pd0.25Pt0.75(NO2)4]3 | 3275 3236 3148 | 2963 | 1597 1582 1146 | 1431 1394 1332 | 1050 | 827 |
| Compound | Measured | Calculated |
|---|---|---|
| [RhEn3]2[Pd(NO2)4]3 | 36.8% | 36.53% |
| [RhEn3]2[Pd0.75Pt0.25(NO2)4]3 | 39.2% | 39.33% |
| [RhEn3]2[Pd0.5Pt0.5(NO2)4]3 | 41.7% | 41.89% |
| [RhEn3]2[Pd0.25Pt0.75(NO2)4]3 | 44.3% | 44.26% |
| [RhEn3]2[Pt(NO2)4]3 | 46.2% | 46.43% |
| Sample | Content (Measured/Calculated), % | ||
|---|---|---|---|
| Rh | Pd | Pt | |
| Rh2Pt3/Al2O3 | 0.05/0.051 | - | 0.15/0.149 |
| Rh2Pd3/Al2O3 | 0.08/0.076 | 0.12/0.119 | - |
| Rh2(Pd0.25Pt0.75)3/Al2O3 | 0.05/0.057 | 0.03/0.022 | 0.11/0.121 |
| Rh2(Pd0.5Pt0.5)3/Al2O3 | 0.05/0.062 | 0.05/0.048 | 0.10/0.089 |
| Rh2(Pd0.75Pt0.25)3/Al2O3 | 0.07/0.069 | 0.08/0.081 | 0.06/0.049 |
| Pt/Al2O3 | - | - | 0.16/0.15 |
| Pd/Al2O3 | - | 0.11/0.119 | - |
| Sample | Eg, eV | |
|---|---|---|
| Initial | Aged at 800 °C | |
| Pt/γ-Al2O3 | 2.62 | 1.65 |
| Rh2Pt3/γ-Al2O3 | 2.47 | 1.97 |
| Rh+Pt/γ-Al2O3 | 2.34 | 2.01 |
| Rh2(Pd0.25Pt0.75)3/γ-Al2O3 | 2.47 | 2.08 |
| Rh2(Pd0. 5Pt0.5)3/γ-Al2O3 | 2.44 | 2.17 |
| Rh2(Pd0.75Pt0.25)3/γ-Al2O3 | 2.43 | 2.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedyagin, A.A.; Shubin, Y.V.; Kenzhin, R.M.; Plyusnin, P.E.; Stoyanovskii, V.O. The Attractiveness of the Ternary Rh-Pd-Pt Alloys for CO Oxidation Process. Processes 2020, 8, 928. https://doi.org/10.3390/pr8080928
Vedyagin AA, Shubin YV, Kenzhin RM, Plyusnin PE, Stoyanovskii VO. The Attractiveness of the Ternary Rh-Pd-Pt Alloys for CO Oxidation Process. Processes. 2020; 8(8):928. https://doi.org/10.3390/pr8080928
Chicago/Turabian StyleVedyagin, Aleksey A., Yury V. Shubin, Roman M. Kenzhin, Pavel E. Plyusnin, and Vladimir O. Stoyanovskii. 2020. "The Attractiveness of the Ternary Rh-Pd-Pt Alloys for CO Oxidation Process" Processes 8, no. 8: 928. https://doi.org/10.3390/pr8080928
APA StyleVedyagin, A. A., Shubin, Y. V., Kenzhin, R. M., Plyusnin, P. E., & Stoyanovskii, V. O. (2020). The Attractiveness of the Ternary Rh-Pd-Pt Alloys for CO Oxidation Process. Processes, 8(8), 928. https://doi.org/10.3390/pr8080928






