Synthesis and Characterization of CoxOy–MnCO3 and CoxOy–Mn2O3 Catalysts: A Comparative Catalytic Assessment Towards the Aerial Oxidation of Various Kinds of Alcohols
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Fabrication
2.2. Characterization Techniques
2.3. Catalytic Assessment
2.4. Reutilizing Tests
3. Results and Discussion
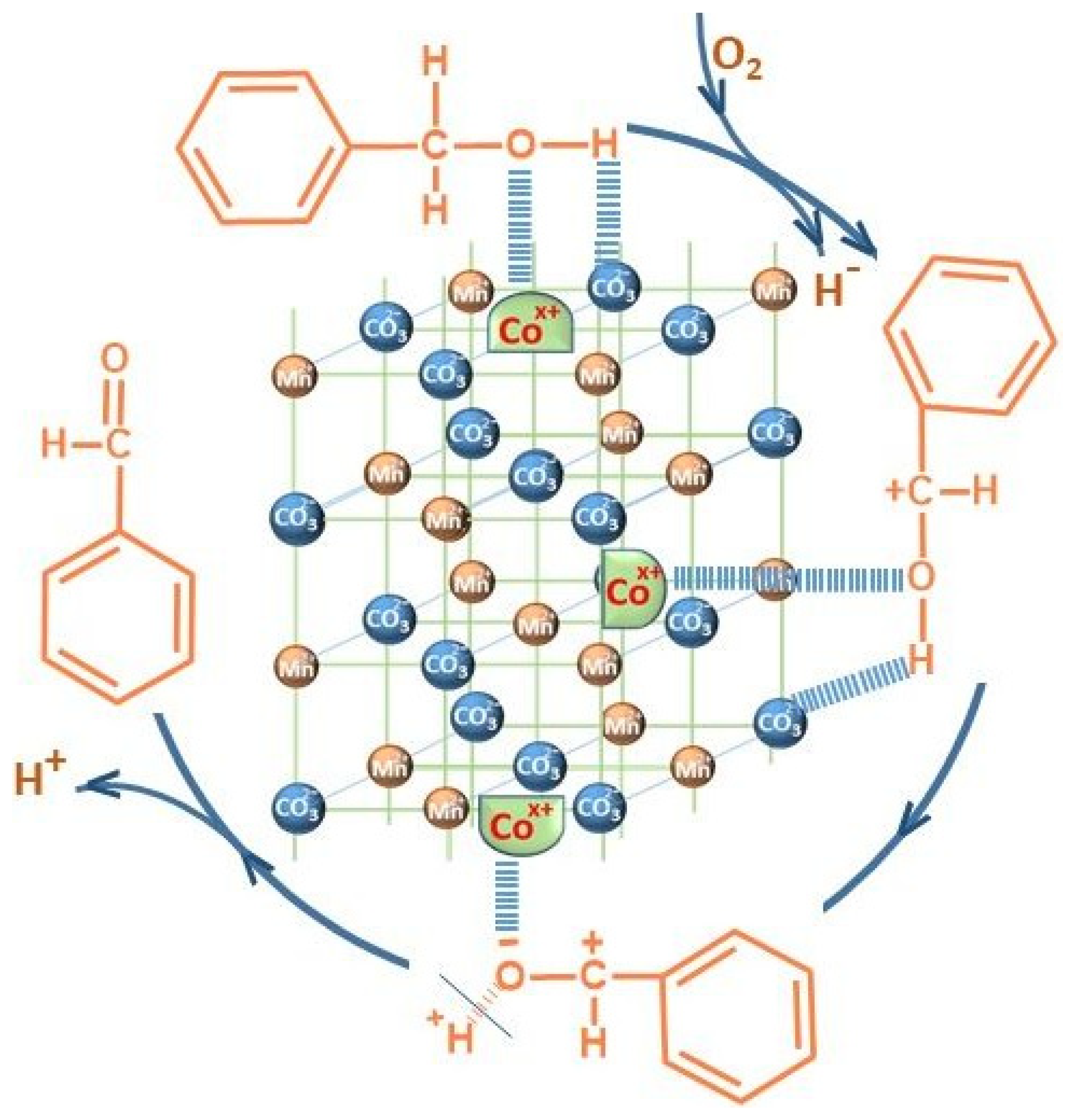
3.1. Characterizations
3.2. Catalytic Performance Results
3.2.1. Influence of Calcination Temperature
3.2.2. Influence of Weight % of CoxOy Promoter
3.2.3. Influence of Temperature
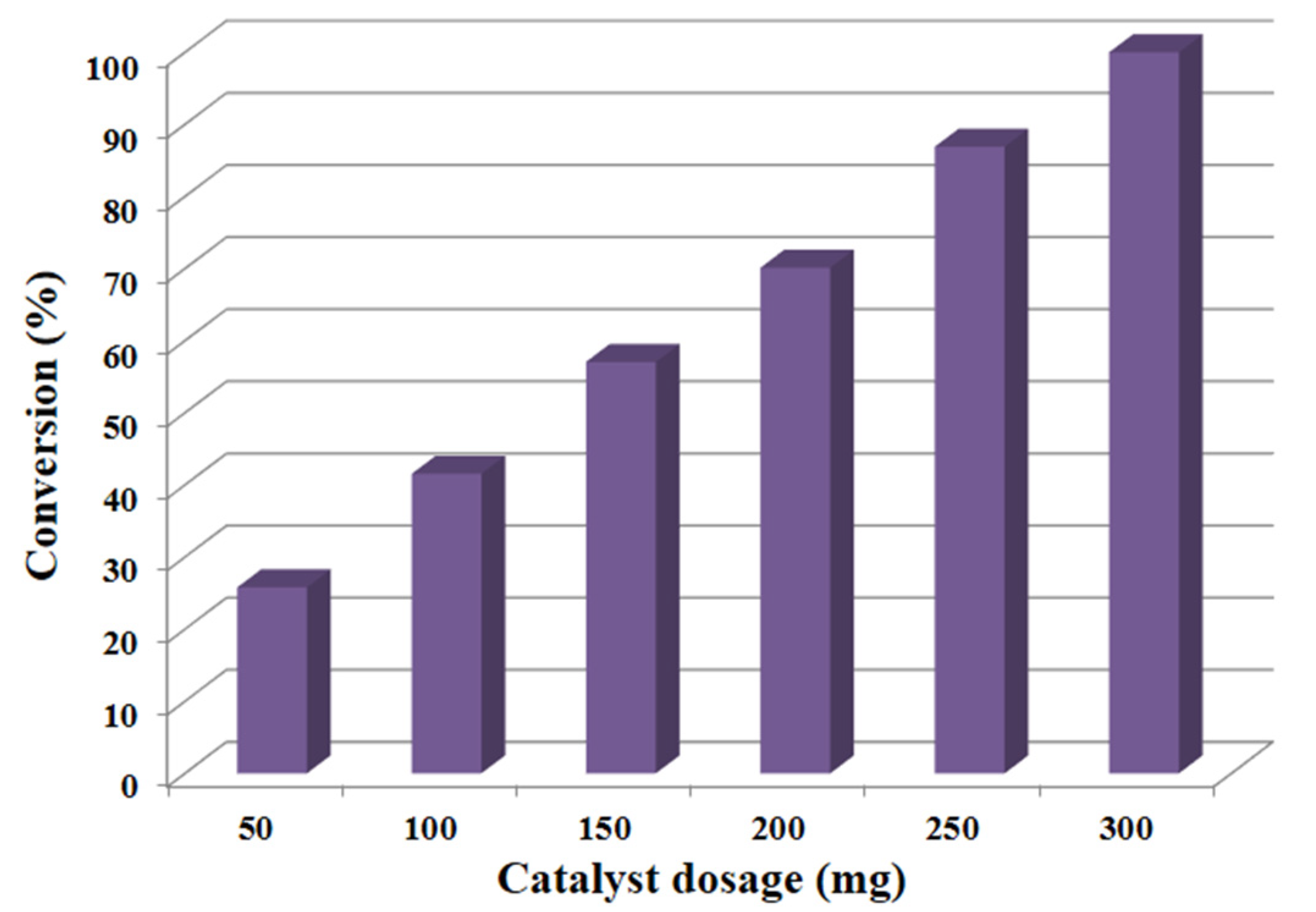
3.2.4. Influence of Dosage of Catalyst
3.3. Recovery Tests
3.4. General Applicability of (1%)CoxOy–MnCO3 Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; Arends, I.; Dijksman, A. New developments in catalytic alcohol oxidations for fine chemicals synthesis. Catal. Today 2000, 57, 157–166. [Google Scholar] [CrossRef]
- Adam, F.; Ooi, W.T. Selective oxidation of benzyl alcohol to benzaldehyde over Co-metalloporphyrin supported on silica nanoparticles. Appl. Catal. A Gen. 2012, 445, 252–260. [Google Scholar] [CrossRef]
- Kamimura, A.; Nozaki, Y.; Ishikawa, S.; Inoue, R.; Nakayama, M. K-birnessite MnO2: A new selective oxidant for benzylic and allylic alcohols. Tetrahedron Lett. 2011, 52, 538–540. [Google Scholar] [CrossRef]
- Albadi, J.; Alihosseinzadeh, A.; Jalali, M.; Shahrezaei, M.; Mansournezhad, A. Highly dispersed cobalt nanoparticles supported on a mesoporous Al2O3: An efficient and recyclable catalyst for aerobic oxidation of alcohols in aqueous media. Mol. Catal. 2017, 440, 133–139. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Dai, Z.; Wang, X.; Zhang, Y.; Li, L.; Chen, P.; Huang, W.; Dong, X. Nanowires assembled from MnCo2O4@C nanoparticles for water splitting and all-solid-state supercapacitor. Nano Res. 2016, 9, 1300–1309. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, Z.; Dai, Y.; Jia, X.; Yu, H.; Yang, Y. Promoting role of bismuth on carbon nanotube supported platinum catalysts in aqueous phase aerobic oxidation of benzyl alcohol. Appl. Catal. B Environ. 2016, 181, 118–126. [Google Scholar] [CrossRef]
- Zhu, J.; Figueiredo, J.L.; Faria, J.L. Au/activated-carbon catalysts for selective oxidation of alcohols with molecular oxygen under atmospheric pressure: Role of basicity. Catal. Commun. 2008, 9, 2395–2397. [Google Scholar] [CrossRef]
- Wusiman, A.; Lu, C.D. Selective oxidation of benzylic, allylic and propargylic alcohols using dirhodium (II) tetraamidinate as catalyst and aqueous tert-butyl hydroperoxide as oxidant. Appl. Organomet. Chem. 2015, 29, 254–258. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mizuno, N. Supported ruthenium catalyst for the heterogeneous oxidation of alcohols with molecular oxygen. Angew. Chem. Int. Ed. 2002, 41, 4538–4542. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Yang, X.; Hu, J.; Peng, L.; Bai, L.; Huo, Q.; Guan, J. Highly efficient single atom cobalt catalyst for selective oxidation of alcohols. Appl. Catal. A 2017, 543, 61–66. [Google Scholar] [CrossRef]
- Cruz, P.; Pérez, Y.; del Hierro, I.; Fajardo, M. Copper, Copper Oxide Nanoparticles and Copper Complexes Supported on Mesoporous SBA-15 as Catalysts in the Selective Oxidation of Benzyl Alcohol in Aqueous Phase. Microporous Mesoporous Mater. 2016, 220, 136–147. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karimi, H.; Koohi, A. Selective oxidation of alcohols over nickel zirconium phosphate. Chin. J. Catal. 2015, 36, 1109–1116. [Google Scholar] [CrossRef]
- Cang, R.; Lu, B.; Li, X.; Niu, R.; Zhao, J.; Cai, Q. Iron-Chloride Ionic Liquid Immobilized on SBA-15 for Solvent-Free Oxidation of Benzyl Alcohol to Benzaldehyde with H2O2. Chem. Eng. Sci. 2015, 137, 268–275. [Google Scholar] [CrossRef]
- Jiang, N.; Ragauskas, A.J. Vanadium-catalyzed selective aerobic alcohol oxidation in ionic liquid [bmim] PF6. Tetrahedron Lett. 2007, 48, 273–276. [Google Scholar] [CrossRef]
- Öztürk, Ö.F.; Zümreoğlu-Karan, B.; Karabulut, S. Solvent-free oxidation of benzyl alcohol over chromium orthoborate. Catal. Commun. 2008, 9, 1644–1648. [Google Scholar] [CrossRef]
- Biradar, A.V.; Dongare, M.K.; Umbarkar, S.B. Selective oxidation of aromatic primary alcohols to aldehydes using molybdenum acetylide oxo-peroxo complex as catalyst. Tetrahedron Lett. 2009, 50, 2885–2888. [Google Scholar] [CrossRef]
- Goh, T.W.; Xiao, C.; Maligal-Ganesh, R.V.; Li, X.; Huang, W. Utilizing Mixed-Linker Zirconium Based Metal-Organic Frameworks to Enhance the Visible Light Photocatalytic Oxidation of Alcohol. Chem. Eng. Sci. 2015, 124, 45–51. [Google Scholar] [CrossRef]
- Forouzani, M.; Mardani, H.R.; Ziari, M.; Malekzadeh, A.; Biparva, P. Comparative Study of Oxidation of Benzyl Alcohol: Influence of Cu-Doped Metal Cation on Nano ZnO Catalytic Activity. Chem. Eng. J. 2015, 275, 220–226. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Meijboom, R. Selective liquid phase oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide over γ-Al2O3 supported copper and gold nanoparticles. Appl. Surf. Sci. 2017, 398, 19–32. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Zhou, R. Catalytic performance of manganese doped CuO–CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas. Fuel 2016, 163, 56–64. [Google Scholar] [CrossRef]
- Clarke, T.J.; Kondrat, S.A.; Taylor, S.H. Total Oxidation of Naphthalene Using Copper Manganese Oxide Catalysts. Catal. Today 2015, 258 Pt 2, 610–615. [Google Scholar] [CrossRef]
- Kim, S.C.; Park, Y.-K.; Nah, J.W. Property of a highly active bimetallic catalyst based on a supported manganese oxide for the complete oxidation of toluene. Powder Technol. 2014, 266, 292–298. [Google Scholar] [CrossRef]
- Feng, Z.; Xie, Y.; Hao, F.; Liu, P.; Luo, H.a. Catalytic oxidation of cyclohexane to KA oil by zinc oxide supported manganese 5, 10, 15, 20-tetrakis (4-nitrophenyl) porphyrin. J. Mol. Catal. A Chem. 2015, 410, 221–225. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Burange, A.S.; Kale, S.R.; Jayaram, R.V. Oxidation of alkyl aromatics to ketones by tert-butyl hydroperoxide on manganese dioxide catalyst. Tetrahedron Lett. 2012, 53, 2989–2992. [Google Scholar] [CrossRef]
- Pei, J.; Han, X.; Lu, Y. Performance and kinetics of catalytic oxidation of formaldehyde over copper manganese oxide catalyst. Build. Environ. 2015, 84, 134–141. [Google Scholar] [CrossRef]
- Reddy, V.G.; Jampaiah, D.; Chalkidis, A.; Sabri, Y.M.; Mayes, E.L.; Bhargava, S.K. Highly dispersed cobalt oxide nanoparticles on manganese oxide nanotubes for aerobic oxidation of benzyl alcohol. Catal. Commun. 2019, 130, 105763. [Google Scholar] [CrossRef]
- Li, M.; Fu, X.; Peng, L.; Bai, L.; Wu, S.; Kan, Q.; Guan, J. Synthesis of Three-Dimensional-Ordered Mesoporous Cobalt Oxides for Selective Oxidation of Benzyl Alcohol. ChemSelect 2017, 2, 9486–9489. [Google Scholar] [CrossRef]
- Zhu, J.; Kailasam, K.; Fischer, A.; Thomas, A. Supported cobalt oxide nanoparticles as catalyst for aerobic oxidation of alcohols in liquid phase. ACS Catal. 2011, 1, 342–347. [Google Scholar] [CrossRef]
- Taghavimoghaddam, J.; Knowles, G.P.; Chaffee, A.L. Mesoporous Silica SBA-15 Supported Co3O4 Nanorods as Efficient Liquid Phase Oxidative Catalysts. Top. Catal. 2012, 55, 571–579. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, S.M.; Tremel, W.; Tahir, M.N.; Adil, S.F. A highly reduced graphene oxide/ZrOx–MnCO3 or–Mn2O3 nanocomposite as an efficient catalyst for selective aerial oxidation of benzylic alcohols. RSC Adv. 2017, 7, 55336–55349. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Alzahrani, A.Y.; Al-Warthan, A.; Siddiqui, M.R.H.; Adil, S.F. Mixed Zinc/Manganese on Highly Reduced Graphene Oxide: A Highly Active Nanocomposite Catalyst for Aerial Oxidation of Benzylic Alcohols. Catalysts 2017, 7, 391. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Alharthi, A.I.; Varala, R.; Siddiqui, M.R.H.; Adil, S.F. Ag2O nanoparticles/MnCO3,–MnO2 or–Mn2O3/highly reduced graphene oxide composites as an efficient and recyclable oxidation catalyst. Arab. J. Chem. 2019, 12, 54–68. [Google Scholar] [CrossRef]
- Gac, W. The influence of silver on the structural, redox and catalytic properties of the cryptomelane-type manganese oxides in the low-temperature CO oxidation reaction. Appl. Catal. B Environ. 2007, 75, 107–117. [Google Scholar] [CrossRef]
- Hu, H.; Xu, J.-y.; Yang, H.; Liang, J.; Yang, S.; Wu, H. Morphology-Controlled Hydrothermal Synthesis of MnCO3 Hierarchical Superstructures with Schiff Base as Stabilizer. Mater. Res. Bull. 2011, 46, 1908–1915. [Google Scholar] [CrossRef]
- Maslen, E.; Streltsov, V.; Streltsova, N.; Ishizawa, N. Electron density and optical anisotropy in rhombohedral carbonates. III. Synchrotron X-ray studies of CaCO3, MgCO3 and MnCO3. Acta Crystallogr. Sect. B Struct. Sci. 1995, 51, 929–939. [Google Scholar] [CrossRef]
- Dubal, D.; Dhawale, D.; Salunkhe, R.; Pawar, S.; Lokhande, C. A novel chemical synthesis and characterization of Mn3O4 thin films for supercapacitor application. Appl. Surf. Sci. 2010, 256, 4411–4416. [Google Scholar] [CrossRef]
- Zhu, C.; Saito, G.; Akiyama, T. A new CaCO 3-template method to synthesize nanoporous manganese oxide hollow structures and their transformation to high-performance LiMn 2 O 4 cathodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 7077–7082. [Google Scholar] [CrossRef]
- Sreethawong, T.; Ngamsinlapasathian, S.; Yoshikawa, S. Facile surfactant-aided sol–gel synthesis of mesoporous-assembled Ta2O5 nanoparticles with enhanced photocatalytic H 2 production. J. Mol. Catal. A Chem. 2013, 374, 94–101. [Google Scholar] [CrossRef]
- Smoláková, L.; Kout, M.; Koudelková, E.; Čapek, L. Effect of Calcination Temperature on the Structure and Catalytic Performance of the Ni/Al2O3 and Ni–Ce/Al2O3 Catalysts in Oxidative Dehydrogenation of Ethane. Ind. Eng. Chem. Res. 2015, 54, 12730–12740. [Google Scholar] [CrossRef]
- Kunkalekar, R.; Salker, A. Low temperature carbon monoxide oxidation over nanosized silver doped manganese dioxide catalysts. Catal. Commun. 2010, 12, 193–196. [Google Scholar] [CrossRef]
- Su, F.Z.; Liu, Y.M.; Wang, L.C.; Cao, Y.; He, H.Y.; Fan, K.N. Ga–Al Mixed-Oxide-Supported Gold Nanoparticles with Enhanced Activity for Aerobic Alcohol Oxidation. Angew. Chem. Int. Ed. 2008, 120, 340–343. [Google Scholar] [CrossRef]
- Ishida, T.; Takamura, R.; Takei, T.; Akita, T.; Haruta, M. Support effects of metal oxides on gold-catalyzed one-pot N-alkylation of amine with alcohol. Appl. Catal. A 2012, 413, 261–266. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Kendall, T.A.; Martin, S.T.; Friend, C.M.; Vlassak, J.J. Heteroepitaxial nucleation and oriented growth of manganese oxide islands on carbonate minerals under aqueous conditions. Environ. Sci. Technol. 2005, 39, 1239–1249. [Google Scholar] [CrossRef]
- Tang, Q.; Gong, X.; Wu, C.; Chen, Y.; Borgna, A.; Yang, Y. Insights into the nature of alumina-supported MnOOH and its catalytic performance in the aerobic oxidation of benzyl alcohol. Catal. Commun. 2009, 10, 1122–1126. [Google Scholar] [CrossRef]
- Jha, A.; Mhamane, D.; Suryawanshi, A.; Joshi, S.M.; Shaikh, P.; Biradar, N.; Ogale, S.; Rode, C.V. Triple nanocomposites of CoMn2O4, Co3O4 and reduced graphene oxide for oxidation of aromatic alcohols. Catal. Sci. Technol. 2014, 4, 1771–1778. [Google Scholar] [CrossRef]
- Ragupathi, C.; Vijaya, J.J.; Narayanan, S.; Jesudoss, S.; Kennedy, L.J. Highly Selective Oxidation of Benzyl Alcohol to Benzaldehyde with Hydrogen Peroxide by Cobalt Aluminate Catalysis: A Comparison of Conventional and Microwave Methods. Ceram. Int. 2015, 41, 2069–2080. [Google Scholar] [CrossRef]
- Assal, M.E.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tremel, W.; Nawaz Tahir, M.; Adil, S.F. Synthesis and Comparative Catalytic Study of Zirconia–MnCO3 or–Mn2O3 for the Oxidation of Benzylic Alcohols. ChemOpen 2016, 6, 112–120. [Google Scholar]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Venkata, S.J.; Alzahrani, A.Y.; Al-Warthan, A.; Al-Tamrah, S.A.; Siddiqui, M.R.H.; Hashmi, S.A.; et al. Silver-doped manganese based nanocomposites for aerial oxidation of alcohols. Mater. Express 2018, 8, 35–54. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Kuniyil, M.; Khan, M.; Shaik, M.R.; Alwarthan, A.; Labis, J.P.; Siddiqui, M.R.H. Synthesis and Comparative Catalytic Study of Zinc Oxide (ZnOx) Nanoparticles Promoted MnCO3, MnO2 and Mn2O3 for Selective Oxidation of Benzylic Alcohols Using Molecular Oxygen. Mater. Express 2017, 7, 79–92. [Google Scholar] [CrossRef]
- Yang, X.; Wu, S.; Hu, J.; Fu, X.; Peng, L.; Kan, Q.; Huo, Q.; Guan, J. Highly efficient N-doped magnetic cobalt-graphene composite for selective oxidation of benzyl alcohol. Catal. Commun. 2016, 87, 90–93. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Wang, H.; Peng, F. Controllable synthesis and catalytic performance of graphene-supported metal oxide nanoparticles. Chin. J. Catal. 2014, 35, 952–959. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hasheminasab, H.R. Vanadium phosphorus oxide catalyst promoted by cobalt doping for mild oxidation of benzyl alcohol to benzaldehyde in the liquid phase. Appl. Catal. A 2014, 482, 189–197. [Google Scholar] [CrossRef]
- Panwar, V.; Kumar, P.; Ray, S.S.; Jain, S.L. Organic inorganic hybrid cobalt phthalocyanine/polyaniline as efficient catalyst for aerobic oxidation of alcohols in liquid phase. Tetrahedron Lett. 2015, 56, 3948–3953. [Google Scholar] [CrossRef]
- Peyrovi, M.; Mahdavi, V.; Salehi, M.; Mahmoodian, R. Oxidation of alcohols with tert-butylhydroperoxide catalyzed by Co (II) complexes immobilized between silicate layers of bentonite. Catal. Commun. 2005, 6, 476–479. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Solvent free clean selective oxidation of alcohols catalyzed by mono transition metal (Co, Mn, Ni)-substituted Keggin-phosphomolybdates using hydrogen peroxide. Appl. Catal. A 2013, 459, 59–64. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Li, W.; Liu, C.; Wang, F.; He, J.; Guild, C.; Jin, J.; Kriz, D.; Miao, R. Aerobic oxidation of alcohols over Ru-Mn-Ce and Ru-Co-Ce catalysts: The effect of calcination temperature. Appl. Catal. A 2017, 535, 77–84. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Ataee-Kachouei, T.; Moeini, F. A Novel and Active Catalyst Ag/ZnO for Oxidant-Free Dehydrogenation of Alcohols. Mater. Res. Bull. 2015, 72, 98–105. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; An, Y.; Wang, X.; Xu, G.; Chen, Y.; Dai, L. Promotional synergistic effect of Sn doping into a novel bimetallic Sn-W oxides/graphene catalyst for selective oxidation of alcohols using aqueous H2O2 without additives. Appl. Catal. A 2018, 558, 26–33. [Google Scholar] [CrossRef]
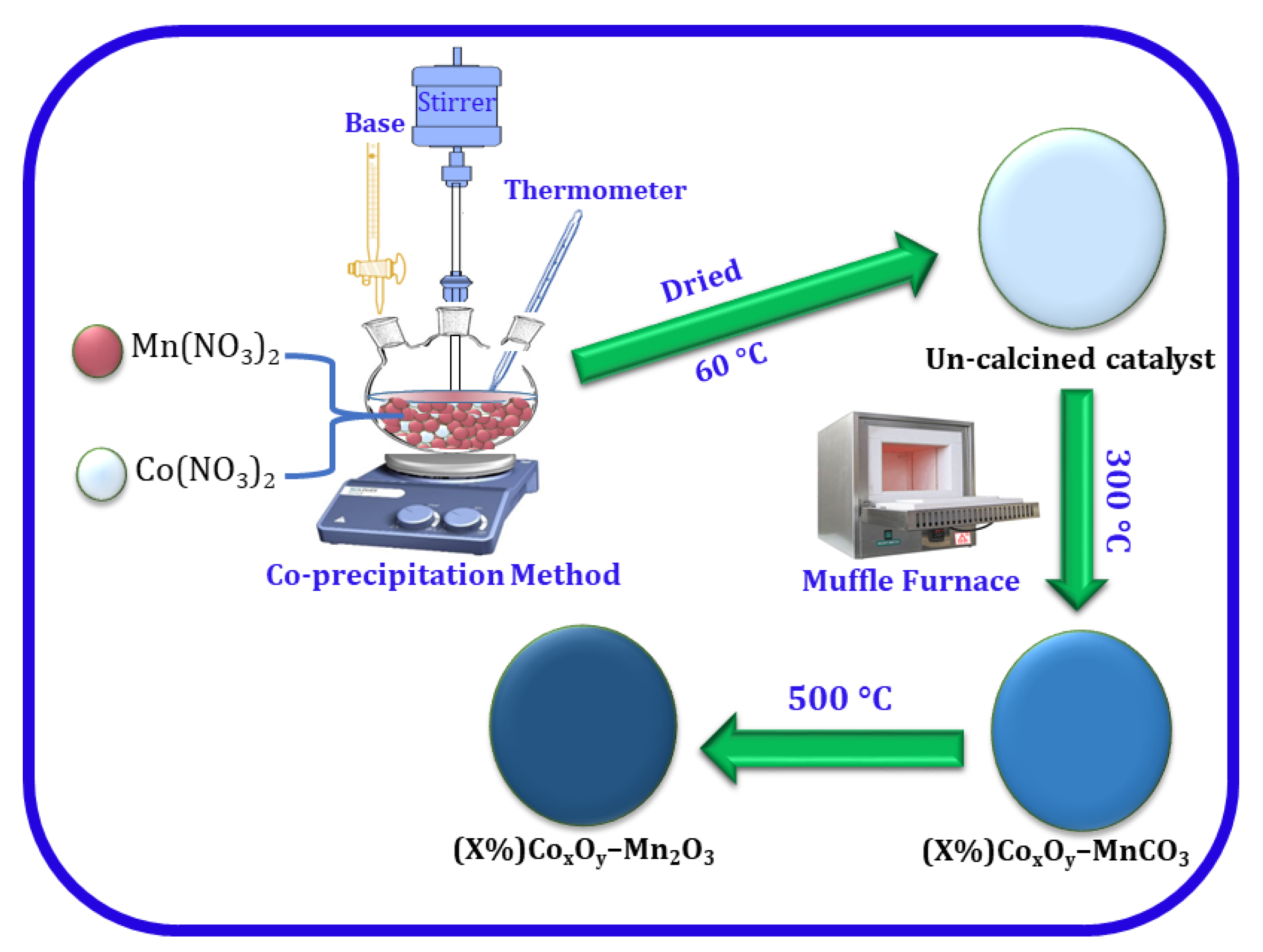
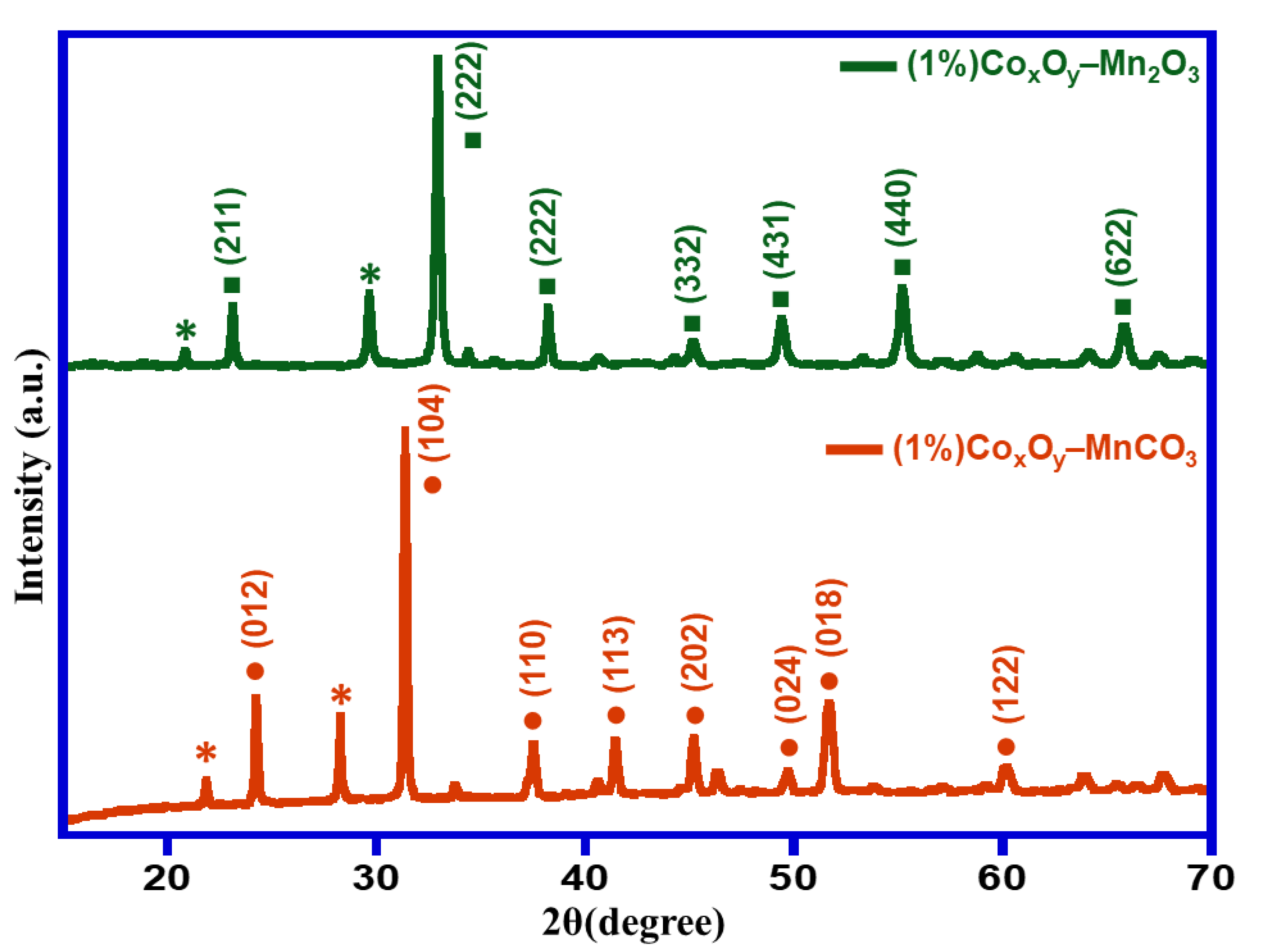

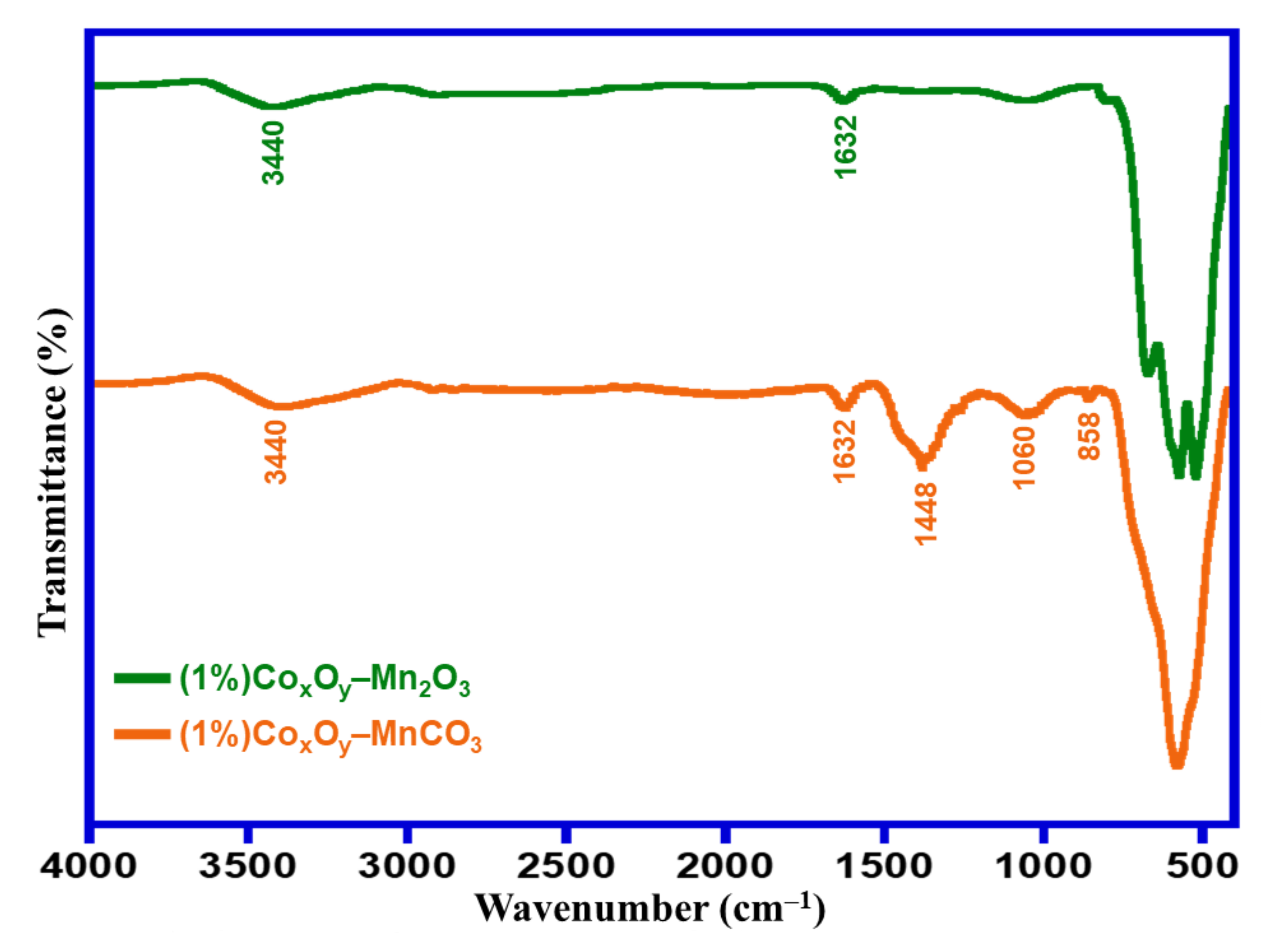


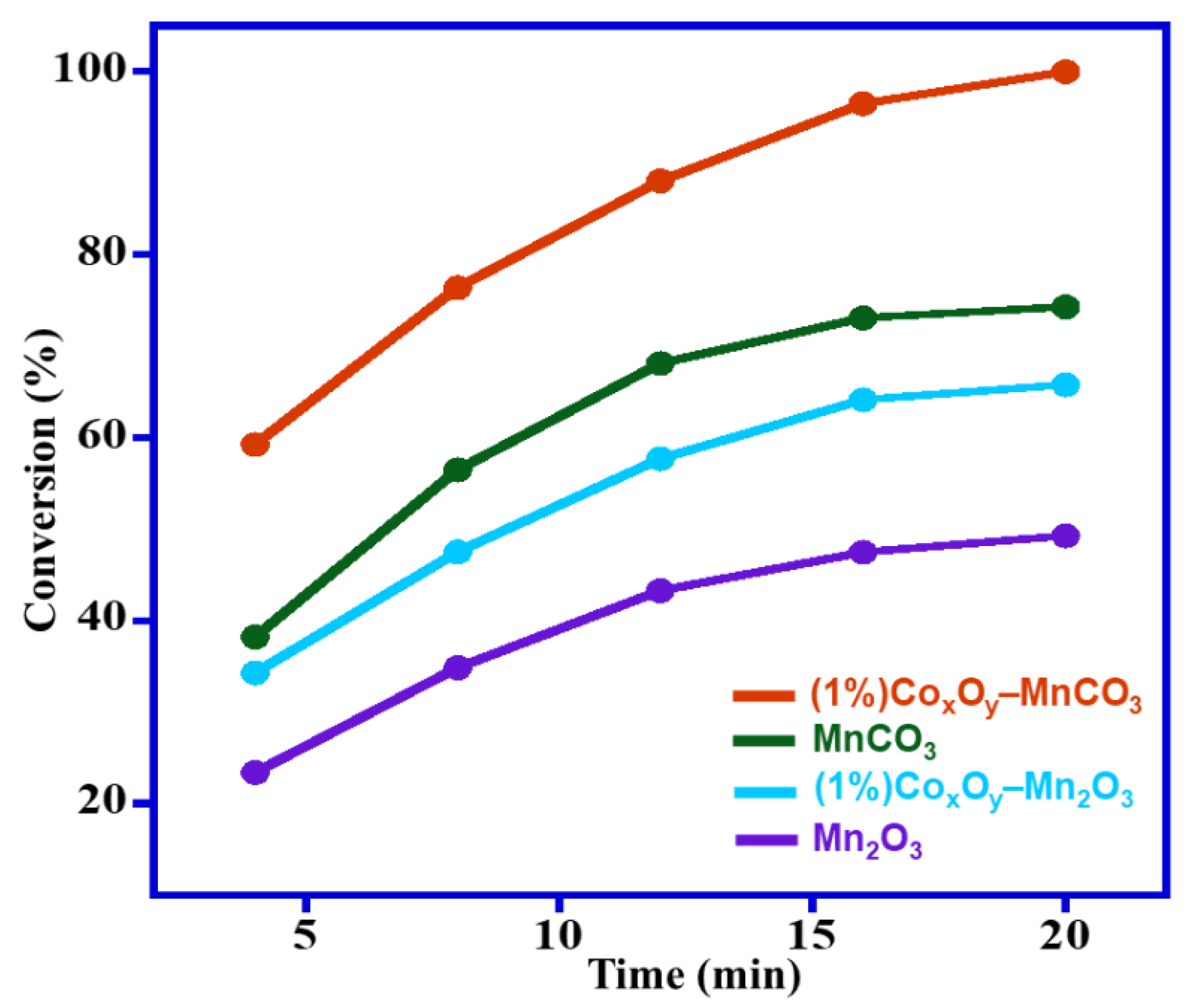



| Entry | Catalyst | Calcination Temperature (°C) | Specific Surface Area (m2/g) | Conversion (%) | Specific Activity (mmol/g·h) | Selectivity (%) |
|---|---|---|---|---|---|---|
| 1 | MnCO3 | 300 | 85.2 | 74.3 | 14.9 | >99 |
| 2 | Mn2O3 | 500 | 24.1 | 49.3 | 9.9 | >99 |
| 3 | (1%)CoxOy–MnCO3 | 300 | 108.4 | 100.0 | 20.0 | >99 |
| 4 | (1%)CoxOy–Mn2O3 | 500 | 56.7 | 65.8 | 13.2 | >99 |
| Entry | CoxOy (%) | Catalyst | Conversion (%) | Specific Activity (mmol/g·h) | Selectivity (%) |
|---|---|---|---|---|---|
| 1 | 0 | (0%)CoxOy–MnCO3 | 74.3 | 14.9 | >99 |
| 2 | 1 | (1%)CoxOy–MnCO3 | 100.0 | 20.0 | >99 |
| 3 | 3 | (3%)CoxOy–MnCO3 | 94.2 | 18.8 | >99 |
| 4 | 5 | (5%)CoxOy–MnCO3 | 83.1 | 16.6 | >99 |
| 5 | 7 | (7%)CoxOy–MnCO3 | 65.4 | 13.1 | >99 |
| Entry | Temperature (°C) | Conversion (%) | Specific Activity (mmol/g·h) | Selectivity (%) |
|---|---|---|---|---|
| 1 | RT | 35.7 | 7.1 | >99 |
| 2 | 40 | 56.3 | 11.3 | >99 |
| 3 | 60 | 70.9 | 14.2 | >99 |
| 4 | 80 | 84.5 | 16.9 | >99 |
| 5 | 100 | 100.0 | 20.0 | >99 |
| Entry | Quantity (mg) | Conversion (%) | Specific Activity (mmol/g·h) | Selectivity (%) |
|---|---|---|---|---|
| 1 | 50 | 25.8 | 30.9 | >99 |
| 2 | 100 | 41.6 | 24.9 | >99 |
| 3 | 150 | 57.1 | 22.8 | >99 |
| 4 | 200 | 70.1 | 21.0 | >99 |
| 5 | 250 | 86.9 | 20.8 | >99 |
| 6 | 300 | 100.0 | 20.0 | >99 |
| Catalyst | Conversion (%) | Selectivity (%) | Time (h) | Temperature (°C) | Specific Activity (mmol/g·h) | Ref. | |
|---|---|---|---|---|---|---|---|
| 1. | (1%)CoxOy–MnCO3 | 100.0 | >99 | 0.3 | 100 | 20.0 | Herein |
| 2. | MnCoO–(1%)RGO | 78.0 | 100.0 | 2 | 140 | 12.6 | [47] |
| 3. | ZrOx(1%)–MnCO3 | 100.0 | >99 | 0.5 | 100 | 13.3 | [49] |
| 4. | Ag2O(1%)–MnO2 | 100.0 | >99 | 2.0 | 100 | 3.3 | [50] |
| 5. | ZnOx(1%)–MnCO3 | 100.0 | >99 | 0.42 | 100 | 16.0 | [51] |
| 6. | ZrOx(1%)–MnCO3/(1%)HRG | 100.0 | >99 | 0.15 | 100 | 44.4 | [32] |
| 7. | Ag2O(1%)–MnO2/(5%)HRG | 100.0 | >99 | 0.58 | 100 | 11.4 | [34] |
| 8. | ZnOx(1%)–MnCO3/(1%)HRG | 100.0 | >99 | 0.12 | 100 | 57.1 | [33] |
| 9. | CoAl2O4 | 96.0 | 98.9 | 8 | 80 | 1.2 | [48] |
| 10. | Co/NG | 89.5 | 97.3 | 8 | 100 | 4.5 | [52] |
| 11. | Co3O4-SBA-15 | 31.0 | 73.0 | 12 | reflux | 5.2 | [31] |
| 12. | CoOx/RGO-HP | 96.0 | >99 | 6 | 110 | 14.8 | [53] |
| 13. | Co/Al2O3 | 93.0 | - | 1 | reflux | 18.6 | [4] |
| 14. | VPO-Co | 66.0 | 74.0 | 8 | 90 | 8.3 | [54] |
| 15. | CoPc/PANI | 82.0 | - | 4 | 65 | 2.1 | [55] |
| 16. | Co3O4/MnO2 | 93.0 | 99.0 | 6 | 100 | 6.2 | [28] |
| 17. | [Co(bpy)2]2+ | 53.0 | 100.0 | 8 | reflux | 4.0 | [56] |
| 18. | PMo11Co | 56.5 | 90.9 | 24 | 90 | 11.8 | [57] |
| 19. | Ru-Co-Ce | 92.3 | 100 | 6 | 60 | 12.3 | [58] |
| Entry | Alcohols | Carbonyls | Time (minutes) | Conversion (%) –Selectivity (%) |
|---|---|---|---|---|
| 1 |  |  | 20 | 100 – >99 |
| 2 |  |  | 25 | 100 – >99 |
| 3 |  |  | 25 | 100 – >99 |
| 4 |  |  | 30 | 100 – >99 |
| 5 |  |  | 30 | 100 – >99 |
| 6 |  |  | 35 | 100 – >99 |
| 7 |  |  | 35 | 100 – >99 |
| 8 |  |  | 35 | 100 – >99 |
| 9 |  |  | 40 | 100 – >99 |
| 10 |  |  | 40 | 100 – >99 |
| 11 |  |  | 45 | 100 – >99 |
| 12 |  |  | 45 | 100 – >99 |
| 13 |  |  | 50 | 100 – >99 |
| 14 |  |  | 55 | 100 – >99 |
| 15 |  |  | 65 | 100 – >99 |
| 16 |  |  | 70 | 100 – >99 |
| 17 |  |  | 70 | 100 – >99 |
| 18 |  |  | 70 | 100 – >99 |
| 19 |  |  | 75 | 100 – >99 |
| 20 |  |  | 95 | 100 – >99 |
| 21 |  |  | 30 | 100 – >99 |
| 22 |  |  | 35 | 100 – >99 |
| 23 |  |  | 45 | 100 – >99 |
| 24 |  |  | 35 | 100 – >99 |
| 25 |  |  | 40 | 100 – >99 |
| 26 |  |  | 35 | 100 – >99 |
| 27 |  |  | 45 | 100 – >99 |
| 28 |  |  | 75 | 100 – >99 |
| 29 |  |  | 190 | 100 – >99 |
| 30 |  |  | 240 | 100 – >99 |
| 31 |  |  | 130 | 100 – >99 |
| 32 |  |  | 90 | 100 – >99 |
| 33 |  |  | 230 | 100 – >99 |
| 34 |  |  | 250 | 100 – >99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alduhaish, O.; Adil, S.F.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Manqari, K.M.; Sekou, D.; Khan, M.; Khan, A.; Dewidar, A.Z.; et al. Synthesis and Characterization of CoxOy–MnCO3 and CoxOy–Mn2O3 Catalysts: A Comparative Catalytic Assessment Towards the Aerial Oxidation of Various Kinds of Alcohols. Processes 2020, 8, 910. https://doi.org/10.3390/pr8080910
Alduhaish O, Adil SF, Assal ME, Shaik MR, Kuniyil M, Manqari KM, Sekou D, Khan M, Khan A, Dewidar AZ, et al. Synthesis and Characterization of CoxOy–MnCO3 and CoxOy–Mn2O3 Catalysts: A Comparative Catalytic Assessment Towards the Aerial Oxidation of Various Kinds of Alcohols. Processes. 2020; 8(8):910. https://doi.org/10.3390/pr8080910
Chicago/Turabian StyleAlduhaish, Osamah, Syed Farooq Adil, Mohamed E. Assal, Mohammed Rafi Shaik, Mufsir Kuniyil, Khalid M. Manqari, Doumbia Sekou, Mujeeb Khan, Aslam Khan, Ahmed Z. Dewidar, and et al. 2020. "Synthesis and Characterization of CoxOy–MnCO3 and CoxOy–Mn2O3 Catalysts: A Comparative Catalytic Assessment Towards the Aerial Oxidation of Various Kinds of Alcohols" Processes 8, no. 8: 910. https://doi.org/10.3390/pr8080910
APA StyleAlduhaish, O., Adil, S. F., Assal, M. E., Shaik, M. R., Kuniyil, M., Manqari, K. M., Sekou, D., Khan, M., Khan, A., Dewidar, A. Z., Al-Warthan, A., & Siddiqui, M. R. H. (2020). Synthesis and Characterization of CoxOy–MnCO3 and CoxOy–Mn2O3 Catalysts: A Comparative Catalytic Assessment Towards the Aerial Oxidation of Various Kinds of Alcohols. Processes, 8(8), 910. https://doi.org/10.3390/pr8080910










